Abstract
The fungal genus Trichoderma is highly valued in agriculture for its versatile roles, mainly as a biocontrol agent against plant pathogens. Recently, its use as a natural biofertilizer has gained attention, as Trichoderma spp. promotes crop growth and improves yield by enhancing the rhizosphere environment and activating plant defences. Globally, over 250 Trichoderma-based products dominate 60–90% of the market, but their efficacy can decline during transportation and storage. Additionally, concerns about their impact on native soil biodiversity have led to interest in using locally adapted, native strains. The novel native strain of Trichoderma sp. STP8 (formerly T. koningiopsis agg. STP8) previously showed strong antagonism against Sclerotinia sclerotiorum and promoted lettuce growth in greenhouse conditions. This study evaluated Trichoderma sp. STP8’s effectiveness in field-grown lettuce, revealing yield increases of 16.6% to 30.5%. The most significant gains occurred when Trichoderma sp. STP8 was applied before head formation, 26 days after planting. That was in one treatment with two applications (at seedling planting and after 26 days) and another with three applications (at sowing, at seedling planting, and after 26 days). These results demonstrate Trichoderma sp. STP8’s potential as a sustainable biocontrol and biofertilizer agent for lettuce, encouraging further research across different agricultural systems, including hydroponics and soil-less medium.
1. Introduction
Lettuce (Lactuca sativa L., Asteraceae) is a popular leafy vegetable worldwide, cultivated on approximately 1,260,000 hectares with a total yearly production of around 28 million tons (alongside with chicory). According to official data, in Europe it is cultivated on 127,311 hectares, producing about 3.3 million tons per year [1]. In Croatia, it is grown on 300 hectares of open fields and 64 hectares in greenhouses, with an annual total production of 6932 tons (1650 t in greenhouse) [2]. Lettuce is recognized as an important functional food due to its low calories, fat, and sodium content, while being rich in vitamins, folate, dietary fibres, and essential minerals such as phosphorus (P), calcium (Ca), magnesium (Mg), and iron (Fe) [3,4]. Head lettuce contains on average about 94–96% water, along with 2.0–2.3% sugars, 0.5–0.6% crude cellulose, 0.4–0.6% mineral substances, and 1.0–1.3% crude protein [5]. Its leaves are particularly high in vitamins C, E, and B complex, and it is a good source of potassium (K) while containing low sodium levels (Na). The content of minerals varies among different lettuce types and is influenced by soil conditions. Additionally, lettuce contains bioactive compounds such as polyphenols, carotenoids, and chlorophyll, which offer various health benefits [6].
The growth cycle of lettuce is highly dependent on temperature, with crops typically reaching maximum development during the warmer part of a year in about 45–60 days after transplanting, depending on the variety. In contrast, winter-grown lettuce may take between 90 and 120 days until harvest. Elevated temperatures, combined with high light intensity and longer daylight hours, accelerate growth by promoting faster leaf development, resulting in broader leaves and quicker head formation [5]. Due to its short growth cycle, it is possible to cultivate 2–3 crops of lettuce per year.
Fungi from the genus Trichoderma (Hypocreales, Ascomycota) are rhizosphere inhabitants, the members of soil and plant mycobiota, playing a crucial ecological role in both natural and agricultural ecosystems, including the bioremediation of polluted environments [7]. As one of a dominant component of rhizosphere microbiota across virtually all terrestrial ecosystems, Trichoderma spp. can enhance overall plant health by creating a favourable rhizosphere environment that supports nutrient availability and suppresses pathogens. Their agricultural importance primarily lies in their strong antagonism against various soil-borne plant pathogenic fungi, which has driven their commercial success as bio-fungicides, typically applied as soil treatments and seed coatings [8,9,10]. But some Trichoderma spp. colonize the root surface, even penetrating the epidermis and a few cell layers below in the root tissue, demonstrating their high opportunistic potential by establishing a mycorrhizal relationship with the plant host [11,12]. Due to that, Trichoderma spp. can alleviate extrinsic as well as intrinsic stresses and activate plants’ natural defence mechanisms to preserve the health of cultivated plants, which, as new studies have shown, can be heritable [7,13,14,15]. Also, Trichoderma spp. may cause significant biochemical changes in plant contents of carbohydrates, amino acids, organic acids, and lipids improving dry weight biomass [16]. In addition, their potential to improve water and nutrient uptake contributes to increased plant height, root and shoot growth, and faster seed germination as well as sprouting. Owing to this fertilization potential, Trichoderma spp. has gained even greater agricultural utilization for green technologies, marketed as plant inoculants or plant strengthening agents, and biofertilizers [17,18].
Beside the well-known positive effects of Trichoderma spp. application on soil-grown crops, recent research also confirmed positive effects on lettuce growth, yield, and mineral accumulation in hydroponic [19,20,21] and aquaponic growing systems [22]. Application of Trichoderma spp. in these systems also enhances the efficiency of mineral fertilizers and helps reduce the amounts required.
The availability and distribution of Trichoderma-based biofertilizers are more widespread than commonly recognized and are expanding, partly because they are easier to register, since they are not classified as pesticides, and face less regulatory pressure to reduce chemical pesticide use [23]. Due to the shortened life span of the Trichoderma product during transportation, storage, and application, a possible weakened biological control effect may occur after application in the field [10,24]. Moreover, the negative impact of Trichoderma species from bioproducts on the biodiversity of not only native Trichoderma species but also other organisms (e.g., plants, bacteria, other fungi) is receiving more attention in research studies [7,23,25,26,27]. Therefore, the application of an autochthonous Trichoderma sp. strain is possibly more appropriate than different commercial Trichoderma spp. products containing an allochthones strain.
