Cloning and Functional Analysis of the Aldehyde Dehydrogenase Gene VvALDH in the IAA Synthesis Pathway of Volvariella volvacea
Abstract
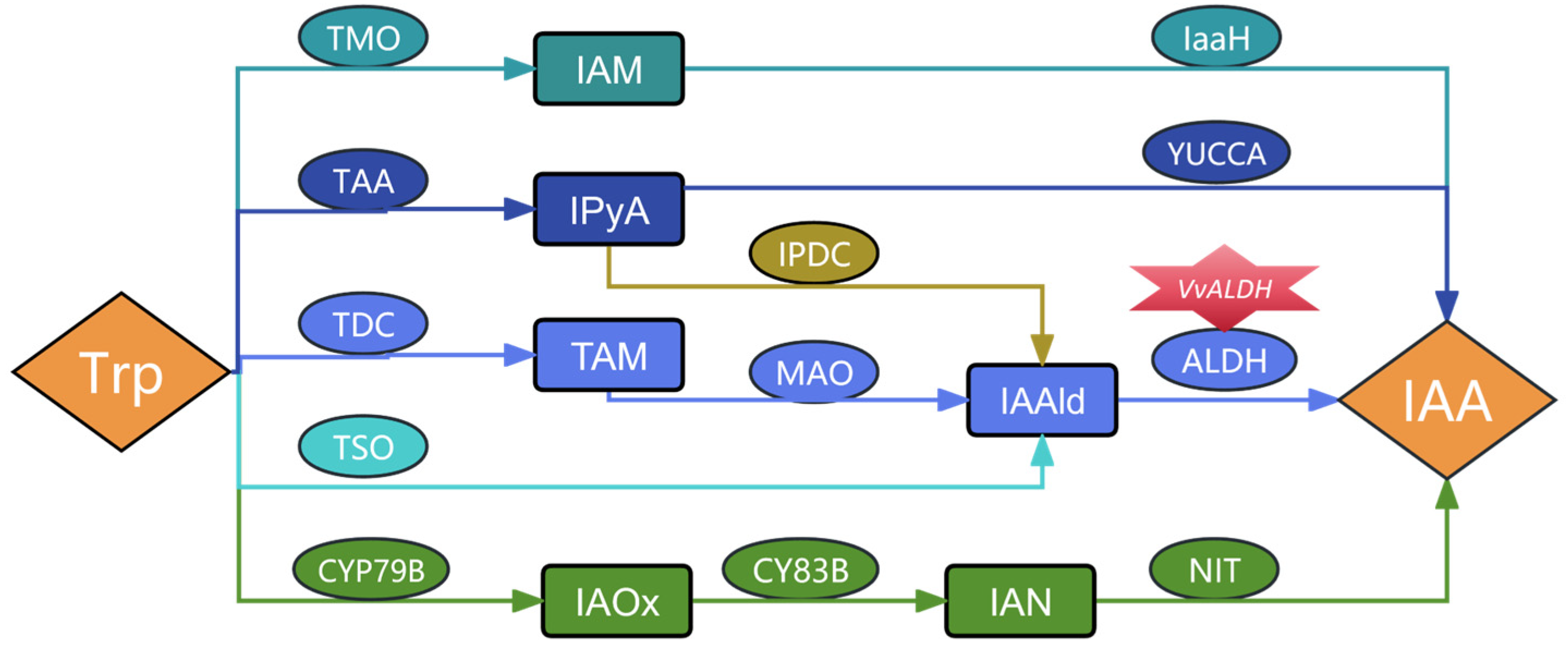
1. Introduction
2. Materials and Methods
2.1. Medium
2.2. Cloning and Bioinformatics Analysis of VvALDH
2.3. Vector Construction and Agrobacterium-Mediated Genetic Transformation
2.3.1. Construction of VvALDH Gene Overexpression and Silencing Vectors
2.3.2. Establishment of an Agrobacterium-Mediated Transformation System for V. volvacea
2.3.3. Verification of Transformants
2.4. Determination of Transformant Traits
2.4.1. Determination of Mycelial Characteristics and Growth Rate
2.4.2. Determination of Primordium Indices and Fruiting Body Yield of Transformants
2.4.3. Detection of Key Genes in the IAA Synthesis Pathway of V. volvacea
2.5. Data Analysis
3. Results
3.1. Cloning and Bioinformatics Analysis of VvALDH
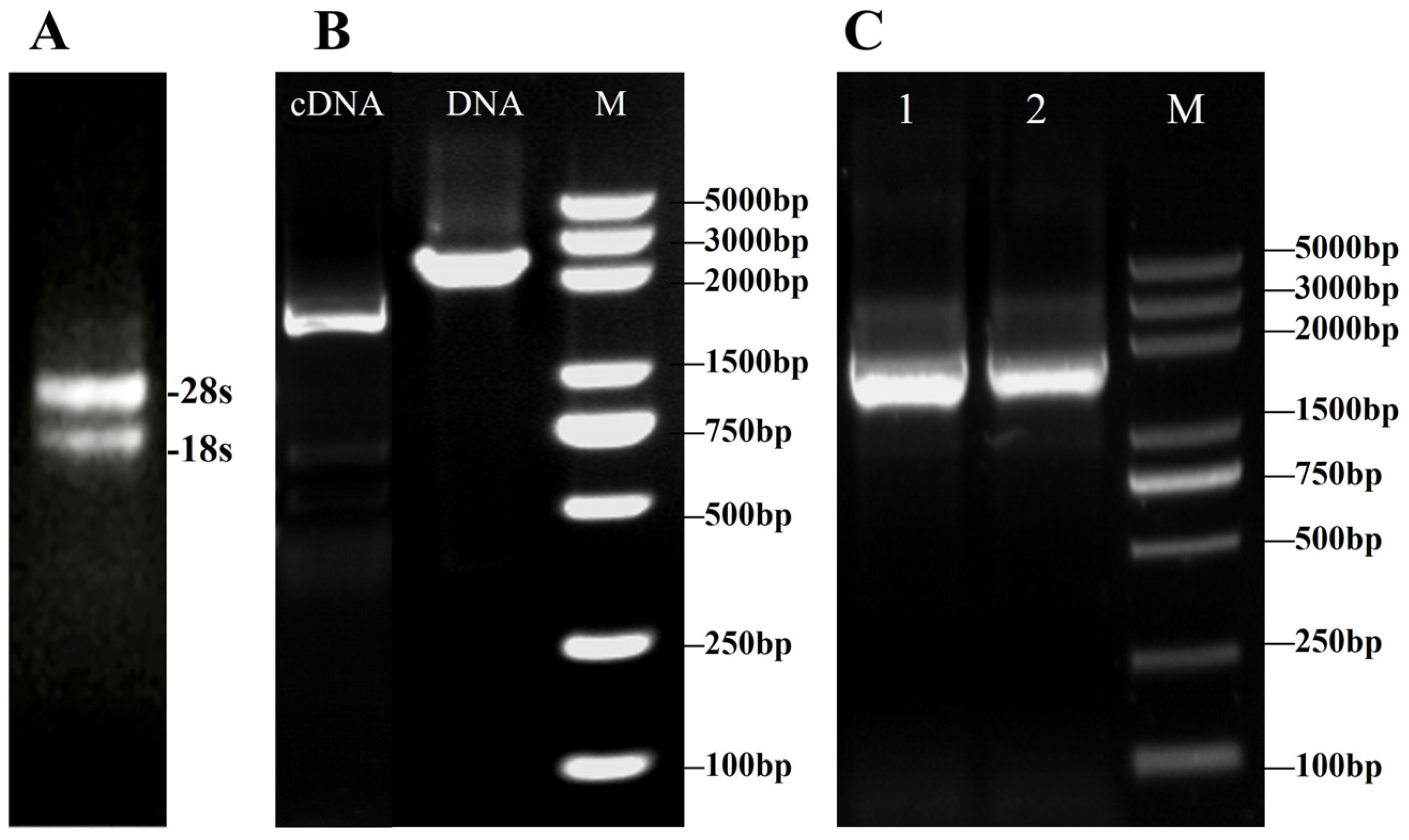
3.1.1. Cloning of VvALDH Gene
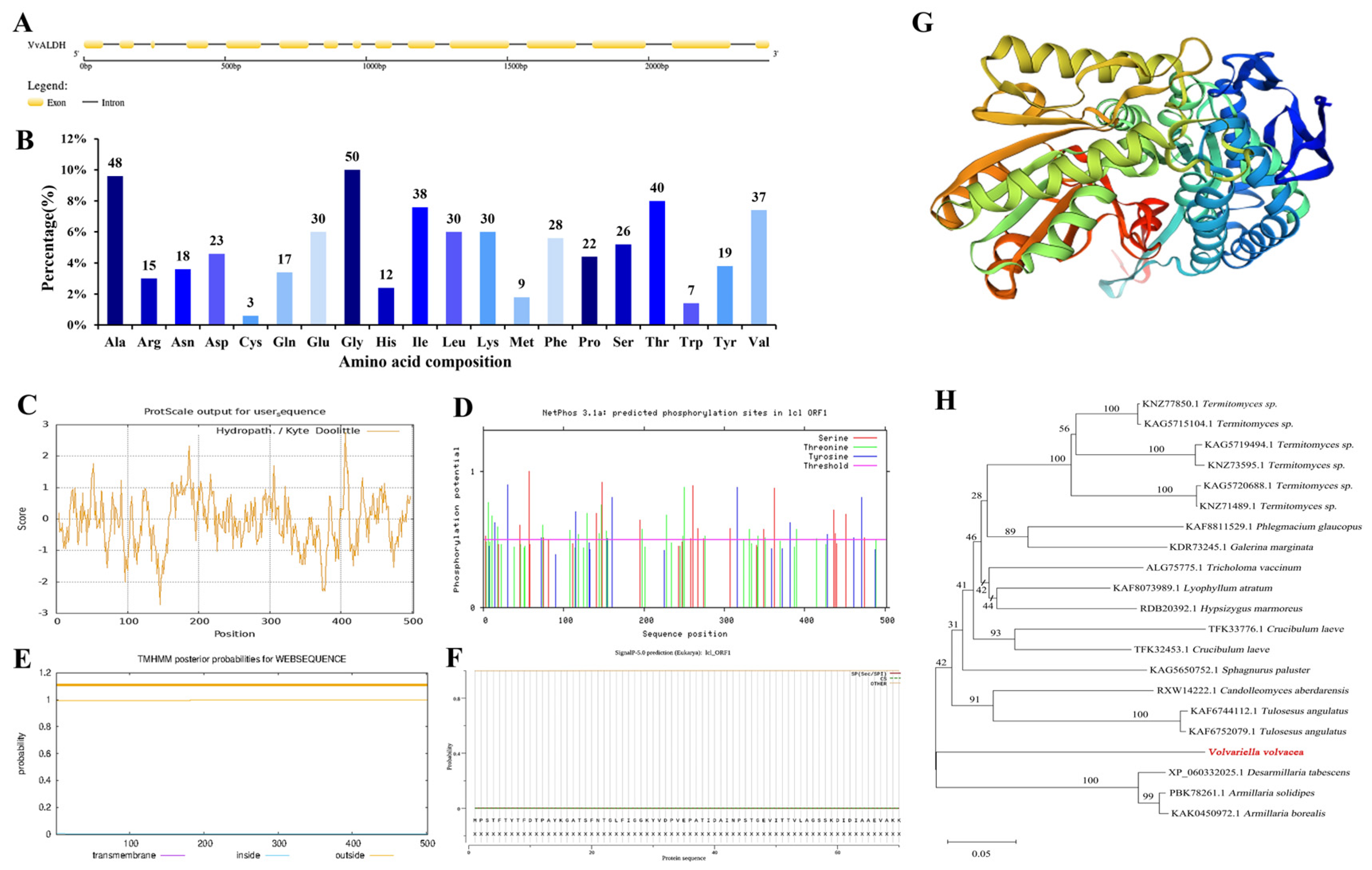
3.1.2. Bioinformatics Analysis of VvALDH Gene
3.2. Construction of Overexpression and RNAi Vectors for VvALDH in V. volvacea and Establishment of Genetic Transformation System
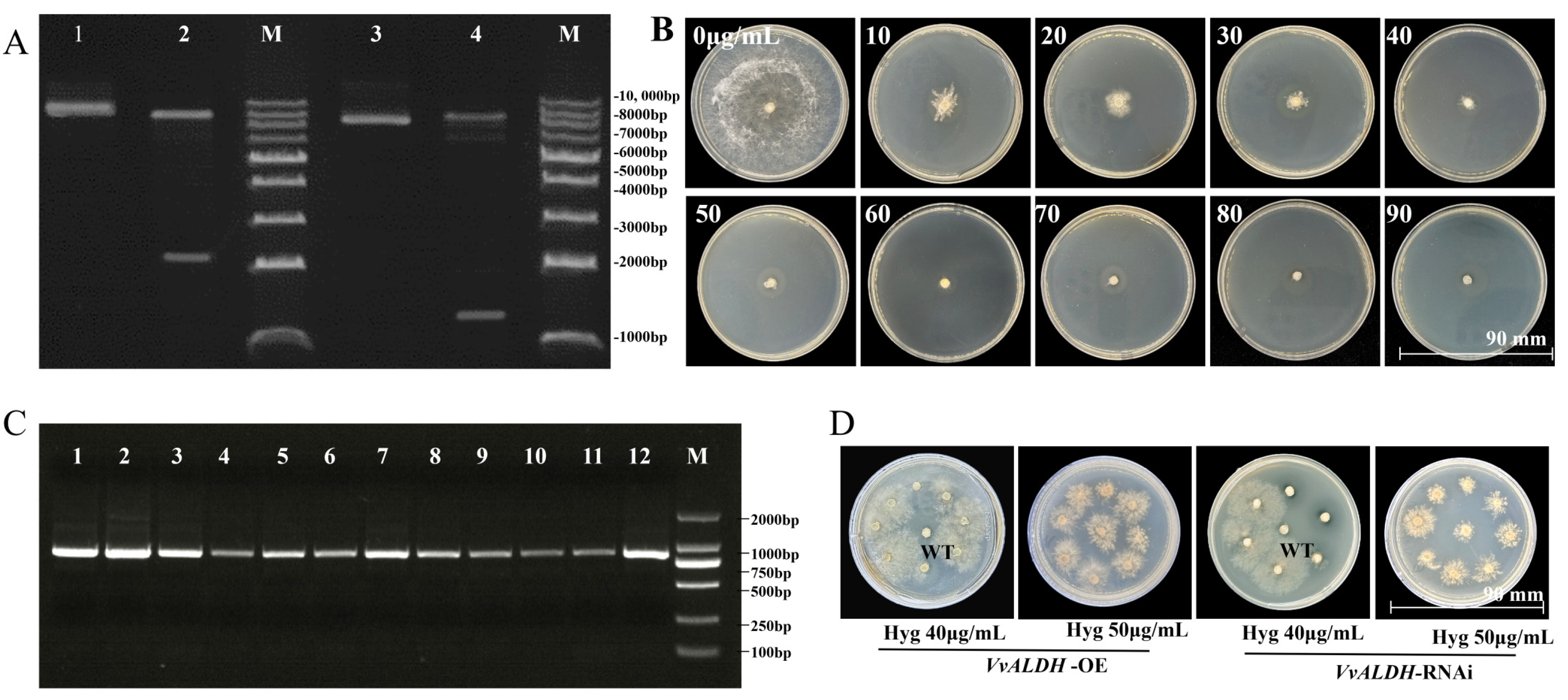
3.2.1. Validation of VvALDH Overexpression and Silencing Vectors, and Hyg Sensitivity Assay
3.2.2. Quantitative Validation of VvALDH Overexpression and RNAi Transformants
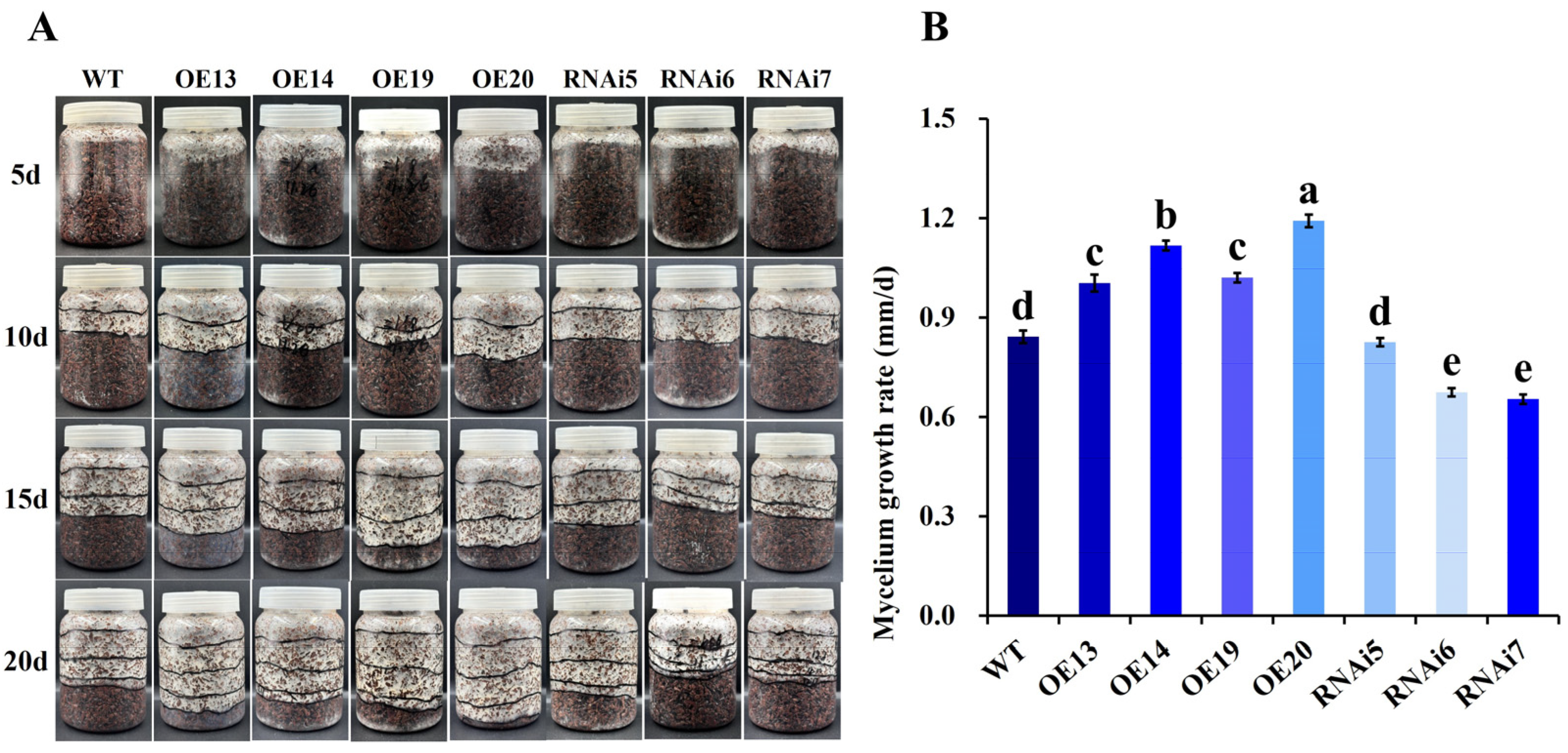
3.2.3. Effects of VvALDH on Mycelial Morphology and Growth Rate
3.2.4. Effect of VvALDH on Mycelial Growth Rate in Growth Substrate
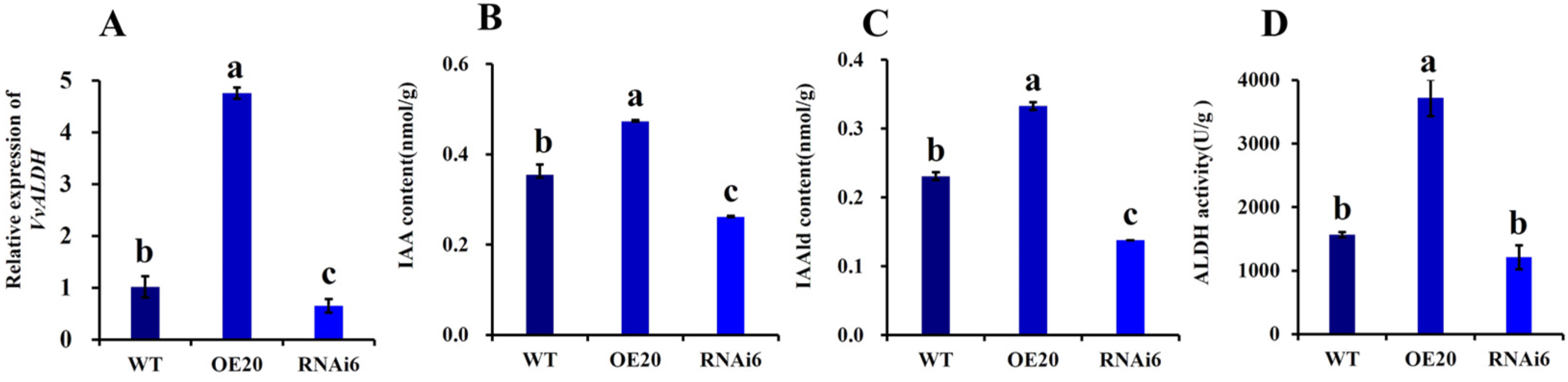
3.3. Quantitative Analysis of VvALDH Expression During Primordium Formation and Its Impact on IAA Biosynthesis
