Molecular Identification and Pathogenicity of Fusarium Fungi Causing Potato Dry Rot in Shanxi Province, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Pathogen Isolation and Purification
2.2. Molecular Characterization
2.3. Morphological Characterization
2.4. Determination of Pathogenicity
2.5. Data Statistics and Analysis
3. Results
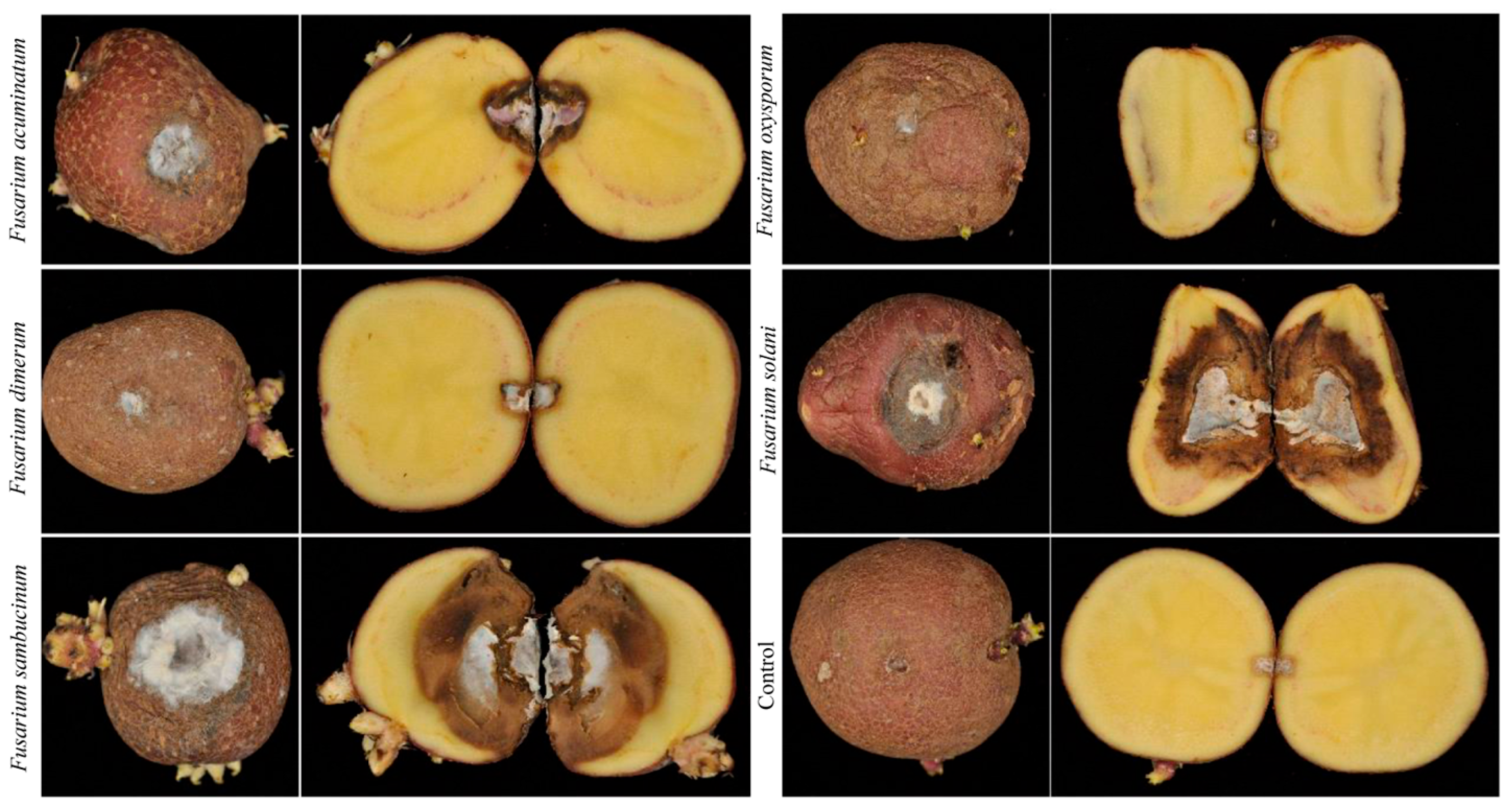
3.1. Characterization of Potato Dry Rot Disease Symptoms During Storage
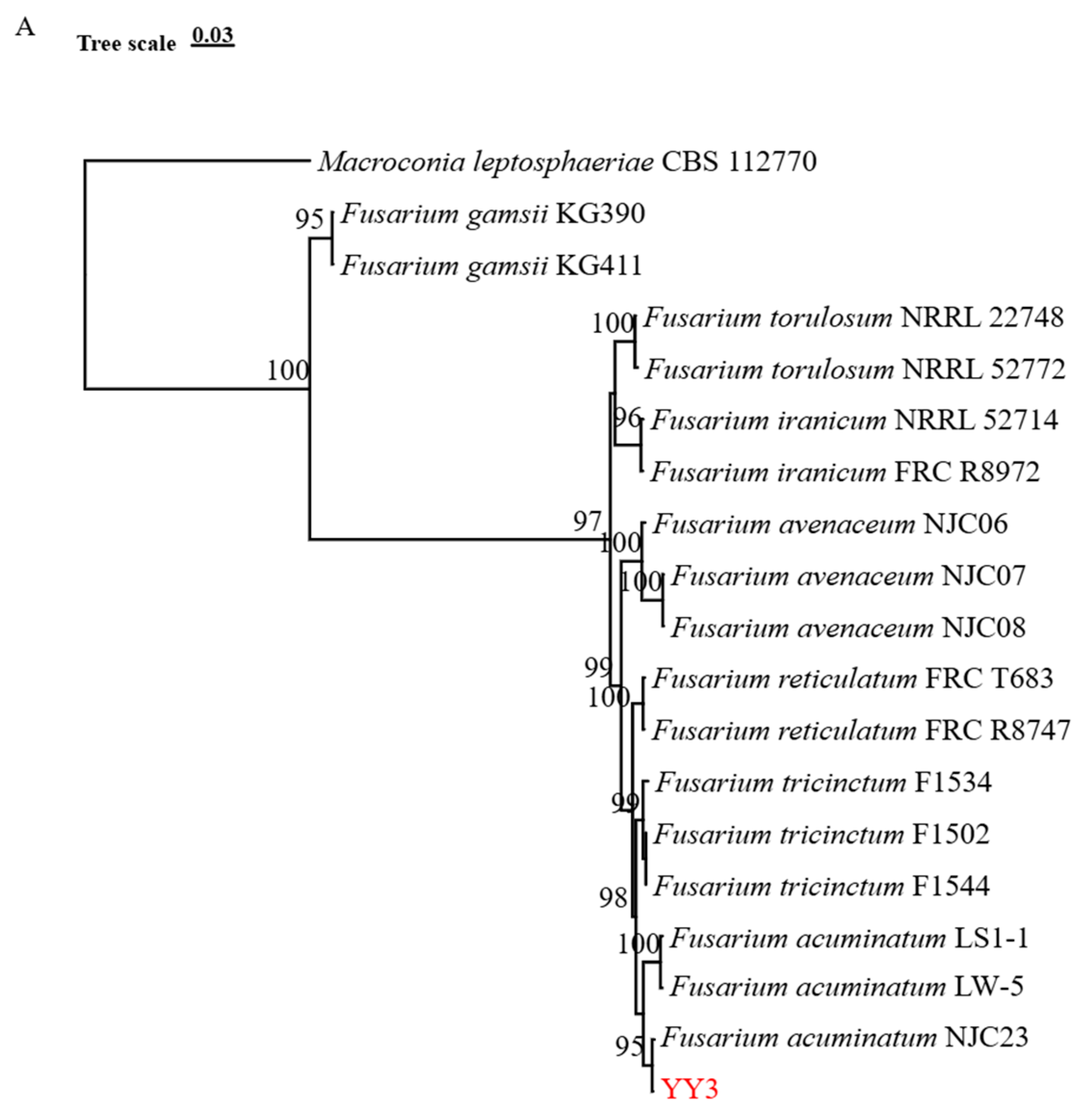
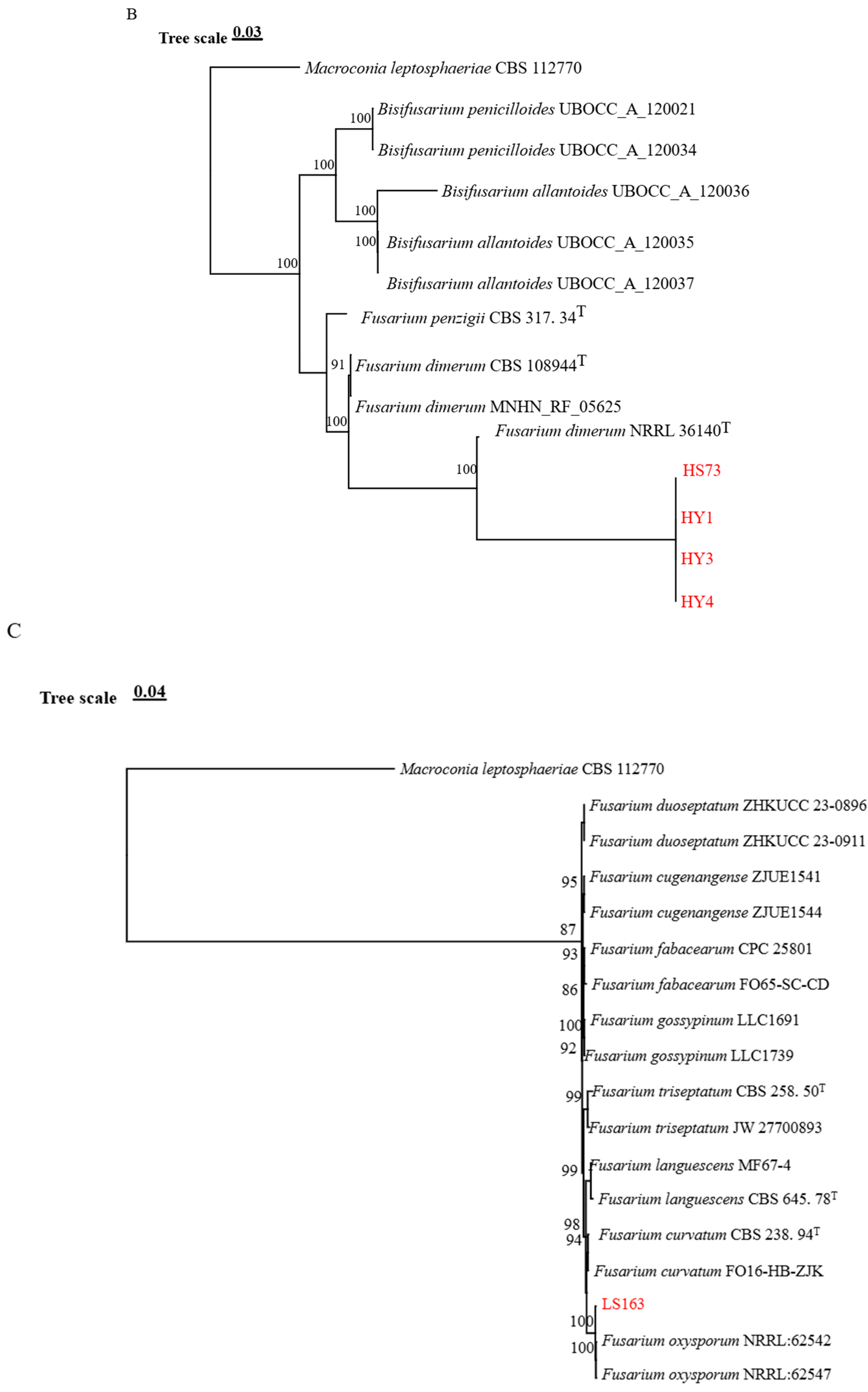
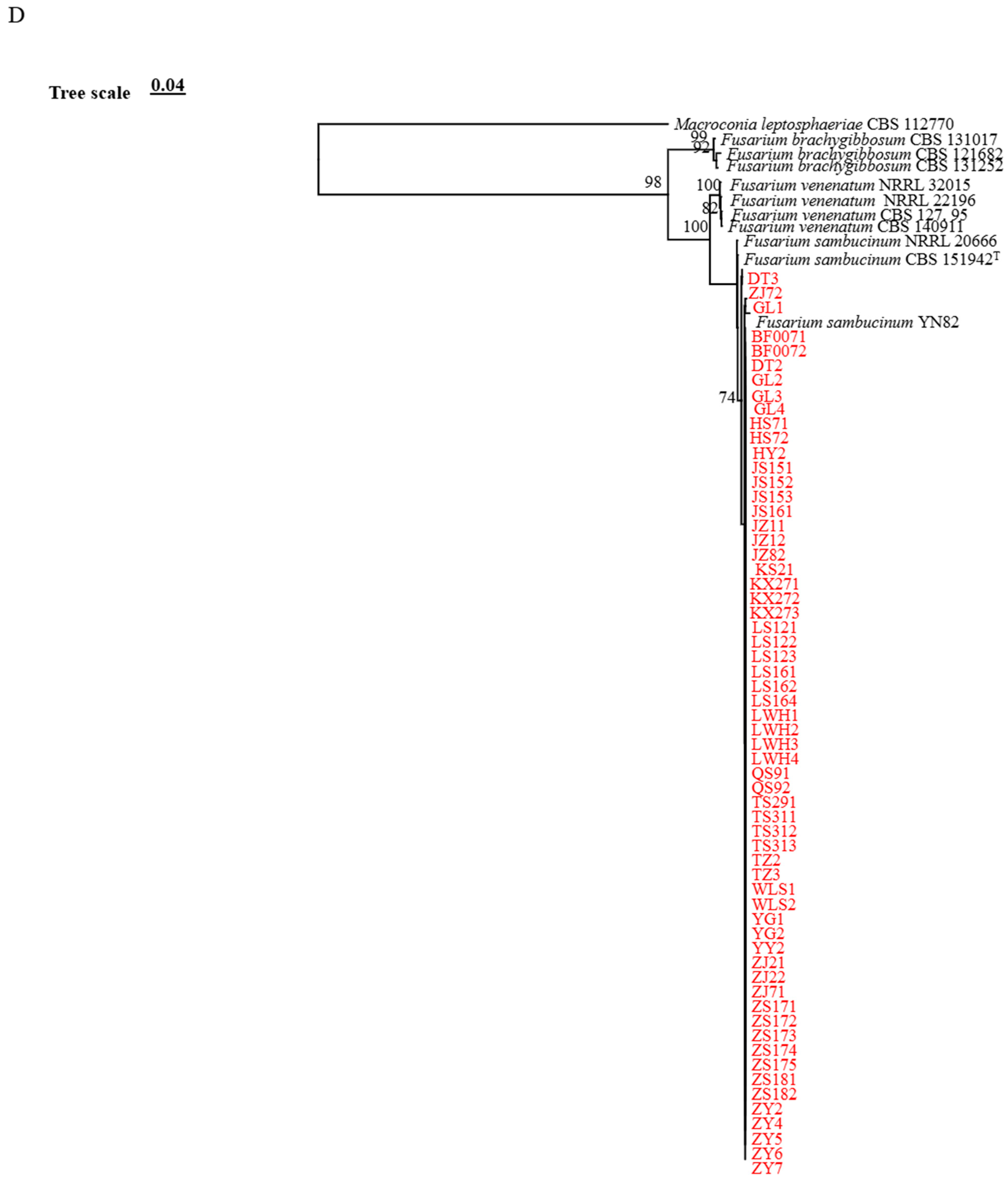
3.2. Phylogenetic Analysis
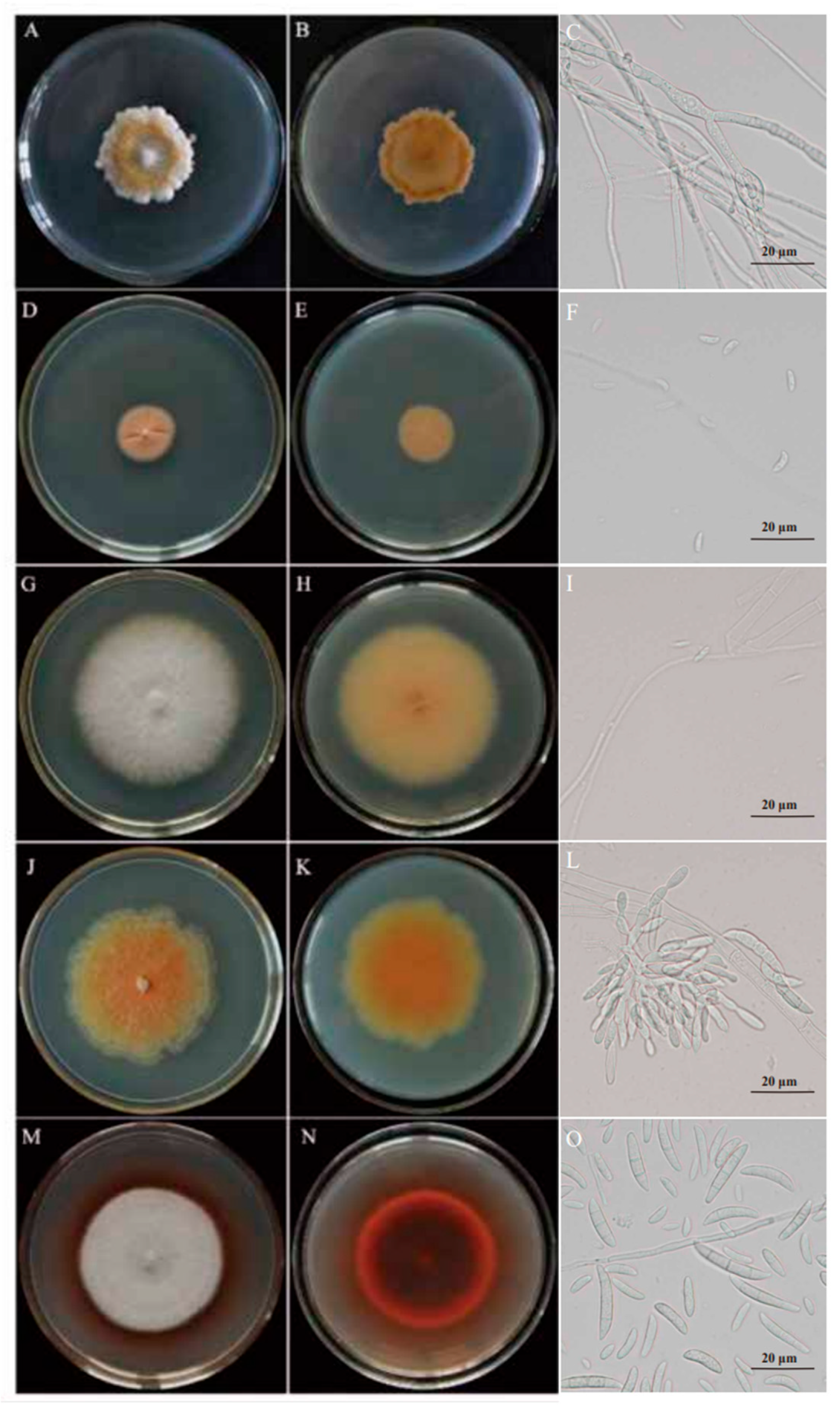
3.3. Morphology of the Potato Dry-Rot Pathogens
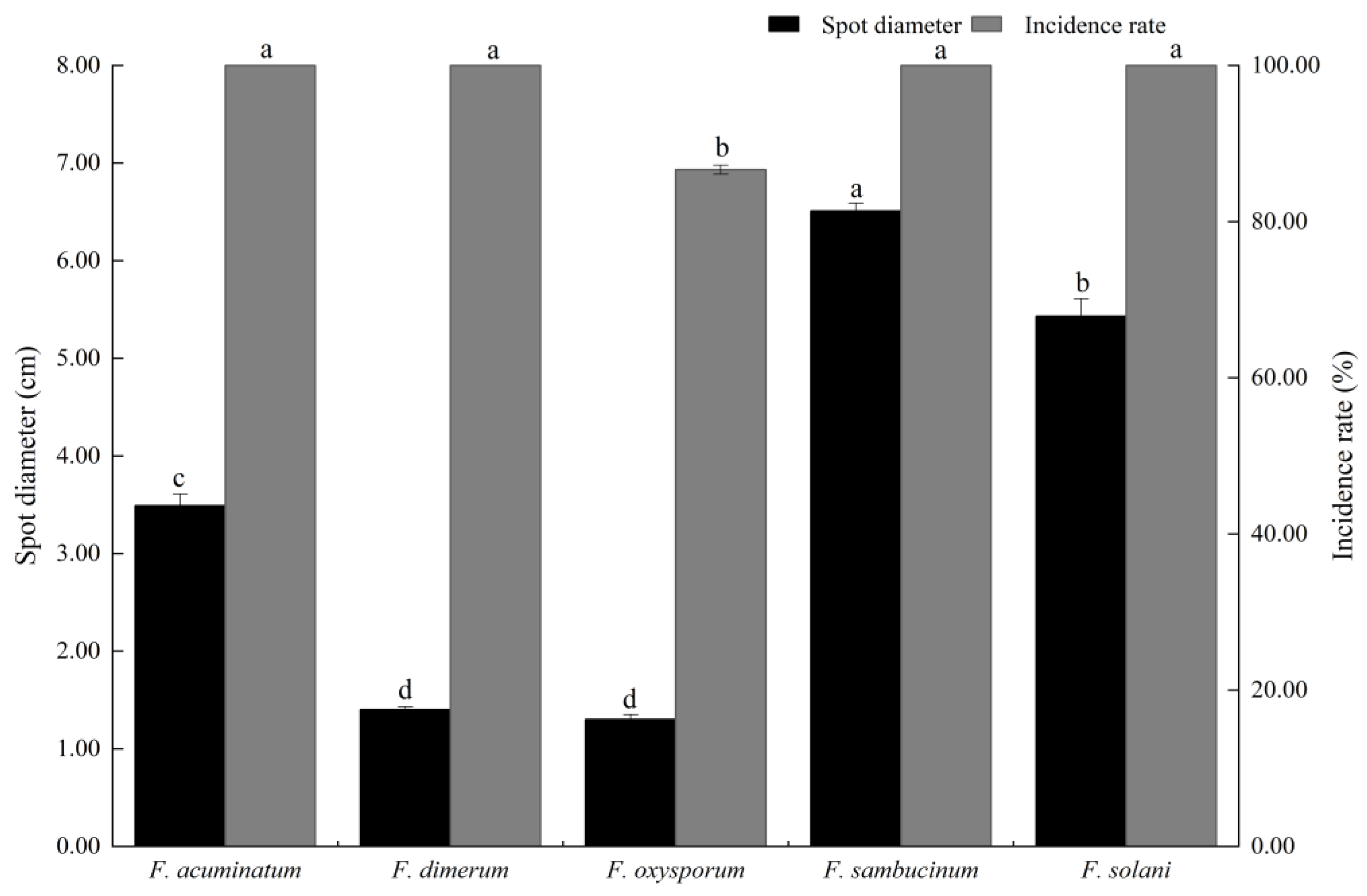
3.4. Pathogenicity Analysis
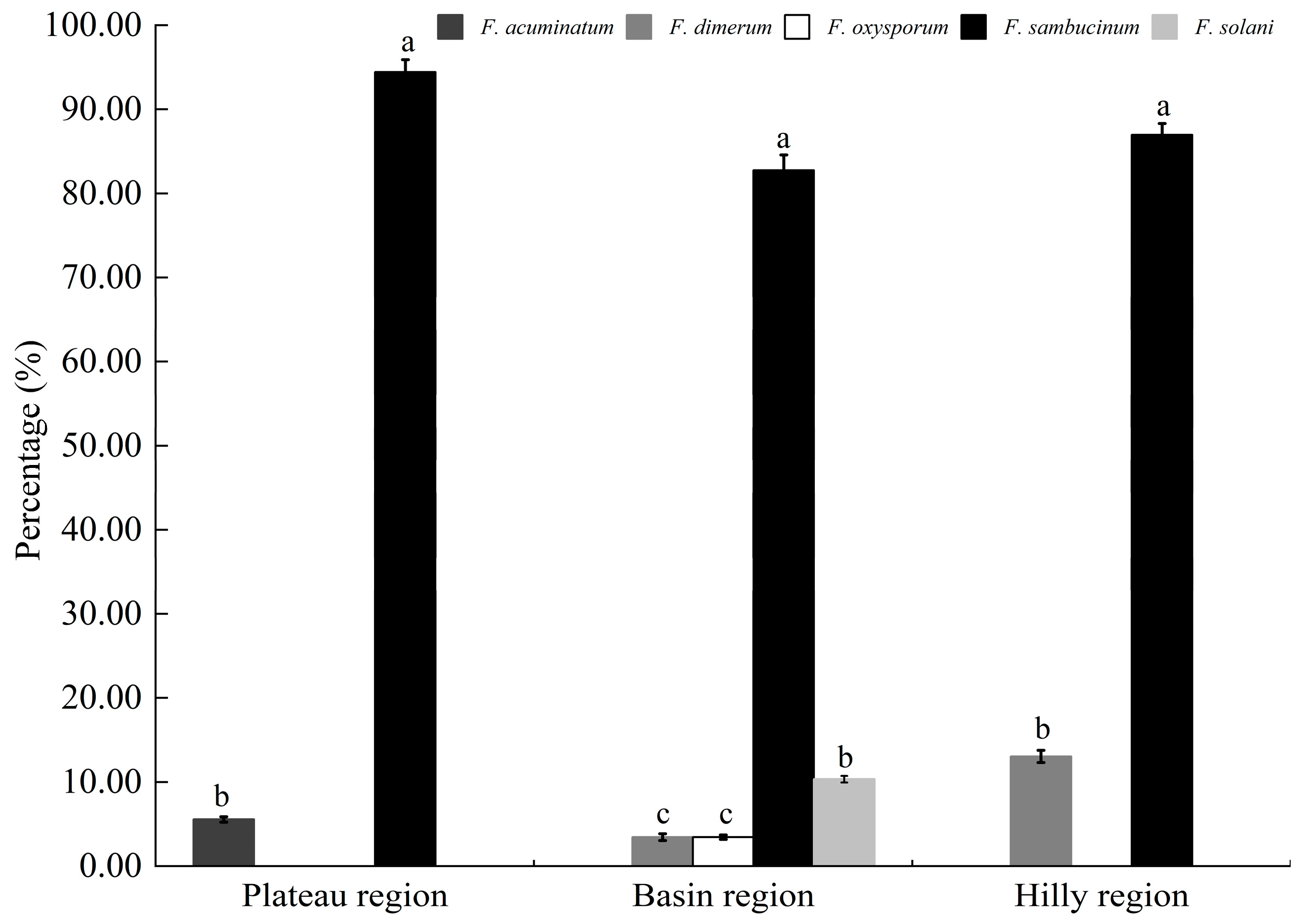
3.5. Composition and Distribution of the Pathogens Causing Potato Dry Rot in Three Potato-Growing Areas
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qu, D.Y.; Xie, K.Y.; Jin, L.P.; Pang, W.F.; Bian, C.S.; Duan, S.G. Development of potato industry and food security in China. Sci. Agric. Sin. 2005, 38, 358–362. [Google Scholar] [CrossRef]
- Gao, Y.L.; Xu, J.; Liu, N.; Zhou, Q.; Ding, X.H.; Zhan, J.S.; Cheng, X.Y.; Huang, J.; Lu, Y.W.; Yang, Y.H. Current status and management strategies for potato insect pests and diseases in China. Plant Prot. 2019, 45, 106–111. [Google Scholar] [CrossRef]
- Du, M.R.; Ren, X.Y.; Sun, Q.H.; Wang, Y.; Zhang, R.F. Characterization of Fusarium spp. causing potato dry rot in China and susceptibility evaluation of Chinese potato germplasm to the pathogen. Potato Res. 2012, 55, 175–184. [Google Scholar] [CrossRef]
- Fan, Y.L.; Zhang, W.N.; Kang, Y.C.; Shi, M.F.; Yang, X.Y.; Yu, H.F.; Zhang, R.Y.; Liu, Y.H.; Qin, S.H. Physiological and dynamic transcriptome analysis of two potato varieties reveal response of lignin and MAPK signal to dry rot caused by Fusarium sulphureum. Sci. Hortic. 2021, 289, 110470. [Google Scholar] [CrossRef]
- Xue, H.L.; Liu, Q.L.; Yang, Z.M. Pathogenicity, mycotoxin production, and control of potato dry rot caused by Fusarium spp.: A review. J. Fungi 2023, 9, 843. [Google Scholar] [CrossRef]
- Li, B.Y.; Ma, S.M. Pathogens of soybean root rot and screening of resistant sources. J. Plant Prot. 2000, 27, 91–92. [Google Scholar] [CrossRef]
- Wu, H.Y.; Sun, S.R.; Fan, Z.W.; Liu, C.G.; Yang, T.Y.; Zhu, J.H. Research condition and prevention countermeasures of maize stalk rot. J. Maize Sci. 2007, 15, 129–132. [Google Scholar] [CrossRef]
