The Occurrence of Colletotrichum karstii and C. fructicola Causes Anthracnose on Endangered Ethnic Vegetable Yunnanopilia longistaminea in Yunnan, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Sites, Diseased Sample Collection and Pathogen Isolation
2.2. Pathogenicity Test
2.3. Morphological Identification
2.4. DNA Extraction, Sequencing, and Analysis
3. Results
3.1. Symptomatology and Fungal Isolation
3.2. Pathogenicity Test
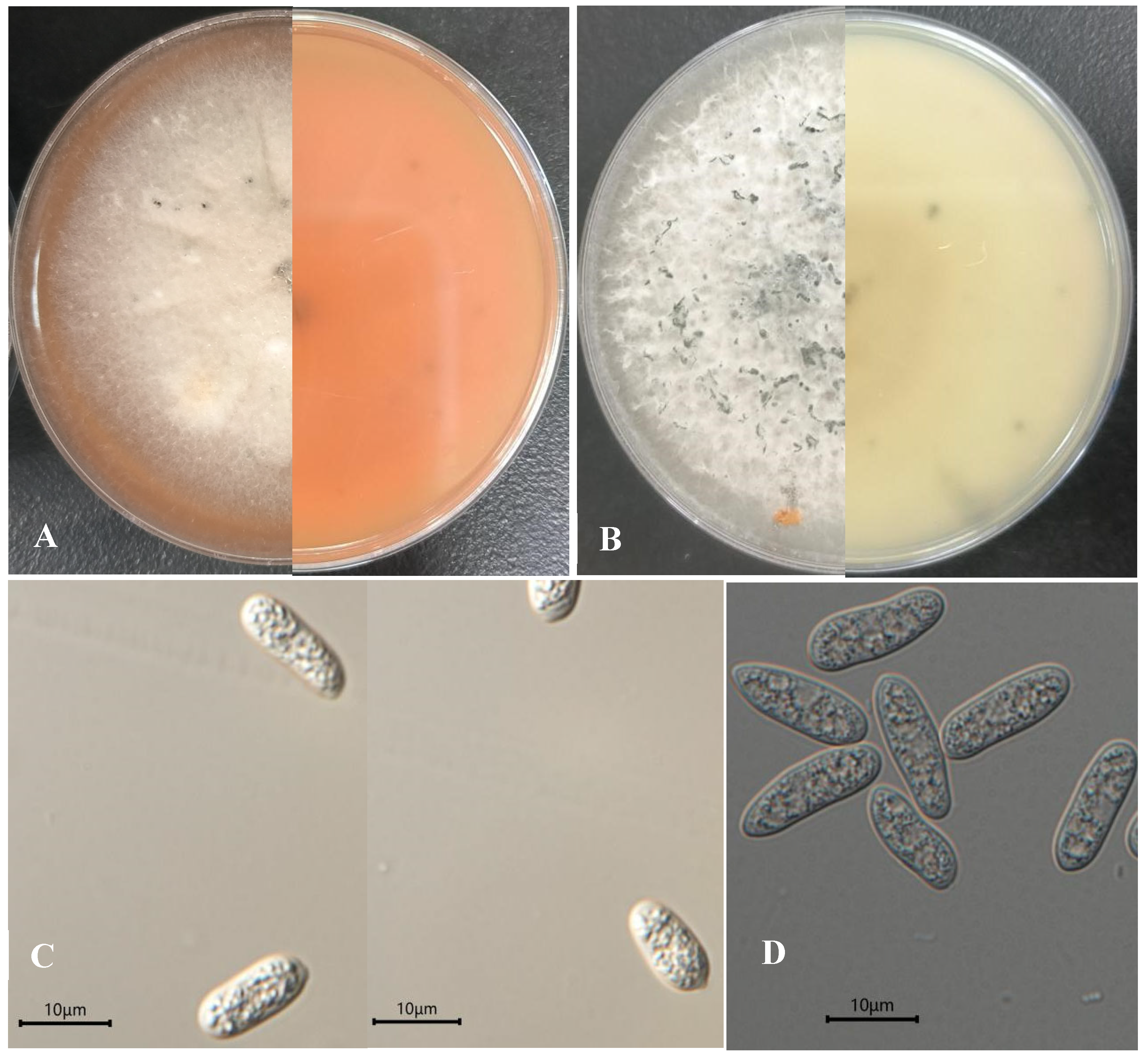
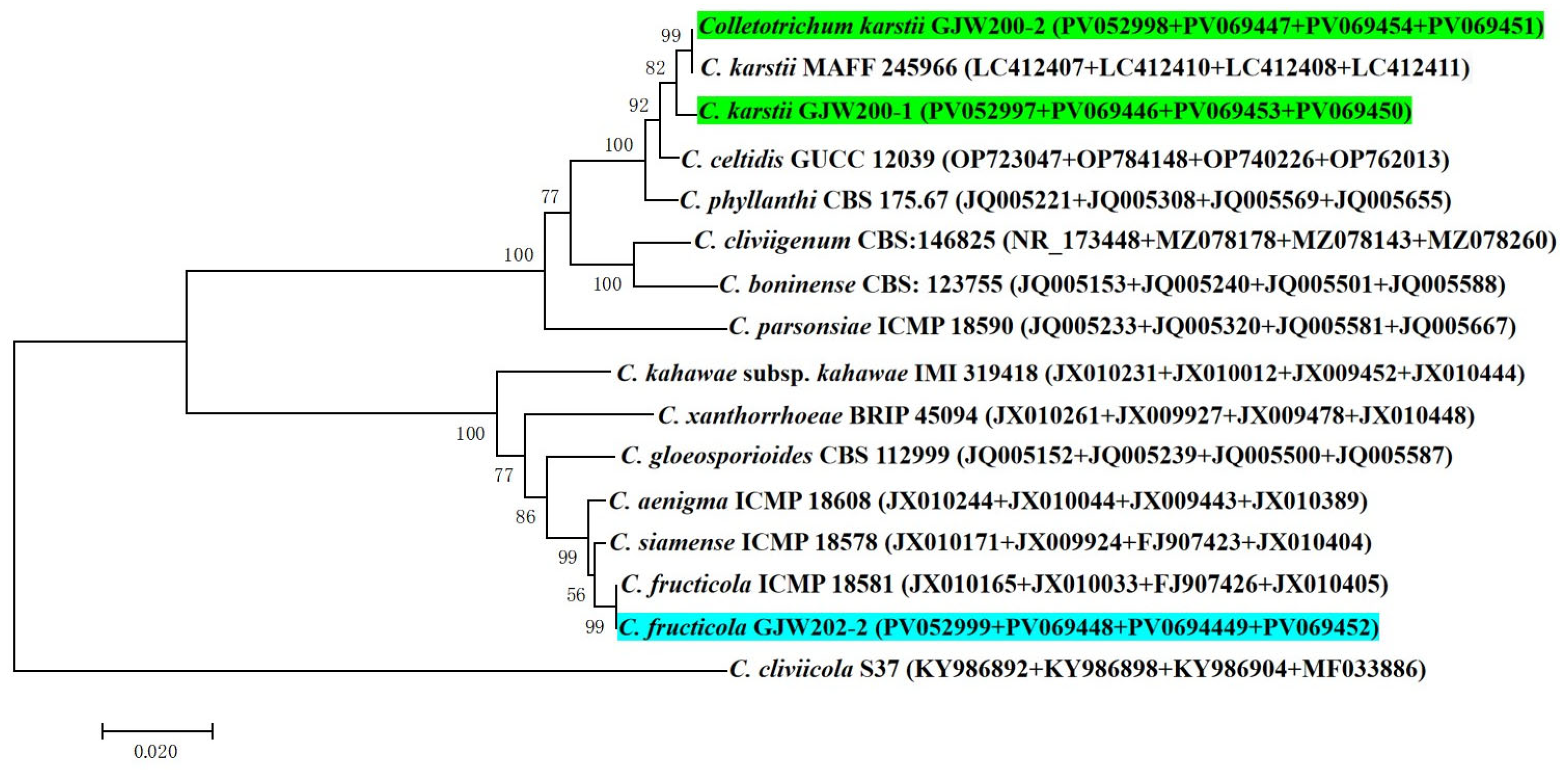
3.3. Morphological and Molecular Identification of the Pathogen
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lian, Y.; Bai, Y.; Huang, Z.; Ali, M.; Wang, J.; Chen, H. Spatio-temporal changes and habitats of rare and endangered species in Yunnan Province based on MaxEnt Model. Land 2024, 13, 240. [Google Scholar] [CrossRef]
- Zhu, H. A biogeographical comparison between Yunnan, southwest China, and Taiwan, southeast China, with implications for the evolutionary history of the east Asian flora. Ann. Mo. Bot. Gard. 2016, 101, 750–771. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mitttermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2002, 403, 853–858. [Google Scholar] [CrossRef]
- Trew, B.T.; Maclean, I.M.D. Vulnerability of global biodiversity hotspots to climate change. Glob. Ecol. Biogeogr. 2021, 30, 768–783. [Google Scholar] [CrossRef]
- Ren, H.; Guo, Z.H. Progress and prospect of biodiversity conservation in China. Ecol. Sci. 2021, 40, 247–252. [Google Scholar]
- Yang, L.; Yang, G.S.; Ma, H.Y.; Wang, Y.H.; Shen, S.K. Phylogenetic placement of Yunnanopilia (Opiliaceae) inferred from molecular and morphological data. J. Systemat. Evol. 2018, 56, 48–55. [Google Scholar] [CrossRef]
- Le, C.T.; Liu, B.; Barrett, R.L.; Lu, L.M.; Wen, J.; Chen, Z.D. Phylogeny and a new tribal classification of Opiliaceae (Santalales) based on molecular and morphological evidence. J. Systemat. Evol. 2018, 56, 56–66. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Li, D.Z. Yunnanopilia—A primitive new genus of Opiliaceae from Yunnan Plateau, China and its biogeographic significance. Acta Bot. Yunnanica 2000, 22, 248–250. [Google Scholar]
- Wu, Z.S.; Wang, Y.H. Analysis of nutritional components in tender leaves and stems of wild plant Yunnanopilia longistaminea. J. Plant Resour. Environ. 2005, 47, 491–977. [Google Scholar]