A stimulant effect of Trichoderma spp. was noted in the greenhouse experiments with lettuce [28,29,30,31,32]. Yet, there is still limited research on its beneficial effects when used in open-field lettuce cultivation. Our recent greenhouse study also demonstrated the strong biofungicidal potential of a native Croatian strain, a novel strain of Trichoderma sp. strain STP8 (previously as T. koningiopsis agg. STP8), against Sclerotinia sclerotiorum in association with lettuce plants, as well as biostimulant effects that resulted in increased lettuce yields [33]. The obtained results led to the setup of a field trial with the aim of investigating the potential of the Trichoderma sp. STP8 strain as a lettuce yield promoter and its influence on mineral content. The hypothesis posited that the Trichoderma sp. STP8 strain could enhance growth parameters without compromising plant quality.
2. Materials and Methods
2.1. Isolation and Identification of the Trichoderma sp. Strain
In this study, the novel fungal strain Trichoderma sp. STP8 was utilized. The isolate was obtained from humus soil at the experimental vegetable garden site and was originally isolated from the lettuce roots infected with Sclerotinia sclerotiorum [33]. The axenic culture was maintained on potato dextrose agar (PDA) media and stored in the temporary laboratory collection under the code STP8 (Trichoderma sp.). The strain was taxonomically determined by molecular methods, and the process of DNA isolation, amplification of ITS, rpb2 and tef1 gene regions, and sequencing is presented in detail in the previous work [33].
The Basic Local Alignment Search Tool (BLAST, https://blast.ncbi.nlm.nih.gov, accessed on 6 October 2025) was used for searching similar sequences in GenBank. Sequence alignment of the dataset was achieved individually on ITS, rpb2 and tef1 using MAFFT vers. 7.490 [34,35], available as the Geneious Prime plugin (Geneious Prime 2025.0.3 Build 2024-11-22 (Biometers, Oakland, New Zealand)). The ITS dataset was not used in the further phylogenetic analysis due to the weak barcode potential of this DNA region in the genus Trichoderma [36]. The best substitution model was selected by Smart Model Selection in the PhyML online resource (http://www.atgc-montpellier.fr/phyml/, accessed on 7 October 2025) [37], TN93 for the rpb2 dataset and HKY85 for the tef1 dataset, respectively. All species included in the phylogenetic analysis are listed in Table 1, with Protocrea farinosa designated as the outgroup [38]. After being aligned and trimmed, concatenation of rpb2 and tef1 alignments was accomplished using Geneious Prime 2025.0.2. The concatenated alignment contained 1400 character positions including gaps, with 819 character positions for rpb2 and 584 characters positions for tef1. Phylogenetic analysis of concatenated rpb2 and tef1 datasets was conducted using maximum likelihood (ML) in PhyML 3.3.20180621, available as the Geneious Prime plugin [39], by applying the bootstrap approximation with 1000 replicates. Bayesian inference (BI) analysis was performed in MrBayes 3.2.6, available as the Geneious Prime plugin [40] under the GTR + G substitution model. BI analysis was executed for 6,000,000 generations, sampling trees and other parameters every 1500 generations. The default numbers of chains (four) and heating parameters were used. Posterior probabilities (BPPs) were calculated after discarding the first 1000 sampled trees. The phylogenetic trees were visualized and annotated using FigTree vers. 1.4.3 (http://tree.bio.ed.ac.uk/software/figtree/, accessed on 13 October 2025).

Table 1.
List of sequences included in the phylogenetic analysis.
2.2. Field Trial
The field trial was conducted at the Maksimir experimental field (45°49′ N, 16°02′ E), Department of Vegetable Crops, Faculty of Agriculture in Zagreb, Croatia. Prior to the experiment, comprehensive soil analysis was performed (Table 2), revealing a neutral soil pH (pH H2O: 7.5; nKCl: 6.86), low humus content (2.22%), but sufficient nitrogen (0.2% N) and potassium (25.5 mg K2O·100 g−1) levels, while phosphorus was abundant (41.1 mg P2O5·100 g−1). The chemical properties were assessed on air-dried, ground, and homogenized soil samples using standardized methods: soil pH was measured electrometrically with a combined pH electrode in a 1:2.5 soil-to-water suspension, humus content was determined by the Tyurin method, potassium and phosphorus levels were analyzed via the Egner–Riehm–Domingo method [52], and nitrogen content was assessed using the Kjeldahl method [53].

Table 2.
Results of soil chemical analysis at the Maksimir experimental field.
Based on available meteorological data from the Maksimir meteorological station, from May to July, the average monthly air temperature ranged between 15.9 and 21.2 °C, which are optimal for outdoor lettuce cultivation. Rainy days occurred in May and June, totaling 13 days each, and 11 days in July. The average monthly precipitation was 136.1 mm in May, 57.7 mm in June, and 145.7 mm in July [54].