- Xu, Y.H.; Li, Y.; Xu, X.Y. Progress in research on Fusarium wilt of tomato. J. Northeast Agric. Univ. 2008, 39, 128–134. [Google Scholar] [CrossRef]
- Li, Y.T.; Xia, X.N.; Zhao, Q.M.; Dong, P. The biocontrol of potato dry rot by microorganisms and bioactive substances: A review. Physiol. Mol. Plant Pathol. 2022, 122, 101919. [Google Scholar] [CrossRef]
- Wang, W.Z.; Min, F.X.; Yang, S.; Wei, Q.; Guo, M.; Gao, Y.; Hu, L.S.; Sheng, W.M. Research progress on potato dry rot disease in China and its control measures. China Veg. 2020, 40, 22–29. [Google Scholar] [CrossRef]
- Chen, S.Z.; Chen, T.X.; Ji, X.X.; Yang, C.D.; Chen, X.R. Identification of the pathogen of the potato dry rot disease and screening of fungicides in the Minxian district of Gansu Province. Pratacultural Sci. 2017, 34, 2218–2225. [Google Scholar] [CrossRef]
- Li, J.H.; Wang, D.; Chai, Z.X.; Lester, W.B. Isolation and identification of the dominant pathogens causing potato Fusarium dry rot in Gansu Province. Acta Phytopathol. Sin. 2011, 41, 456–463. [Google Scholar] [CrossRef]
- Liu, Z.P.; Wang, L.X.; Li, R.X.; Liu, C.F.; Li, Y.J. Identification and biological character test of wheat common rot pathogen. Acta Agric. Boreali-Sin. 2002, 17, 44–48. [Google Scholar] [CrossRef]
- O’Donnell, K.; Whitaker, B.; Laraba, I.; Proctor, R.; Brown, D.; Broders, K.; Kim, H.; McCormick, S.P.; Busman, M.; Aoki, T.; et al. DNA sequence-based identification of Fusarium: A work in progress. Plant Dis. 2021, 106, 1597–1609. [Google Scholar] [CrossRef]
- O’Donnell, K.; Kistler, H.C.; Cigelnik, E.; Ploetz, R.C. Multiple evolutionary origins of the fungus causing Panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA 1998, 95, 2044–2049. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Bashyal, B.M.; Shanmugam, V.; Lal, M.K.; Kumar, R.; Sharma, S.; Vinod; Gaikwad, K.; Singh, B.; Aggarwal, R. Impact of Fusarium dry rot on physicochemical attributes of potato tubers during postharvest storage. Postharvest Biol. Technol. 2021, 181, 111638. [Google Scholar] [CrossRef]
- Fu, J.N.; Liu, X.H.; Cai, F.D.; Kou, L.P. Identification of pathogenic fungus causing a decay of stored pomegranate fruits using molecular biology technique. Acta Hortic. Sin. 2007, 34, 877–882. [Google Scholar] [CrossRef]
- Min, F.X.; Wang, X.D.; Hu, L.S.; Wei, Q.; Dong, X.Z.; Li, F.L.; Li, H.C.; Liu, W.T.; Guo, M. Identification of species and pathogenicity of the Fusarium on potato in Heilongjiang Province. Plant Prot. 2010, 36, 112–115. [Google Scholar] [CrossRef]