- Xu, L.P.; Tang, H.Y.; Jia, P.; Li, Q.; Liu, Y.; Zhang, J.Z. Research advances in Yunnanopilia longistaminea of Opiliaceae in Yunnan. Chin Wild Plant Resour. 2014, 33, 44–46. [Google Scholar]
- Wen, Q.; Fang, F.; Li, Q.; Wang, Q.; Ding, Y. Domestication, cultivation and demonstration effect of six tropical forest vegetables in Yuanjiang. J. West. China For. Sci. 2006, 35, 108–112. [Google Scholar]
- Yang, G.S.; Yang, L.; Wang, Y.H.; Shen, S.K. Physiological epicotyl dormancy and its alleviation in seeds of Yunnanopilia longistaminea: The first report of physiological epicotyl dormancy in China. PeerJ 2017, 5, e3435. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.L.; Shi, C.; Cai, N.N.H.; Ci, X.T.; Peng, J.Y.; Duan, A.A.; Wang, D.W. The complete chloroplast genome of Yunnanopilia longistaminea (Opiliaceae), an endemic species in southwest China. Mitochondrial DNA Part. B 2019, 4, 3624–3625. [Google Scholar] [CrossRef]
- Wang, Y.C.; Shen, B.Q.; Yang, L.; Wang, D.W. Integrated analysis of the transcriptome and metabolome in young and mature leaves of Yunnanopilia longistaminea. Plant Biotechnol. Rep. 2022, 16, 553–564. [Google Scholar] [CrossRef]
- Liu, X.; Li, L.F.; Wang, W.J.; Zhang, H.Y.; Li, X.J.; Gu, M.; Zhou, D.M.; Hou, H.X.; Li, Y.J. Effects of substrate fertilization and root cutting on biomass and photosynthetic pigments of Yunnanopilia longistaminea seedlings. J. Xiamen Univ. (Nat. Sci.) 2023, 62, 873–880. [Google Scholar]
- de Castro, M.T.; Montalvão, S.C.L.; Wolf, V.R.S. First report of Umbaspis regularis (Newstead, 1911) (Hemiptera: Diaspididae) associated with Agonandra brasiliensis Miers ex Benth. & Hook.f. (Opiliaceae) in the Brazilian Cerrado. Entomol. Commun. 2022, 4, ec04033. [Google Scholar]
- Talhinhas, P.; Baroncelli, R. Hosts of Colletotrichum. Mycosphere 2023, 14, 158–261. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Ksack, K.E.; Pietro, A.D.; Spanu, P.D.; Rudd, J.J. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Balendres, M.A.; Mendoza, J.; Dela Cueva, F. Characteristics of Colletotrichum musae PHBN0002 and the susceptibility of popular banana cultivars to postharvest anthracnose. Indian. Phytopathol. 2020, 73, 57–64. [Google Scholar] [CrossRef]
- Ranasinghe, L.S.; Jayawardena, B.; Abeywickrama, K. Use of waste generated from cinnamon bark oil (Cinnamomum zeylanicum Blume) extraction as a post harvest treatment for Embul banana. Food Agric. Environ. 2003, 1, 340–344. [Google Scholar]