3.4. Effect of VvALDH on Yield and Productivity
3.5. Detection of Key Genes in the IAA Synthesis Pathway of and Content of Intermediate Products in V. volvacea
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chang, T.T.; Zha, L.; Yu, C.X.; Chen, M.J.; Zhou, S.; Qin, D.; Wu, Y.Y.; Fan, S.; Zhao, Y. Expanding the understanding of Volvariella volvacea autolysis at 4 °C with transcriptomics and metabolomics. Sci. Hortic. 2024, 336, 113386. [Google Scholar] [CrossRef]
- Zhang, J.X. Science and Development of China’s Edible Mushroom Industry; China Agricultural Publishing House: Beijing, China, 2009; pp. 40–47. [Google Scholar]
- Zhao, X.; Yu, C.X.; Zhao, Y.; Liu, S.J.; Wang, H.; Wang, C.G.; Guo, L.G.; Chen, M.J. Changes in Mannitol Content, Regulation of Genes Involved in Mannitol Metabolism, and the Protective Effect of Mannitol on Volvariella volvacea at Low Temperature. BioMed Res. Int. 2019, 2019, 1493721. [Google Scholar] [CrossRef]
- Zan, Y.X.; Shao, Y.Z.; Yang, Y.; Wei, J.; Sun, L.; Cui, F.J.; Li, W.; Zhu, H.G.; Sun, W.J.; Zhang, J.S. Improving overall postharvest quality of straw mushroom using an accessible and low-cost strategy. J. Food Compos. Anal. 2024, 128, 106036. [Google Scholar] [CrossRef]
- Qin, H.J.; Chen, P.; Wang, Q.; Zhang, J. The Chemical Composition, Biological Activity and Cultivation Situation of Straw Mushroom. Food Ind. 2017, 38, 203–206. [Google Scholar]
- Li, H.P.; Luo, X.; Ma, L.; Jiang, Y.; Wang, L.; Yang, H.P.; Qu, S.X. Dynamic characteristics of nutrient and microbial community structure in culture substrate during Volvariella volvacea cultivation. Jiangsu Agric. Sci. 2023, 51, 149–157. [Google Scholar] [CrossRef]