- Wei, W.; Zhu, J.H.; Zhang, H.L.; Yang, Z.H. Identification of the pathogens causing potato dry rot in Hebei and Inner Mongolia. J. Plant Prot. 2013, 40, 296–300. [Google Scholar] [CrossRef]
- Ye, Q.M.; Wang, G.C. On Fusarium dry rot of potato in Zhejiang. Acta Phytopathol. Sin. 1995, 25, 148. [Google Scholar] [CrossRef]
- Boyd, A. Dry-rot disease of the potato: VII. the effect of storage temperature upon subsequent susceptibility of tubers. Ann. Appl. Biol. 1952, 39, 351–357. [Google Scholar] [CrossRef]
- Theron, D.; Holz, G. Effect of temperature on dry rot development of potato tubers inoculated with different Fusarium spp. Potato Res. 1990, 33, 109–117. [Google Scholar] [CrossRef]
- Estrada Jr, R.; Gudmestad, N.; Rivera, V.; Secor, G. Fusarium graminearum as a dry rot pathogen of potato in the USA: Prevalence, comparison of host isolate aggressiveness and factors affecting aetiology. Plant Pathol. 2010, 59, 1114–1120. [Google Scholar] [CrossRef]
- Elsherbiny, E.A.; Dawood, D.H.; Elsebai, M.F.; Mira, A.; Taher, M.A. Control of dry rot and resistance induction in potato tubers against Fusarium sambucinum using red onion peel extract. Postharvest Biol. Technol. 2023, 195, 112119. [Google Scholar] [CrossRef]
- Pozo, M.G.; Vaessen, C.V.; Kourmpetli, S.; Terry, L.A.; Medina, A. Effect of temperature, relative humidity, and incubation time on the mycotoxin production by Fusarium spp. responsible for dry rot in potato tubers. Toxins 2024, 16, 414. [Google Scholar] [CrossRef]
- Aydin, M.H.; Inal, B. Comparative susceptibility of some commercial potato cultivars to Fusarium sambucinum and F. solani isolates causing tuber dry rot. Appl. Ecol. Environ. Res. 2018, 16, 4879–4892. [Google Scholar] [CrossRef]
- Azil, N.; Stefanczyk, E.; Sobkowiak, S.; Chihat, S.; Boureghda, H.; Sliwka, J. Identification and pathogenicity of Fusarium spp. associated with tuber dry rot and wilt of potato in Algeria. Eur. J. Plant Pathol. 2021, 159, 495–509. [Google Scholar] [CrossRef]
- Gachango, E.; Hanson, L.E.; Rojas, A.; Hao, J.J.; Kirk, W.W. Fusarium spp. causing dry rot of seed potato tubers in Michigan and their sensitivity to fungicides. Plant Dis. 2012, 96, 1767–1774. [Google Scholar] [CrossRef]
- Gavrilova, O.; Orina, A.; Trubin, I.; Gagkaeva, T. Identification and pathogenicity of Fusarium fungi associated with dry rot of potato tubers. Microorganisms 2024, 12, 598. [Google Scholar] [CrossRef]