- Wang, K.; Liu, F.; Cai, L. A name list of common agricultural phytopathogenic fungi in China. Mycosystema 2022, 41, 361–386. [Google Scholar]
- Han, S.L.; Wang, M.M.; Ma, Z.Y.; Raza, M.; Zhao, P.; Liang, J.M.; Wang, J.W.; Cai, L. Fusarium diversity associated with diseased cereals in China, with an updated phylogenomic assessment of the genus. Stud. Mycol. 2023, 104, 87–148. [Google Scholar] [CrossRef]
- Yang, R.; Li, Y.; Zhao, H.; Sun, X.; Chen, W.; Li, P.; Li, X.; Wu, C.; Ma, M.; Gong, G. Identification and characterization of Colletotrichum species associated with maize in Sichuan, China. J. Fungi 2024, 10, 799. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lin, L.; Cao, J.; Gan, M.; Fan, X. Three new species of Colletotrichum (Glomerellales, Glomerellaceae) associated with walnut (Juglans regia) anthracnose from China. MycoKeys 2024, 108, 147–167. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Taylor, A.S.; Tongson, E.; Edwards, J.; Vaghefi, N.; Ades, P.K.; Crous, P.W.; Taylor, P.W.J. Identification and pathogenicity of Colletotrichum species associated with twig dieback of citrus in Western Australia. Plant Pthol. 2024, 73, 1194–1212. [Google Scholar] [CrossRef]
- Guo, J.W.; Yang, L.F.; Liu, Y.H.; Yang, J.; Wang, H.F.; Li, L.; Liu, Y.H.; Li, W.J. First report of pseudostem black spot caused by Pestalotiopsis microspora on tsao-ko in Yunnan, China. Plant Dis. 2016, 100, 1021. [Google Scholar] [CrossRef]
- Santos, M.C.; Viteri, L.O.; Araujo, S.H.; Mourão, D.C.; Câmara, M.P.; Amaral, A.G.; Oliveira, E.E.; Santos, G.R.d. Molecular characterization and pathogenicity of Colletotrichum on banana fruits: Wound effects on virulence and cross-infection. Microbiol. Res. 2025, 16, 4. [Google Scholar] [CrossRef]
- Doilom, M.; Guo, J.-W.; Phookamsak, R.; Mortimer, P.E.; Karunarathna, S.C.; Dong, W.; Liao, C.-F.; Yan, K.; Pem, D.; Suwannarach, N.; et al. Screening of phosphate-solubilizing fungi from air and soil in Yunnan, China: Four novel species in Aspergillus, Gongronella, Penicillium, and Talaromyces. Front. Microbiol. 2020, 11, 585215. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetesapplication to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- Guerber, J.C.; Liu, B.; Correll, J.C.; Johnston, P.R. Characterization of diversity in Colletotrichum acutatum sensu lato by sequence analysis of two gene introns, mtDNA and intron RFLPs, and mating compatibility. Mycologia 2003, 95, 872–885. [Google Scholar] [CrossRef]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Jiang, N.; Fan, X.L.; Tian, C.M. Identification and characterization of leaf inhabiting fungi from Castanea plantations in China. J. Fungi 2021, 7, 64. [Google Scholar] [CrossRef]