- Davis, R.; Taylor, A.; Nally, R.; Benson, K.F.; Stamets, P.; Jensen, G.S. Differential Immune Activating, Anti-Inflammatory, and Regenerative Properties of the Aqueous, Ethanol, and Solid Fractions of a Medicinal Mushroom Blend. J. Inflamm. Res. 2020, 13, 117–131. [Google Scholar] [CrossRef]
- Zhao, F.Y.; Cheng, Z.H.; Tan, Q.F.; Zhu, J.N.; Sun, W.H.; Zhang, W.W.; Fu, J.M. Effects of Mannitol on Production Characteristics and ROS Scavenging Ability of Volvariella volvacea Subcultured Strains. Sci. Agric. Sin. 2024, 57, 190–203. [Google Scholar] [CrossRef]
- Hou, L.J.; Li, Y.; Chen, M.J.; Li, Z.P. Improved fruiting of the straw mushroom (Volvariella volvacea) on cotton waste supplemented with sodium acetate. Appl. Microbiol. Biotechnol. 2017, 101, 8533–8541. [Google Scholar] [CrossRef]
- Hou, L.J.; Li, Z.P.; Li, C.T.; Li, Z.P.; Lin, J.S.; Ma, L.; Jiang, N.; Qu, S.X.; Li, H.P.; Li, Y. Enhanced enzymatic hydrolysis of cellulose from substrate and Indole-3-Acetic Acid content-during the fruiting body differentiation stage by sodium acetate addition. Front. Fungal Biol. 2021, 2, 746313. [Google Scholar] [CrossRef]
- Xiao, T.T.; Wang, Q.; Jun, J.X.; Chen, M.J.; Chen, H.; Zhang, J.J.; Song, X.X.; Huang, J.C. Effects of different sodium salts on mycelial growth and yield of Agaricus bisporus. Shanghai J. Agric. 2021, 37, 53–59. [Google Scholar] [CrossRef]
- Patten, C.L.; Blakney, A.C.; Coulson, T.D. Activity, distribution and function of indole-3-acetic acid biosynthetic pathways in bacteria. Crit. Rev. Microbiol. 2013, 39, 395–415. [Google Scholar] [CrossRef]
- Chen, B.Z. Differential Expression Analysis of Genes Related to Growth Hormone Metabolism in the Seedling of Straw Mushroom. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2012. [Google Scholar]