- Gherbawy, Y.A.; Hussein, M.A.; Hassany, N.A.; Shebany, Y.M.; Hassan, S.; El-Dawy, E. Phylogeny and pathogenicity of Fusarium solani species complex (FSSC) associated with potato tubers. J. Basic Microbiol. 2021, 61, 1133–1144. [Google Scholar] [CrossRef]
- Heltoft, P.; Brierley, J.L.; Lees, A.K.; Sullivan, L.; Lynott, J.; Hermansen, A. The relationship between soil inoculum and the development of Fusarium dry rot in potato cultivars Asterix and Saturna. Eur. J. Plant Pathol. 2016, 146, 711–714. [Google Scholar] [CrossRef]
- Heltoft, P.; Molteberg, E.L.; Nærstad, R.; Hermansen, A. Effect of maturity level and potato cultivar on development of Fusarium dry rot in Norway. Potato Res. 2015, 58, 205–219. [Google Scholar] [CrossRef]
- Peters, J.C.; Lees, A.K.; Cullen, D.W.; Sullivan, L.; Stroud, G.P.; Cunnington, A.C. Characterization of Fusarium spp. responsible for causing dry rot of potato in Great Britain. Plant Pathol. 2008, 57, 262–271. [Google Scholar] [CrossRef]
- Stefanczyk, E.; Sobkowiak, S.; Brylinska, M.; Sliwka, J. Diversity of Fusarium spp. associated with dry rot of potato tubers in Poland. Eur. J. Plant Pathol. 2016, 145, 871–884. [Google Scholar] [CrossRef]
- Niu, X.; Li, J.; Zhang, J.; Shen, B.; Chai, Z.; Wang, D. Changes of Fusarium in rhizosphere soil under potato continuous cropping systems in arid-irrigated area of Gansu Province. Acta Prataculturae Sin. 2011, 20, 236–243. [Google Scholar] [CrossRef]
- Belete, E.; Ayalew, A.; Ahmed, S. Associations of biophysical factors with faba bean root rot (Fusarium solani) epidemics in the northeastern highlands of Ethiopia. Crop Prot. 2013, 52, 39–46. [Google Scholar] [CrossRef]
- Zhang, L.J.; Wang, C.; Min, G.M.; Yang, X.M. Research progress in pea root rot. Plant Prot. 2019, 45, 82–90. [Google Scholar] [CrossRef]
- Gálvez, L.; Palmero, D. Fusarium dry rot of garlic bulbs caused by Fusarium proliferatum: A review. Horticulturae 2022, 8, 628. [Google Scholar] [CrossRef]
- Peters, R.D.; Sturz, A.V.; Carter, M.R.; Sanderson, J.B. Developing disease-suppressive soils through crop rotation and tillage management practices. Soil Tillage Res. 2003, 72, 181–192. [Google Scholar] [CrossRef]
- Uwamahoro, F.; Berlin, A.; Bucagu, C.; Bylund, H.; Yuen, J. Ralstonia solanacearum causing potato bacterial wilt: Host range and cultivars’ susceptibility in Rwanda. Plant Pathol. 2020, 69, 559–568. [Google Scholar] [CrossRef]