- Truong, H.H.; Sato, T.; Ishikawa, S.; Minoshima, A.; Nishimura, T.; Hirooka, Y. Three Colletotrichum species responsible for anthracnose on Synsepalum dulcificum (Miracle Fruit). Int. J. Phytopathol. 2018, 7, 89–101. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. Mega7: Molecular evolutionary genetics analysis Version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Wang, J.; Zhai, Z.; Xu, G.; Zhang, R.; Zhang, X.; Hu, R. Optimizing sampling points and path planning for soil monitoring in agricultural land. Agronomy 2024, 14, 2947. [Google Scholar] [CrossRef]
- Yang, Y.L.; Cai, L.; Yu, Z.; Liu, Z.; Hyde, K.D. Colletotrichum species on Orchidaceae in southwest China. Cryptogam. Mycol. 2011, 32, 229–253. [Google Scholar]
- Sharma, G.; Shenoy, B.D. Colletotrichum fructicola and C. siamense are involved in chilli anthracnose in India. Arch. Phytopathol. Plant Protect. 2014, 47, 1179–1194. [Google Scholar] [CrossRef]
- Barrus, M.F. Variation in varieties of beans in their susceptibility to anthracnose. Phytopathology 1911, 1, 190–195. [Google Scholar]
- Chen, C.; Dickman, M.B. Dominant active Rac and dominant negative Rac revert the dominant active Ras phenotype in Colletotrichum trifolii by distinct signaling pathways. Mol. Microbiol. 2004, 51, 1493–1507. [Google Scholar] [CrossRef]
- Chen, C.; Ha, Y.; Min, J.; Memmott, S.D.; Dickman, M.B. Cdc42 is required for proper growth and development in the fungal pathogen Colletotrichum trifolii. Eukaryot. Cell 2006, 5, 155–166. [Google Scholar] [CrossRef]
- Dickman, M.B.; Buhr, T.L.; Warwar, V.; Truesdell, G.; Huang, C. Molecular signals during the early stages of alfalfa anthracnose. Can. J. Bot. 1995, 73, 1169–1177. [Google Scholar] [CrossRef]
- Dickman, M.B.; Yarden, O. Protein kinases and phosphatases in filamentous fungi. Fungal Genet. Biol. 1999, 26, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Takano, Y.; Kikuchi, T.; Kubo, Y.; Hamer, J.E.; Mise, K.; Furusawa, I. The Colletotrichum lagenarium MAP kinase gene CMK1 regulates diverse aspects of fungal pathogenesis. Mol. Plant-Microbe Interact. 2000, 13, 374–383. [Google Scholar] [CrossRef]