Commercial natural/untreated seeds of Batavia type lettuce cv. Bataille (Nunhems Netherlands BV, Nunhem, Netherlands) were sown on 24 April 2023 into a polystyrene tray with 104 sites, each filled with 32 mL of autoclaved potting mixture substrate Potground H (Klasmann-Deilmann, Geeste, Germany). One seed was placed per hole, and then 0.5 mL of the Trichoderma sp. STP8 spore suspension at a concentration of 4 × 106 spores mL−1 was applied using a micropipette (Eppendorf Research, Stevenage, UK). Seeds that did not receive this treatment served as controls and for variants where the treatment was omitted.
Seedlings with 3–4 true leaves were manually transplanted at the open field on 25 May 2023, with an intra-row and between-row spacing of 30 cm, resulting in a plant density of approximately 11.1 plants per square meter.
The trial was set up using a randomized complete block design with five replicates, each containing 20 plants, totaling eight treatment variants as shown in Table 3. The Trichoderma sp. STP8 spore suspension, at a concentration of 4 × 106 spore mL−1 was applied by pouring the suspension directly along the root collar into the soil using a knapsack sprayer (Santaj plastika, Valpovo, Croatia).

Table 3.
Experimental variants of Trichoderma sp. strain STP8 suspension application.
Due to the adequate nutrient supply in the soil, fertilization was not applied during soil preparation or lettuce cultivation. The plants were harvested on 7 July, 43 days after planting, and the following morphological parameters were measured: head weight, diameter, and leaf length and width.
2.3. Mineral Content Determination
Determination of the plant material mineral content was carried out in the laboratory of the Department of Plant Nutrition, Faculty of Agriculture. Dry matter (DM) is determined by the gravimetric method, where the total dry matter is expressed as a percentage (%) and constitutes the entire amount of matter from the product that does not evaporate under certain conditions. It is carried out in a heated dryer at a temperature of 105 °C. The method is intended for determining the total dry matter in fruit and vegetable products. It is calculated according to the following equation:
where m0 represents the mass of the container and auxiliary material (quartz sand, glass rod and lid) (g); m1 is the mass of the container with the tested sample before drying (g) and m2 is the mass of the container with the residue after drying (g).
Dry matter (%) = (m2 − m0)/(m1 − m0) × 100
The amount of macroelements is expressed as a percentage of dry matter (% DM) for N, P, K, Ca, and Mg, regarding the variable water content of collected samples. Total nitrogen in plant material is determined by the Kjeldahl method. Dried plant material samples were digested with concentrated HNO3 by Ethos Up Microwave Digestion System(MILESTONE1200MegaMicrowave Digester, Milestone Srl, Sorisole, Italy). After digestion, the phosphorus content was determined spectrophotometrically, and potassium was determined by flame photometer, while calcium and magnesium were analyzed using an atomic absorption spectrophotometer (AAS) (Thermo Fisher Scientific, Ahaus, Germany) [55].
To convert phosphorus and potassium from their oxide form (P2O5 and K2O) to their elemental form, the following equations were used:
P = P2O5 × 0.436
K = K2O × 0.830.
2.4. Assessment of Antagonism on Agar Culture Plates
To evaluate how the Trichoderma sp. strain STP8 competes with native soil phytopathogenic fungi in situ, Alternaria solani Sorauer, Fusarium culmorum (W.G. Sm.) Sacc, F. solani (Mart.) Sacc., and Sclerotinia sclerotiorum (Lib.) de Bary were isolated from soil where the lettuce was planted. Soil fungi were isolated by using the serial dilution plating technique as described by Shivanand et al. [56]. The antagonistic effects of Trichoderma sp. STP8 on the growth inhibition of A. solani, F. culmorum, F. solani, and S. sclerotiorum were investigated according to Porras et al. [57] using the dual-culture method. The fungal cultures were grown on PDA (Biolife, Milan, Italy) at 21 °C in 90 mm diameter Petri dishes. Mycelial discs with a diameter of 6 mm were removed from the edge of the seven-day-old cultures and transferred to 90 mm diameter Petri dishes containing PDA to form dual cultures. A mycelial disc of pathogen was placed on one side of a PDA plate, while a disc of Trichoderma sp. STP8 was placed on the opposite side. For the control plates, a sterile agar disc was used instead of the Trichoderma sp. STP8 mycelial disc. Each treatment was carried out in five plates with three replicates (N = 60). The dishes were sealed with paraffin tape (Parafilm, Brand GMBH + CO KG, Wertheim, Germany) and incubated in the dark at 20 °C ± 1 °C for seven days.
On the seventh day, radial fungal colony growth in the direction of the opposite colony was measured manually using a ruler. The maximum and minimum radial growth was measured, and the average radial growth was calculated. The average radial growth was used to calculate the inhibition index (I %) as follows:
where I is the inhibition percentage; C is the radial growth of pathogen (mm) alone (control); T is the radial growth of pathogen (mm) in the presence of Trichoderma sp. STP8 [58]. An index value of 50% or more is considered as excellent performance.
I (%) = ((C − T)/C) × 100
2.5. Statistical Analysis
Statistical analysis of the achieved results on morphological properties and mineral content was performed in SAS® Software v.9.4 [59]. The differences between the experimental treatments were analyzed by analysis of variance (ANOVA). Determined differences between the average values were compared by the LSD t-test and Duncan’s multiple-range test at significance levels p ≤ 0.05 and p ≤ 0.01.