- Peters, R.D.; MacLeod, C.; Seifert, K.A.; Martin, R.A.; Hale, L.R.; Grau, C.R.; MacInnis, S. Pathogenicity to potato tubers of Fusarium spp. isolated from potato, cereal and forage crops. Am. J. Potato Res. 2008, 85, 367–374. [Google Scholar] [CrossRef]


| Amplified Region | Primer Name | Sequence (5′-3′) | Annealing Temperatures (°C) |
|---|---|---|---|
| TEF1 | EF-1T | ATGGGTAAGGAAGACAAGAC | 57.5 |
| EF-2T | GGAAGTACCAGTGATCATGTT | ||
| RPB1 | Fa | CAYAARGARTCYATGATGGGWC | 55 |
| G2R | GTCATYTGDGTDGCDGGYTCDCC | ||
| RPB2 | 7cf | ATGGGYAARCAAGCYATGGG | 55 |
| 11ar | GCRTGGATCTTRTCRTCSACC |
| Species | Strain No. | Host | GenBank Accession No. | ||
|---|---|---|---|---|---|
| TEF1 | RPB1 | RPB2 | |||
| F. acuminatum | YY3 | S. tuberosum | PV382882 | PV854763 | PV854833 |
| NJC23 | Allium sativum | OL741722 | OL741716 | OL741720 | |
| LW-5 | - | OP838084 | OP838085 | OP838086 | |
| LS1-1 | Wheat | OP105209 | OP785217 | OP785270 | |
| F. gamsii | KG411 | Triticum aestivum | ON960708 | ON960644 | ON960692 |
| KG390 | Triticum aestivum | ON960707 | ON960643 | ON960691 | |
| F. torulosum | NRRL 22748 | - | OL772877 | OL773029 | OL773181 |
| NRRL 52772 | - | OL772887 | OL773039 | OL773191 | |
| F. iranicum | NRRL 52714 | - | OL772883 | OL773035 | OL773187 |
| FRC R8972 | - | OL772864 | OL773016 | OL773168 | |
| F. avenaceum | NJC06 | Kiwi tree | OL439731 | OL439737 | OL439740 |
| NJC07 | Kiwi tree | OL439732 | OL439738 | OL439741 | |
| NJC08 | Kiwi tree | OL439733 | OL439739 | OL439742 | |
| F. reticulatum | FRC T683 | - | OL772860 | OL773012 | OL773164 |
| FRC R8747 | - | OL772859 | OL773011 | OL773163 | |
| F. tricinctum | F1502 | - | OL964794 | OL658681 | OL658756 |
| F1534 | - | OL964796 | OL658676 | OL658761 | |
| F1544 | - | OL964791 | OL658669 | OL658768 | |
| F. dimerum | HS73 | S. tuberosum | PV382890 | PV854767 | PV854837 |
| HY1 | S. tuberosum | PV382889 | PV854766 | PV854836 | |
| HY3 | S. tuberosum | PV382888 | PV854765 | PV854835 | |
| HY4 | S. tuberosum | PV382887 | PV854764 | PV854834 | |
| MNHN_RF_05625 | - | MW811085 | MW811058 | MW811070 | |
| NRRL 36140 T | - | HM347133 | HM347203 | HM347218 | |
| CBS 108944 T | - | KR673912 | KM232212 | KR674020 | |
| F. penzigii | CBS 317.34 T | - | EU926324 | KM232211 | KM232362 |
| B. allantoides | UBOCC_A_120037 | - | MW811088 | MW811055 | MW811073 |
| UBOCC_A_120036 | - | MW811087 | MW811087 | MW811072 | |
| UBOCC_A_120035 | - | MW811075 | MW811046 | MW811060 | |