- Gong, Y.H.; Wei, X.X.; Xiao, Y.; Zhou, Y.; Bian, Y.B. Quantitative metabolomic gene quantification of growth hormone synthesis pathways in heat-tolerant and heat-sensitive Lentinula edodes strains under heat stress conditions. In Chinese Society of Mycology. Abstracts of the 2024 Annual Meeting of the Mycological Society of China; National Key Laboratory of Agricultural Microbial Resources Discovery and Utilisation, Institute of Applied Fungi, Huazhong Agricultural University, Hongshan Laboratory: Hubei, China, 2024. [Google Scholar] [CrossRef]
- Zhou, S.S.; Wang, G.Z.; Luo, Y.; Ma, C.J.; Gong, Y.H.; Bian, Y.B.; Zhou, Y. Auxin and auxin analogues enhancing the thermotolerance of Lentinula edodes. J. Mycol. 2018, 37, 1723–1730. [Google Scholar] [CrossRef]
- Tang, J.T.; Li, Y.K.; Zhang, L.L.; Mu, J.T.; Jiang, X.Y.; Fu, H.L.; Zhang, Y.F.; Cui, H.F.; Yu, X.P.; Ye, Z.H. Biosynthetic Pathways and Functions of Indole-3-Acetic Acid in Microorganisms. Microorganisms 2023, 11, 2077. [Google Scholar] [CrossRef]
- Puspendu, S.; Frank, K. Characterization of indole-3-pyruvic acid pathway-mediated biosynthesis of auxin in Neurospora crassa. PLoS ONE 2018, 13, e0192293. [Google Scholar] [CrossRef]
- Zhu, Q.J. Establishment of Agrobacterium Tumefaciens-Mediated Silencing System for URA3 Gene in Shiitake Mushroom. Master’s Thesis, Shanghai Ocean University, Shanghai, China, 2021. [Google Scholar] [CrossRef]
- Mu, D.S.; Li, C.Y.; Zhang, X.C.; Li, X.B.; Shi, L.; Hong, R.; Zhao, M.W. Functions of the nicotinamide adenine dinucleotide phosphate oxidase family in Ganoderma lucidum: An essential role in ganoderic acid biosynthesis regulation, hyphal branching, fruiting body development, and oxidative-stress resistance. Environ. Microbiol. 