3. Results
3.1. The Trichoderma sp. Strain Identification
In this study, a novel native strain Trichoderma sp. STP8, originated from lettuce roots. To identify Trichoderma sp. strain STP8 at the species rank, we followed the guidelines of Cai i Druzhinina [36] using three DNA barcode sequence data (ITS, rpb2, and tef1 gene regions). Megablast search of the NCBIs GenBank nucleotide database using the rpb2 and tef1 sequences of Trichoderma sp. strain STP8 showed that several closest hits belong to T. hongkuii C. L. Zhang, T. neohongkuii C. L. Zhang and T. parahongkuii C. L. Zhang. Phylogenetic analysis of Trichoderma sp. STP8 and closely related taxa confirmed clear affiliation of Trichoderma sp. STP8 with Koningii clade (Figure 1). Comparison of Trichoderma sp. STP8 sequence data to the reference (type) strains of T. hongkuii, T. neohongkuii and T. parahongkuii resulted in 100% identity in rpb2 with T. hongkuii, and ≥99% to T. neohongkuii and T. parahongkuii (Table 4). Since rpb2 is solely not efficient in molecular species recognition of a number of Trichoderma spp., e.g., T. caribbaeum, T. istrianum and others (www.trichoderma.info, accessed on 9 October 2025), we used in our analysis also of the tef1 DNA region to ascertain the best possible taxonomic position for the Trichoderma sp. STP8 strain. All three aforementioned closely related species were ≤97% similar in the tef1 DNA region to the Trichoderma sp. STP8 strain (Table 5). Concatenate phylogenetic analysis of both rpb2 and tef1 datasets showed the strong support of the Trichoderma sp. strain STP8 to the T. hongkuii species group, with T. neohongkuii as a sister species (MLBP = 60; BIPP = 0.8) (Figure 1). Detailed phylogenetic and taxonomic analyses that will include all phenetic datasets are still in progress.
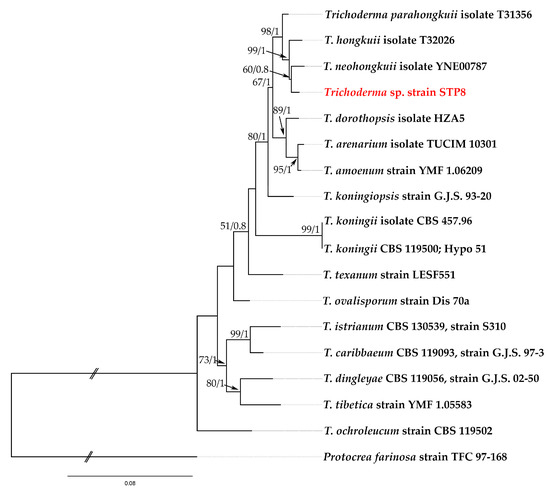
Figure 1.
Phylogenetic tree generated by maximum likelihood (ML) analysis using the concatenated sequences of the rpb2 and tef1 loci of the Trichoderma sp. species clustered in a Koningii clade. Notes are marked with maximum likelihood bootstrap proportions ≥50% (left) and Bayesian inference posterior probability values ≥0.8 (right) (MLBP/BIPP). Protocrea farinosa was used as an outgroup. Croatian strain Trichoderma sp. STP8 is marked in red color.

Table 4.
Similarity of rpb2 sequences (%) between Trichoderma sp. strain STP8 and closely related species.

Table 5.
Similarity of tef-1 sequences (%) between Trichoderma sp. strain STP8 and closely related species.
3.2. Lettuce Phenotypic Characteristics
In this study, the lettuce cultivar Bataille responded well to the inoculation of Trichoderma sp. STP8 in terms of biomass accumulation and marketable yield. In all variants of Trichoderma sp. STP8 application, treated plants showed the best quality in terms of morphological parameters as the head weight, diameter, and leaf width were significantly increased in relation to the control. The greatest effect was achieved by applying the Trichoderma sp. STP8 suspension twice or three times. The best effect on head weight was achieved in treatment B2 followed by treatment A3. The best effect on head diameter was achieved in treatment A3, A2 and B2. The increment of leaf width was greatest in treatment A1. Although the treated plants showed an increment of leaf length, it was not statistically significant (Table 6).

Table 6.
Effect of Trichoderma sp. strain STP8 on the phenotypic characteristics of lettuce in field growth.
The Trichoderma sp. strain STP8 enhanced growth parameters without compromising plant quality as the hypothesis posited.
3.3. Lettuce Mineral Content
The application of Trichoderma sp. STP8 increased the dry matter biomass and mineral content of lettuce leaves (Figure 2A–F) compared to the untreated control, but also with significant differences between treatments (i.e., time and number of treatments). The significantly highest DM content (6.60%) was determined in treatments A2 and A1, when plants were treated at sowing and planting. In contrast, these plants had the lowest nitrogen, phosphorus, and magnesium contents (2.24, 0.50 and 0.20% DM), even lower than control plants (Figure 2A). Nitrogen and phosphorus contents were highest in treatment C1, when plants were treated only once, 26 days after planting. The content was 20.4 to 40.1% higher than in other treatments, and 28.3% higher than in the control for nitrogen, and 27.3 to 5.1% and 18.2% for phosphorus, respectively (Figure 2B,C). Multiple Trichoderma sp. STP8 applications positively affected potassium, calcium, and magnesium contents in lettuce leaves, so the highest values of these minerals were recorded in plants that were treated three times (treatment A3). When compared to the other treatments, contents were higher between 2.5 and 41.8% for potassium, from 19.2 to 46.2% for calcium, and from 6.5 to 35.5% for magnesium. Regarding control plants, the values were 7.9, 19.2, and 16.1% higher (Figure 2D–F).