| B. penicilloides | UBOCC_A_120021 | - | MW811081 | MW811051 | MW811066 |
| UBOCC_A_120034 | - | MW811080 | MW811050 | MW811065 | |
| F. oxysporum | LS163 | S. tuberosum | PV382883 | PV854762 | PV854832 |
| NRRL:62542 | - | KC808229 | KC808302 | KC808365 | |
| NRRL:62547 | - | KC808224 | KC808304 | KC808367 | |
| F. triseptatum | CBS 258.50 T | Ipomoea batatas | MH484964 | MW928820 | MH484873 |
| JW 277008 | - | MZ921888 | MZ921662 | MZ921757 | |
| F. languescens | CBS 645.78 T | S. lycopersicum | MH484971 | MW928813 | MH484880 |
| MF67-4 | Dioscorea esculenta | PQ774592 | PQ774580 | PQ774586 | |
| F. curvatum | CBS 238.94 T | Beaucarnea sp. | MH484984 | MW928804 | MH484893 |
| FO16-HB-ZJK | - | PQ300823 | PQ296597 | PQ296676 | |
| F. duoseptatum | ZHKUCC 23-0911 | Strelitzia reginae | PQ316056 | PQ468017 | PQ356493 |
| ZHKUCC 23-0896 | Strelitzia reginae | PQ316055 | PQ468016 | PQ356492 | |
| F. fabacearum | CPC 25801 | - | MH485029 | MZ921691 | MH484938 |
| FO65-SC-CD | - | PQ300866 | PQ296640 | PQ296719 | |
| F. gossypinum | LLC1739 | Soil | OP487221 | OP486374 | OP486789 |
| LLC1691 | Soil | OP487220 | OP486373 | OP486788 | |
| F. cugenangense | ZJUE1544 | Citrus unshiu | PX130564 | PV983890 | PX130492 |
| ZJUE1541 | Citrus unshiu | PX130563 | PV983889 | PX130491 | |
| F. sambucinum | BF0071 | S. tuberosum | PV382910 | PV854758 | PV854828 |
| BF0072 | S. tuberosum | PV382892 | PV854757 | PV854827 | |
| CBS 151942 T | Sambucus nigra | PQ260927 | PQ280949 | PQ274212 | |
| DT2 | S. tuberosum | PV382943 | PV854756 | PV854826 | |
| DT3 | S. tuberosum | PV382891 | PV854755 | PV854825 | |
| GL1 | S. tuberosum | PV382948 | PV854754 | PV854824 | |
| GL2 | S. tuberosum | PV382947 | PV854753 | PV854823 | |
| GL3 | S. tuberosum | PV382934 | PV854752 | PV854822 | |
| GL4 | S. tuberosum | PV382924 | PV854751 | PV854821 | |
| HS71 | S. tuberosum | PV382904 | PV854750 | PV854820 | |
| HS72 | S. tuberosum | PV382927 | PV854749 | PV854819 | |
| HY2 | S. tuberosum | PV382951 | PV854748 | PV854818 | |
| JS151 | S. tuberosum | PV382906 | PV854747 | PV854817 | |
| JS152 | S. tuberosum | PV382919 | PV854746 | PV854816 | |
| JS153 | S. tuberosum | PV382937 | PV854745 | PV854815 | |
| JS161 | S. tuberosum | PV382905 | PV854744 | PV854814 | |
| JZ11 | S. tuberosum | PV382908 | PV854743 | PV854813 | |
| JZ12 | S. tuberosum | PV382939 | PV854742 | PV854812 | |
| JZ82 | S. tuberosum | PV382907 | PV854741 | PV854811 | |