2014, 16, 1709–1728. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, Z.H.; Hong, R.; Shi, L.; Shi, D.K.; Li, X.B.; Zhao, M.W. The mitogen-activated protein kinase GlSlt2 regulates fungal growth, fruiting body development, cell wall integrity, oxidative stress and ganoderic acid biosynthesis in Ganoderma lucidum. Fungal Genet. Biol. 2017, 104, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.T.; Ding, Y.T.; Gao, X.Y.; Liu, H.H.; Zhao, K.; Gao, Y.Q.; Qiu, L.Y. Promotion of the growth and plant biomass degrading enzym-es production in solid-state cultures of Lentinula edodes expressing Vitreoscilla hemoglobin gene. J. Biotechnol. 2019, 302, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.Z. Key Regulatory Benchmarks for Fruiting Body Development of Pleurotus ostreatus Shielded by Selection and Functional Assays. Master’s Thesis, Hebei University of Engineering, Handan, China, 2023. [Google Scholar] [CrossRef]
- Sun, J. Mining and Functional Study of Genes Related Tocellulose Degradation in Auricularia heimuer. Ph.D. Thesis, Northeast Forestry University, Harbin, China, 2022. [Google Scholar] [CrossRef]
- Wang, X.T. Study on the Mechanism of Salicylic acid Regulating Triterpenoids Synthesis and Key Gene Function in Sanghuangporus baumii. Ph.D. Thesis, Northeast Forestry University, Harbin, China, 2022. [Google Scholar] [CrossRef]
- Meng, L. Selection of Differentially Expressed Transcription Factors and Functional Analysis of Key Gene During the Morphogenesis of Volvariella volvacea. Ph.D. Thesis, Jilin Agricultural University, Changchun, China, 2013. [Google Scholar]
- Liu, Z.C. Mining of Genes Related to Triterpenoids Synthesisand Study of Key Gene Functions in Sanghuangporusbaumii. Ph.D. Thesis, Northeast Forestry University, Harbin, China, 2022. [Google Scholar] [CrossRef]