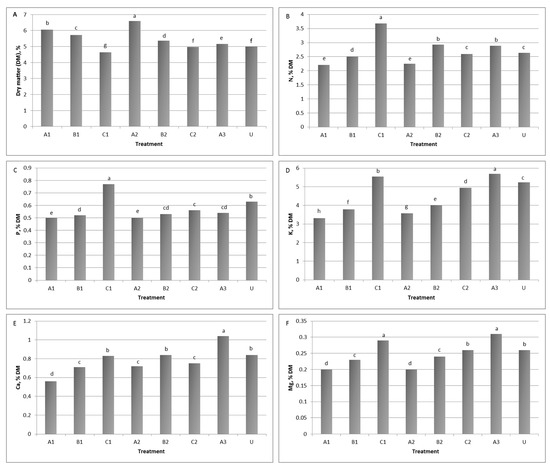
Figure 2.
Effect of Trichoderma sp. strain STP8 on the (A) dry matter (DM) content and mineral content of lettuce leaves’ (B) nitrogen, (C) phosphorus, (D) potassium, (E) calcium, (F) magnesium. The columns with different letters differ significantly at a significance level of p ≤ 0.05.
3.4. Antagonism on Agar Culture Plates
All plants in this field trial remained healthy, without any disease symptoms, even though no protective products were used, while laboratory analysis of soil showed the presence of pathogens A. solani, F. culmorum, F. solani, and S. sclerotiorum.
In vitro tests showed excellent antagonisms of the selected native strain Trichoderma sp. STP8 to tested pathogens found on the trial site, through competitiveness and mycoparasitism. Trichoderma sp. STP8 grew faster and occupied the largest part of the Petri dish in seven days, and inhibited the growth and parasitized colonies of pathogenic species, except for F. culmorum, in the following percentages: A. solani—79%; F. culmorum—44%; F. solani—75%; and S. sclerotiorum—77% (Table 7).

Table 7.
Antagonism of Trichoderma sp. strain STP8 against some phytopathogenic fungi.
3.5. Limitation Subsection
The obtained results are based on a field trial conducted at a single location, utilizing the lettuce cultivar Bataille as the sole crop species, over the course of one growing season, which might restrict broader generalization.
4. Discussion
Although the genus Trichoderma is well-known for its antagonism against phytopathogenic fungi and its beneficial effects on plant growth, these abilities are not uniform across all Trichoderma species or even biotypes of the same species, with some exhibiting minimal or no activity, primarily due to genetic differences among strains. Recent taxonomic advancements have revealed biogeographic biases and substrate preferences among species [36], and diversity studies highlight the ecological specialization of Trichoderma spp., showing that distribution is influenced by microclimate, substrate types, and complex ecological interactions [60,61,62]. Furthermore, the metabolic products vary among strains, often exhibiting selectivity toward specific plant species or varieties [62,63], and the effectiveness of a strain in promoting plant growth depends on factors such as application method, soil composition, environmental conditions, crop rotation history, and plant interactions [63,64,65]. Consequently, employing autochthonous, locally adapted Trichoderma spp. strains may produce more effective results in specific agricultural systems.
In this study, the use of the novel native Trichoderma sp. STP8 strain, originating from lettuce roots collected at the same location where the trial was conducted, resulted in an increased marketable yield of the lettuce variety Bataille, contributing positively to similar research [29]. The significant yield increase within just over two months is particularly noteworthy, as lettuce leaves are the final commercial product. The greatest increases in head weight and diameter were observed when the Trichoderma sp. STP8 strain was applied before head formation, which in this study occurred 26 DAP: 1) treatment B2—Trichoderma sp. STP8 applied twice, at seedling planting and again at 26 DAP; and 2) treatment A3—Trichoderma sp. STP8 applied three times, at sowing, seedling planting and 26 DAP.
The observed yield increases of 16.6 to 30.5% align with the findings of Senger et al. [31], who reported yield improvements of 16 to 22% compared to untreated controls, depending on the lettuce variety and Trichoderma sp. inoculation dose. Additionally, Lima et al. [30] observed a 45% increase in the fresh mass of the aerial part with T. virens and a 15% increase in plant height with T. koningiopsis. It should be emphasized that those studies were conducted in optimal/controlled greenhouse conditions. Similar results in lettuce growth promotion were achieved in a study where T. virens were applied, resulting in a yield increase of 45% to 67% [66].
The dry weight content significantly differed from the control with Trichoderma sp. STP8 application; notably, plants treated once at 26 DAP (treatment C1), showed a 7% reduction in dry matter content. In contrast, other treatments resulted in increases ranging from 3.3% to 24.2%, with the highest increase observed in treatment A2, where Trichoderma sp. STP8 was applied twice, at sowing and seedling planting. Similar findings were reported by Senger et al. [31], who observed dry matter content increases between 17.2% and 26.7% across different lettuce varieties in greenhouse conditions. Additionally, our results are comparable to those of Gutiérrez-Chávez et al. [21], although their study was conducted in a floating system, demonstrating that Trichoderma spp. application reliably enhances the dry matter content of lettuce regardless of the cultivation approach. These findings suggest the broad applicability of Trichoderma spp. across different cultivation systems, which could be valuable for growers seeking effective biological methods to improve yield.