| KS21 | S. tuberosum | PV382901 | PV854740 | PV854810 | |
| KX271 | S. tuberosum | PV382917 | PV854739 | PV854809 | |
| KX272 | S. tuberosum | PV382945 | PV854738 | PV854808 | |
| KX273 | S. tuberosum | PV382921 | PV854737 | PV854807 | |
| LS121 | S. tuberosum | PV382946 | PV854736 | PV854806 | |
| LS122 | S. tuberosum | PV382930 | PV854735 | PV854805 | |
| LS123 | S. tuberosum | PV382914 | PV854734 | PV854804 | |
| LS161 | S. tuberosum | PV382920 | PV854733 | PV854803 | |
| LS162 | S. tuberosum | PV382893 | PV854732 | PV854802 | |
| LS164 | S. tuberosum | PV382896 | PV854731 | PV854801 | |
| LWH1 | S. tuberosum | PV382944 | PV854730 | PV854800 | |
| LWH2 | S. tuberosum | PV382909 | PV854729 | PV854799 | |
| LWH3 | S. tuberosum | PV382923 | PV854728 | PV854798 | |
| LWH4 | S. tuberosum | PV382895 | PV854727 | PV854797 | |
| NRRL 20666 | - | MW233072 | MW233243 | MW233415 | |
| QS91 | S. tuberosum | PV382936 | PV854726 | PV854796 | |
| QS92 | S. tuberosum | PV382913 | PV854725 | PV854795 | |
| TS291 | S. tuberosum | PV382929 | PV854724 | PV854794 | |
| TS311 | S. tuberosum | PV382935 | PV854723 | PV854793 | |
| TS312 | S. tuberosum | PV382940 | PV854722 | PV854792 | |
| TS313 | S. tuberosum | PV382915 | PV854721 | PV854791 | |
| TZ2 | S. tuberosum | PV382903 | PV854720 | PV854790 | |
| TZ3 | S. tuberosum | PV382911 | PV854719 | PV854789 | |
| WLS1 | S. tuberosum | PV382942 | PV854718 | PV854788 | |
| WLS2 | S. tuberosum | PV382894 | PV854717 | PV854787 | |
| YG1 | S. tuberosum | PV382898 | PV854716 | PV854786 | |
| YG2 | S. tuberosum | PV382899 | PV854715 | PV854785 | |
| YN82 | - | OR019814 | OR019820 | OR019826 | |
| YY2 | S. tuberosum | PV382932 | PV854714 | PV854784 | |
| ZJ21 | S. tuberosum | PV382916 | PV854713 | PV854783 | |
| ZJ22 | S. tuberosum | PV382925 | PV854712 | PV854782 | |
| ZJ71 | S. tuberosum | PV382931 | PV854711 | PV854781 | |
| ZJ72 | S. tuberosum | PV382928 | PV854710 | PV854780 | |
| ZS171 | S. tuberosum | PV382897 | PV854709 | PV854779 | |
| ZS172 | S. tuberosum | PV382938 | PV854708 | PV854778 | |
| ZS173 | S. tuberosum | PV382912 | PV854707 | PV854777 | |
| ZS174 | S. tuberosum | PV382922 | PV854706 | PV854776 | |
| ZS175 | S. tuberosum | PV382941 | PV854705 | PV854775 | |
| ZS181 | S. tuberosum | PV382933 | PV854704 | PV854774 | |
| ZS182 | S. tuberosum | PV382918 | PV854703 | PV854773 | |
| ZY2 | S. tuberosum | PV382949 | PV854702 | PV854772 | |
| ZY4 | S. tuberosum | PV382950 | PV854701 | PV854771 | |