- Mao, M.M.; Liu, D.; Zhou, H.M.; Bai, Y.Y.; Hong, P.; Wang, J.L.; Yang, L.B.; Chen, Z.M. ptimization of Ganoderma leucocontextum Fermented Tea Medium Formula Basedon High-yield Extracellular Crude. Sci. Technol. Food Ind. 2024, 45, 93–100. [Google Scholar] [CrossRef]
- Dong, Q.; Zhu, L.D.; Guo, Q.; Fan, X.Q.; Sun, Y.H.; Chen, M.J.; Zhao, Y. Preliminary study on cultivating Volvariella volvacea using a compoundculture medium of Lyophyllum decastes residues and rice straw. Shanghai J. Agric. 2025, 41, 21–29. [Google Scholar] [CrossRef]
- Chen, B.Z. Analysis of the Differential Expression of IAA Metabolism-Related Genes in Fruiting Developmental Stages from Volvariella volvacea. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2012. [Google Scholar]
- Xu, Y. Cloning and Expression of Key Enzyme Genes for Agrobacterium Tumefaciens-Mediated Polysaccharide Biosynthesis in Cordyceps Chrysanthemi. Master’s Thesis, Zhejiang University of Technology, Hangzhou, China, 2020. [Google Scholar] [CrossRef]
- Zhang, J. Screening and Functional Study of Cellulose Degradation Genes in Response to Different Carbon Sources in Auricularia nigra. Ph.D. Thesis, Northeast Forestry University, Harbin, China, 2023. [Google Scholar] [CrossRef]
- Hou, L.J.; Lin, J.S.; Liu, S.H.; Li, R.X.; Ma, L.; Jiang, N.; Qu, S.X.; Li, H.P. Sodium acetate and compound with four kinds of minerals on the mycelium biomass of Volvariella volvacea. Zhejiang J. Agric. 2018, 30, 228–235. [Google Scholar] [CrossRef]
- Liu, Q.M. Promoting Effects of Trichoderma.harzianum NJAU 4742 on Plant Growth and Its Molecular Mechanism. Ph.D. Thesis, Nanjing Agricultural University, Nanjing, China, 2020. [Google Scholar] [CrossRef]