It was determined that Trichoderma spp. in soil affects root morphology and architecture, and stimulates root growth, which consequently contributes to nutrient uptake, particularly nitrate, Ca, Mg, and K [66]. The significant Trichoderma sp. STP8 interaction with the following elements was observed in determined macronutrient contents: nitrogen, phosphorus, potassium, calcium, and magnesium. Achieved results on mineral content in lettuce leaves corroborate findings from Fiorentino et al. [67], where the use of Trichoderma spp. strains led to a notable increase in P, K, and Ca concentrations in lettuce. The increased phosphorous concentrations could be a result of higher phosphorus availability in native soil, induced by Trichoderma spp. producing acids (coumaric, glucuronic and citric acid) [68]. However, these results differ from the study by Gutiérrez-Chávez et al. [21], which found that lettuce grown in a floating system had the highest levels of K, Ca, and Mg in control plants, while the nutrient content in plants treated with Trichoderma spp. was similar or significantly lower. These findings confirm that endophytic Trichoderma spp. can enhance plant uptake efficiency, particularly for nitrogen. Nitrogen can positively impact lettuce by promoting increased growth, yield, and nutrient content [69,70,71]. However, excessive nitrogen availability may diminish lettuce quality by encouraging nitrate accumulation in the aboveground tissues [29]. Importantly, using Trichoderma spp. can reduce reliance on external nitrogen sources, including mineral fertilizers and organic amendments like manure and compost, contributing to more sustainable cultivation practices. When comparing lettuce grown under mineral fertilization with those under organic fertilization, de Lima et al. [72] determined a lower dry matter content in organic lettuce. Also, in organic lettuce there were lower levels of magnesium and phosphorus, but higher levels of calcium and potassium.
Species of Trichoderma are strong plant invaders capable of colonizing a plant internally in an endophytic manner, allowing them to directly influence plant physiology [17]. This intimate Trichoderma–plant relationship induces localized and systemic resistance plant responses to pathogen attack, promoting plant growth and supporting biocontrol. Furthermore, genetic analyses have shown that most of Trichoderma spp. biocontrol activity is mediated through their ability to induce plant defence mechanisms, described as systemic disease resistance. Also, analysis has shown that much or most of the biocontrol activity of these fungi is through their abilities to induce plant systemic disease resistance, and that antagonistic mechanisms, antibiosis and mycoparasitism, were found to be due solely to induced resistance [16,17]. In our research, Trichoderma sp. strain STP8 was very effective in tests in vitro against soil-borne A. solani, F. solani and S. sclerotiorum, with an inhibition index between 73 and 79%. As a true mycoparasite of S. sclerotiorum, Trichoderma sp. STP8 was confirmed in a previous laboratory and greenhouse trial in vivo with lettuce [33]. It should be emphasized that strain Trichoderma sp. STP8 was isolated from soil at the experimental field and was originally isolated from the lettuce roots infected with S. sclerotiorum. This is in accordance with the conclusion of previous studies that the best results are achieved when the used Trichoderma species is isolated from the local areas of the plant and soil [62,63,73]. Moreover, this finding supports the previously postulated idea that the diverse capabilities of Trichoderma spp. are encoded within its genome, like the observed genome coevolution in numerous plant–pathogen interactions [68]. Consequently, Trichoderma spp. is expected to be effective in disease control under specific conditions of temperature, moisture, and nutrient availability. The hybridization of different strains or species of Trichoderma to enhance their beneficial traits has been proposed and commercial mixtures are available [8,68]. Also, in development are other types of consortia, mixtures of Trichoderma spp. strains with other organisms, that are known as bioagents [74,75]. Based on this, we will focus our future research at this experimental location on identifying native Trichoderma species and their capabilities as biostimulants/biofertilizers, and/or biocontrol agents.
5. Conclusions
The positive effects of Trichoderma spp. on plant growth depend on the use of an effective Trichoderma sp. strain, as the mechanism of its action is influenced by its genetic variability; the method of its application on the seed or root and soil; the genetic variability of the treated plant species and their interactions. Consequently, the outcomes can be unpredictable if the aforementioned factors are uncontrolled, potentially resulting in either positive or negative effects. Notably, the ability of Trichoderma spp. to promote growth in short-lived plants like lettuce is particularly remarkable and valuable, offering an eco-friendly approach that requires less financial input by reducing the use of mineral fertilizers, thereby minimizing environmental and health risks while still maintaining crop yields. In this field study conducted at a single location, from where the Trichoderma sp. STP8 strain originated, the lettuce cultivar Bataille responded positively to Trichoderma sp. STP8 inoculation, demonstrating increased biomass accumulation and marketable yield in just one short growing season. Across all Trichoderma sp. STP8 application variants, treated plants exhibited superior morphological parameters, with significant increases in head weight, diameter, and leaf size compared to controls. Additionally, Trichoderma sp. STP8 improved dry matter and mineral content in the lettuce leaves. In vitro tests further confirmed the strain’s efficacy, showing strong antagonistic activity against pathogens present at the trial site through mechanisms of competitiveness and mycoparasitism. This native strain was shown to be a promising biostimulant and biocontrol agent. For future research, we plan to investigate the long-term effects of Trichoderma sp. STP8 application on lettuce yield over multiple growing seasons, as well as its impact on quality and nutritional content. Finally, we will assess the economic feasibility and sustainability of using Trichoderma sp. STP8 in different agricultural conditions, such as lettuce growing in hydroponic and soil-less media, as this can facilitate its broader adoption in practical farming.