- Durrant, W.E.; Dong, X. Systemic acquired resistance. Annu. Rev. Phytopathol. 2004, 42, 185–209. [Google Scholar] [CrossRef] [PubMed]
- Kuc, J. Phytoalexins. Annu. Rev. Phytopathol. 1972, 10, 207–232. [Google Scholar] [CrossRef]
- Löbl, I.; Klausnitzer, B.; Hartmann, M.; Krell, F.-T. The silent extinction of species and taxonomists—An appeal to science policymakers and legislators. Diversity 2023, 15, 1053. [Google Scholar] [CrossRef]
- Grimaldi, D.A.; Engel, M.S. Why descriptive science still matters. BioScience 2007, 57, 646–647. [Google Scholar] [CrossRef]
- Yang, Z.-W.; Lian, Z.-H.; Liu, L.; Fang, B.-Z.; Li, W.-J.; Jiao, J.-Y. Cultivation strategies for prokaryotes from extreme environments. iMeta 2024, 3, e123. [Google Scholar] [CrossRef]
- Wang, R.; Ouyang, D.; Lu, M.; Tang, L.; Chen, X.; Huang, S.P.; Guo, T.; Hsiang, T.; Li, Q. Identification and characterization of Colletotrichum species associated with anthracnose disease of plum. Plant Dis. 2024, 108, 2874–2886. [Google Scholar] [CrossRef]
- Vieira, W.A.d.S.; Costa, C.A.d.; Veloso, J.S.; Lima, W.G.; Correia, K.C.; Michereff, S.J.; Pinho, D.B.; Câmara, M.P.S.; Reis, A. Diversity of Colletotrichum species causing anthracnose in chayote in Brazil, with a description of two new species in the C. magnum Complex. J. Fungi 2024, 10, 847. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, J.-Y.; Xu, X.; Cheng, J.; Zheng, L.; Huang, J.; Li, D. Identification and characterization of Colletotrichum species associated with Camellia oleifera anthracnose in China. Plant Dis. 2020, 104, 474–482. [Google Scholar] [CrossRef]
- Liu, F.; Tang, G.; Zheng, X.; Li, Y.; Sun, X.; Qi, X.; Zhou, Y.; Xu, J.; Chen, H.; Chang, X.; et al. Molecular and phenotypic characterization of Colletotrichum species associated with anthracnose disease in peppers from Sichuan Province, China. Sci. Rep. 2016, 6, e32761. [Google Scholar] [CrossRef]
- Guo, J.-W.; Mohamad, O.A.A.; Wang, X.; Egamberdieva, D.; Tian, B. Editorial: Microbiome associated with plant pathogens, pathogenesis, and their applications in developing sustainable agriculture. Front. Microbiol. 2024, 15, 1423961. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, H.; Luo, H.; Xun, J.; Ma, C.; Yang, H.; Bai, D.; Yousuf, S.; Lyu, H.; Zhang, T.; et al. iMeta Conference 2024: Building an innovative scientific research ecosystem for microbiome and One Health. iMeta 2024, 3, e251. [Google Scholar] [CrossRef]