- Qi, Y.C.; Liu, Y.D.; Sun, X.K.; Zhang, M.K.; Zhang, Q.; Wen, Q.; Qiu, L.Y.; Shen, J.W. Cloning and expression analysis of Pleurotus ostreatus aldehyde dehydrogenasegene PoALDH1 under abiotic stresses. J. Mycol. 2017, 36, 1121–1131. [Google Scholar] [CrossRef]
- Krause, K.; Henke, C.; Asiimwe, T.; Ulbricht, A.; Klemmer, S.; Schachtschabel, D.; Kothe, E. Biosynthesis and Secretion of Indole-3-Acetic Acid and Its Morphological Effects on Tricholoma vaccinum-Spruce Ectomycorrhiza. Appl. Environ. Microbiol. 2015, 81, 7003–7011. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.R. Effect of Blue-Light on Endogenous Auxin and Effective Components of Ganoderma lucidum. Master’s Thesis, Peking Union Medical College, Beijing, China, 2017. [Google Scholar] [CrossRef]
- Ateek, S.; Yamini, M.; Amrita, H. Double agent indole-3-acetic acid (IAA): Mechanistic analysis of indole-3-acetaldehyde dehydrogenase AldA that synthesises IAA, an auxin that aids bacterial virulence. Biosci. Rep. 2021, 41, 598. [Google Scholar] [CrossRef]





| Primer Name | Primer Sequence (5′-3′) | Application |
|---|---|---|
| VvALDH-1F | CCCTCAAACCATGCCTTCTACC | Gene sequence amplification |
| VvALDH-1R | CGCACAAAAGGGCGATTACAA | |
| M13F-47 | CGCCAGGGTTTTCCCAGTCACGAC | Cloning verification |
| M13R-48 (RV-M) | AGCGGATAACAATTTCACACAGGA | |
| VvALDH-OE-F | TGACCATGGTAGATCTATGCCTTCTACCTTCACATACACCTT | Overexpression primer |
| VvALDH-OE-R | ATTCGAGCTGGTCACCTTACAACTTCATACCAAGGTTAAGATGGACG | |
| VvALDH-RNAi-1F | TCCTCTATATAAGGAAGTTCATTTCATTTGGAGAGAACACGGGGGACTCTTGACCATGGTA | RNAi primer |
| VvALDH-RNAi-1R | CTTAAGAAACTTTATTGCCAAATGTTTGAACGATCGGGGAAATTCGAGCTGGTCACCCGTAT | |