Author Contributions
Conceptualisation, visualization, validation, resources, S.T.-P., B.B. and S.S.; methodology, formal analysis, investigation and data curation, all authors; funding acquisition, S.T.-P. and B.B.; writing—original draft, S.T.-P., B.B. and S.S.; writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors would like to thank the support of the institutional funding of research activities from the University of Zagreb.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- FAOSTAT Statistics Database. Available online: https://www.fao.org/faostat/en/#home (accessed on 12 May 2025).
- Croatian Bureau of Statistics. PC-Axis Databases: Agriculture, Hunting, Forestry and Fishing. Available online: https://web.dzs.hr/PxWeb/pxweb/en/Poljoprivreda,%20lov,%20%C5%A1umarstvo%20i%20ribarstvo/Poljoprivreda,%20lov,%20%C5%A1umarstvo%20i%20ribarstvo__Biljna%20proizvodnja/BP3_NUTS2021.px/ (accessed on 12 May 2025).
- Kim, M.J.; Moon, Y.; Tou, J.C.; Mou, B.; Waterland, N.L. Nutritional value, bioactive compounds and health benefits of lettuce (Lactuca sativa L.). J. Food Compos. Anal. 2016, 49, 19–34. [Google Scholar] [CrossRef]
- Medina-Lozano, I.; Bertolín, J.R.; Díaz, A. Nutritional value of commercial and traditional lettuce (Lactuca sativa L.) and wild relatives: Vitamin C and anthocyanin content. Food Chem. 2021, 359, 129864. [Google Scholar] [CrossRef] [PubMed]
- Welbaum, G.E. Family Asteraceae. In Vegetable Production and Practices, 1st ed.; Stubbs, R., Davies, L., Hayden, R., Eds.; CABI Publishing: Wallingfort, UK, 2015; pp. 222–239. [Google Scholar]
- Shi, M.; Gu, J.; Wu, H.; Rauf, A.; Emran, T.B.; Khan, Z.; Mitra, S.; Aljohani, A.S.M.; Alhumaydhi, F.A.; Al-Awthan, Y.S.; et al. Phytochemicals, Nutrition, Metabolism, Bioavailability, and Health Benefits in Lettuce—A Comprehensive Review. Antioxidants 2022, 11, 1158. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Cornejo, H.M.; Schmoll, M.; Esquivel-Ayala, B.A.; González-Esquivel, C.E.; Rocha-Ramírez, V.; Larsen, J. Mechanisms for plant growth promotion activated by Trichoderma in natural and managed terrestrial ecosystems. Microbiol. Res. 2024, 281, 127621. [Google Scholar] [CrossRef] [PubMed]
- Topolovec-Pintarić, S. Trichoderma: Invisible partner for visible impact in agriculture. In Trichoderma: The Most Widely Used Fungicide, 1st ed.; Shah, M.M., Sharif, U., Buhari, T.R., Eds.; Intechopen: London, UK, 2019; pp. 15–30. [Google Scholar]
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-Sciseł, J. Trichoderma: The Current Status of Its Application in Agriculture for the Biocontrol of Fungal Phytopathogens and Stimulation of Plant Growth. Int. J. Mol. Sci. 2022, 23, 2329. [Google Scholar] [CrossRef]
- Kubiak, A.; Wolna-Maruwka, A.; Pilarska, A.A.; Niewiadomska, A.; Piotrowska-Cyplik, A. Fungi of the Trichoderma Genus: Future Perspectives of Benefits in Sustainable Agriculture. Appl. Sci. 2023, 13, 6434. [Google Scholar] [CrossRef]
- Druzhinina, I.S.; Seidl-Seiboth, V.; Herrera-Estrella, A.; Horwitz, B.A.; Kenerley, C.M.; Monte, E.; Mukherjee, P.K.; Zeilinger, S.; Gigoriev, I.V.; Kubicek, C.P. Trichoderma: The genomics of opportunistic success. Nat. Rev. Microbiol. 2011, 9, 749–759. [Google Scholar] [CrossRef]
- Pozo, M.J.; Zabalgogeazcoa, I.; Vazquez de Aldana, B.R.; Martinez-Medina, A. Untapping the potential of plant mycobiomes for applications in agriculture. Curr. Opin. Plant Biol. 2021, 60, 102034. [Google Scholar] [CrossRef]
- Medeiros, H.; Araújo Filho, J.; Freitas, L.; Castillo, P.; Rubio, M.B.; Hermosa, R. Tomato progeny inherit resistance to the nematode Meloidogyne javanica linked to plant growth induced by the biocontrol fungus Trichoderma atroviride. Sci. Rep. 2017, 7, 216. [Google Scholar] [CrossRef]
- Morán-Diez, M.E.; Martínez de Alba, Á.E.; Rubio, M.B.; Hermosa, R.; Monte, E. Trichoderma and the Plant Heritable Priming Responses. J. Fungi 2021, 7, 318. [Google Scholar] [CrossRef]