- Kus, J.V.; Zaton, K.; Sarkar, R.; Cameron, R.K. Age-related resistance in Arabidopsis is a developmentally regulated defense response to Pseudomonas syringae. Plant Cell 2002, 14, 479–490. [Google Scholar] [CrossRef]
- Develey-Riviere, M.P.; Galiana, E. Resistance to pathogens and host developmental stage: A multifaceted relationship within the plant kingdom. New Phytol. 2007, 175, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Mersha, Z.; Zhang, S.; Hau, B. Effects of temperature, wetness duration and leaf age on incubation and latent periods of black leaf mold (Pseudocercospora fuligena) on fresh market tomatoes. Eur. J. Plant Pathol. 2014, 138, 39–49. [Google Scholar] [CrossRef]
- Li, P.; Dai, X.; Wang, S.; Luo, Q.; Tang, Q.; Xu, Z.; Zhao, Z.; Wu, F. Biological characteristics and fungicide screening of Colletotrichum fructicola causing mulberry anthracnose. Microorganisms 2024, 12, 2386. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.-W.; Tian, R.-C.; Yang, C.-L.; Jia, L.; Zhou, S.-Y.; Yang, M.; Li, L.; Gao, P.; Yu, L.; Muhammad, M.; et al. The Occurrence of Colletotrichum karstii and C. fructicola Causes Anthracnose on Endangered Ethnic Vegetable Yunnanopilia longistaminea in Yunnan, China. J. Fungi 2025, 11, 748. https://doi.org/10.3390/jof11100748
Guo J-W, Tian R-C, Yang C-L, Jia L, Zhou S-Y, Yang M, Li L, Gao P, Yu L, Muhammad M, et al. The Occurrence of Colletotrichum karstii and C. fructicola Causes Anthracnose on Endangered Ethnic Vegetable Yunnanopilia longistaminea in Yunnan, China. Journal of Fungi. 2025; 11(10):748. https://doi.org/10.3390/jof11100748
Chicago/Turabian StyleGuo, Jian-Wei, Rong-Chuan Tian, Chun-Lian Yang, Lizhi Jia, Su-Yue Zhou, Min Yang, Lifang Li, Penghua Gao, Lei Yu, Murad Muhammad, and et al. 2025. "The Occurrence of Colletotrichum karstii and C. fructicola Causes Anthracnose on Endangered Ethnic Vegetable Yunnanopilia longistaminea in Yunnan, China" Journal of Fungi 11, no. 10: 748. https://doi.org/10.3390/jof11100748
APA StyleGuo, J.-W., Tian, R.-C., Yang, C.-L., Jia, L., Zhou, S.-Y., Yang, M., Li, L., Gao, P., Yu, L., Muhammad, M., Ding, M.-L., & Shen, S.-K. (2025). The Occurrence of Colletotrichum karstii and C. fructicola Causes Anthracnose on Endangered Ethnic Vegetable Yunnanopilia longistaminea in Yunnan, China. Journal of Fungi, 11(10), 748. https://doi.org/10.3390/jof11100748