| Hyg-F | ATTTGTGTACGCCCGACAGT | Verify the primer |
| Hyg-R | CTCTCGGAGGGCGAAGAATC | |
| β-TUB-F | TCAGGCAGGTGTCCAGAT | Internal reference primer |
| β-TUB-R | TCGGAGAAGAAAGTGCTG | |
| VvALDH-qpcr-F | CGGATATTTGTGCAAGAGGG | Real-time quantitative PCR |
| VvALDH-qpcr-R | GTAGTTGATGCGAACGGGTC | |
| MAO-F | CTTGTTTCGTGGCTCTT | Gene sequence amplification |
| MAO-R | AGGCTGCTATGTCCTCTAC | |
| TSO-F | GCAAGGCGGTGTTCATA | |
| TSO-R | AGCCATAGCCAATCCAG | |
| IPDC-F | CTGGGTTTCTTGGTGAGT | |
| IPDC-R | AGGAATACAATCGGCTTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, M.; Hou, L.; Ma, L.; Jiang, N.; Lin, J.; Qu, S.; Li, H.; Xu, P.; Liu, D.; Ji, W. Cloning and Functional Analysis of the Aldehyde Dehydrogenase Gene VvALDH in the IAA Synthesis Pathway of Volvariella volvacea. J. Fungi 2025, 11, 773. https://doi.org/10.3390/jof11110773
Mao M, Hou L, Ma L, Jiang N, Lin J, Qu S, Li H, Xu P, Liu D, Ji W. Cloning and Functional Analysis of the Aldehyde Dehydrogenase Gene VvALDH in the IAA Synthesis Pathway of Volvariella volvacea. Journal of Fungi. 2025; 11(11):773. https://doi.org/10.3390/jof11110773
Chicago/Turabian StyleMao, Mingjuan, Lijuan Hou, Lin Ma, Ning Jiang, Jinsheng Lin, Shaoxuan Qu, Huiping Li, Ping Xu, Di Liu, and Wei Ji. 2025. "Cloning and Functional Analysis of the Aldehyde Dehydrogenase Gene VvALDH in the IAA Synthesis Pathway of Volvariella volvacea" Journal of Fungi 11, no. 11: 773. https://doi.org/10.3390/jof11110773
APA StyleMao, M., Hou, L., Ma, L., Jiang, N., Lin, J., Qu, S., Li, H., Xu, P., Liu, D., & Ji, W. (2025). Cloning and Functional Analysis of the Aldehyde Dehydrogenase Gene VvALDH in the IAA Synthesis Pathway of Volvariella volvacea. Journal of Fungi, 11(11), 773. https://doi.org/10.3390/jof11110773