| ZY5 | S. tuberosum | PV382926 | PV854700 | PV854770 | |
| ZY6 | S. tuberosum | PV382900 | PV854699 | PV854769 | |
| ZY7 | S. tuberosum | PV382902 | PV854698 | PV854768 | |
| F. brachygibbosum | CBS 121682 | Stone | PQ260819 | PQ280843 | PQ274106 |
| CBS 131017 | Agropyron sp. | PQ260820 | PQ280844 | PQ274107 | |
| CBS 131252 | Triticum sp. | PQ260821 | PQ280845 | PQ274108 | |
| F. venenatum | NRRL 32015 | - | MW233109 | MW233281 | MW233453 |
| NRRL 22196 | - | MW233078 | MW233249 | MW233421 | |
| CBS 140911 | Grass | PQ260954 | PQ280984 | PQ274241 | |
| CBS 127.95 | S. tuberosum | PQ260953 | PQ280983 | PQ274240 | |
| F. solani | HS74 | S. tuberosum | PV382886 | PV854761 | PV854831 |
| KX301 | S. tuberosum | PV382884 | PV854759 | PV854829 | |
| KX302 | S. tuberosum | PV382885 | PV854760 | PV854830 | |
| CBS 102429 | Bark | KM231936 | KM232227 | KM232376 | |
| GR_FS26 | Asparagus root | MT305228 | MT305111 | MT305169 | |
| GR_FS83 | Asparagus root | MT305232 | MT305115 | MT305173 | |
| F. azukicola | NRRL 54364 T | - | JQ670137 | KJ511276 | KJ511287 |
| NRRL 54366 | - | JQ670139 | KJ511277 | KJ511288 | |
| F. catenatum | NRRL:54993 T | - | KC808214 | KC808292 | KC808355 |
| NRRL:54992 | - | KC808213 | KC808291 | KC808354 | |
| F. petroliphilum | NRRL:54995 | - | KC808215 | KC808293 | KC808356 |
| M. leptosphaeriae | CBS 112770 | Cucurbitaria laburni | KM231960 | KM232256 | KM232389 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Shi, Y.; Chen, X.; Du, P.; Zhao, Y.; Wang, L. Molecular Identification and Pathogenicity of Fusarium Fungi Causing Potato Dry Rot in Shanxi Province, China. J. Fungi 2025, 11, 835. https://doi.org/10.3390/jof11120835
Guo J, Shi Y, Chen X, Du P, Zhao Y, Wang L. Molecular Identification and Pathogenicity of Fusarium Fungi Causing Potato Dry Rot in Shanxi Province, China. Journal of Fungi. 2025; 11(12):835. https://doi.org/10.3390/jof11120835
Chicago/Turabian StyleGuo, Jiaru, Yupei Shi, Xi Chen, Peibing Du, Yingli Zhao, and Liang Wang. 2025. "Molecular Identification and Pathogenicity of Fusarium Fungi Causing Potato Dry Rot in Shanxi Province, China" Journal of Fungi 11, no. 12: 835. https://doi.org/10.3390/jof11120835
APA StyleGuo, J., Shi, Y., Chen, X., Du, P., Zhao, Y., & Wang, L. (2025). Molecular Identification and Pathogenicity of Fusarium Fungi Causing Potato Dry Rot in Shanxi Province, China. Journal of Fungi, 11(12), 835. https://doi.org/10.3390/jof11120835