- Woo, S.L.; Hermosa, R.; Lorito, M.; Monte, E. Trichoderma: A multipurpose, plant- beneficial microorganism for eco-sustainable agriculture. Nat. Rev. Microbiol. 2022, 21, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Sood, M.; Kapoor, D.; Kumar, V.; Sheteiwy, M.S.; Ramakrishnan, M.; Landi, M.; Araniti, F.; Sharma, A. Trichoderma: The “Secrets” of a Multitalented Biocontrol Agent. Plants 2020, 9, 762. [Google Scholar] [CrossRef]
- Harman, G.E. Trichoderma—Not just for biocontrol anymore. Phytoparasitica 2011, 39, 103–108. [Google Scholar] [CrossRef]
- Zin, N.A.; Badaluddin, N.A. Biological functions of Trichoderma spp. for agriculture applications. Ann. Agric. Sci. 2020, 65, 168–178. [Google Scholar] [CrossRef]
- Moreira, V.D.A.; Oliveira, C.E.D.S.; Jalal, A.; Gato, I.M.B.; Oliveira, T.J.S.S.; Boleta, G.H.M.; Giolo, V.M.; Vitoria, L.S.; Tamburi, K.V.; Filho, M.C.M.T. Inoculation with Trichoderma harzianum and Azospirillum brasilense increases nutrition and yield of hydroponic lettuce. Arch. Microbiol. 2022, 204, 440. [Google Scholar] [CrossRef]
- Shabani, E.; Alemzadeh Ansari, N.; Fayezizadeh, M.R.; Caser, M. Can Trichoderma harzianum be used to enhance the yield and nutrient uptake of Lactuca sativa cv “Lollo Rosso” in floating systems? Food Sci. Nut. 2024, 12, 4800–4809. [Google Scholar] [CrossRef]
- Gutiérrez-Chávez, A.; Robles-Hernández, L.; Guerrero, B.I.; González-Franco, A.C.; Medina-Pérez, G.; Acevedo-Barrera, A.A.; Hernández-Huerta, J. Potential of Trichoderma asperellum as a Growth Promoter in Hydroponic Lettuce Cultivated in a Floating-Root System. Plants 2025, 14, 382. [Google Scholar] [CrossRef]
- Patloková, K.; Pokluda, R. Optimization of Plant Nutrition in Aquaponics: The Impact of Trichoderma harzianum and Bacillus mojavensis on Lettuce and Basil Yield and Mineral Status. Plants 2024, 13, 291. [Google Scholar] [CrossRef] [PubMed]
- Martinez, Y.; Ribera, J.; Schwarze, F.W.M.R.; De France, K. Biotechnological development of Trichoderma-based formulations for biological control. App. Microbiol. Biotech. 2023, 107, 5595–5612. [Google Scholar] [CrossRef]
- Yao, X.; Guo, H.; Zhang, K.; Zhao, M.; Ruan, J.; Chen, J. Trichoderma and its role in biological control of plant fungal and nematode disease. Front. Microbiol. 2023, 14, 1160551. [Google Scholar] [CrossRef]
- Kubicek, C.P.; Bissett, J.; Druzhinina, I.; Kullnig-Gradinger, C.; Szakacs, G. Genetic and metabolic diversity of Trichoderma: A case study on Southeast Asian isolates. Fungal Genet. Biol. 2003, 38, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Migheli, Q.; Balmas, V.; Komoñ-Zelazowska, M.; Scherm, B.; Fiori, S.; Kopchinskiy, A.G.; Kubicek, C.P.; Druzhinina, I.S. Soils of a Mediterranean hot spot of biodiversity and endemism (Sardinia, Tyrrhenian Islands) are inhabited by pan-European, invasive species of Hypocrea/Trichoderma. Environ. Microbiol. 2009, 1, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Zachow, C.; Berg, C.; Müller, H.; Meincke, R.; Komon-Zelazowska, M.; Druzhinina, I.S.; Kubicek, C.P.; Berg, G. Fungal diversity in the rhizosphere of endemic plant species of Tenerife (Canary Islands): Relationship to vegetation zones and environmental factors. ISME J. 2009, 3, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Saia, S.; Colla, G.; Raimondi, G.; Di Stasio, E.; Cardarelli, M.; Bonini, P.; De Pascale, S.; Rouphael, Y. An endophytic fungi-based biostimulant modulated lettuce yield, physiological and functional quality responses to both moderate and severe water limitation. Sci. Hort. 2019, 256, 108595. [Google Scholar] [CrossRef]
- Visconti, D.; Fiorentino, N.; Cozzolino, E.; Woo, S.L.; Fagnano, M.; Rouphael, Y. Can Trichoderma-Based Biostimulants Optimize N Use Efficiency and Stimulate Growth of Leafy Vegetables in Greenhouse Intensive Cropping Systems? Agronomy 2020, 10, 121. [Google Scholar] [CrossRef]
- Lima, R.B.; Cabral, C.S.; da Silva, L.R.; de Melo, L.A.M.P.; Muniz, P.H.P.C.; de Mello, S.C.M. Response of Lettuce Cultivars to Inoculation with Trichoderma spp. J. Sci. Res. Rep. 2022, 28, 7–14. Available online: https://www.researchgate.net/publication/359534054 (accessed on 14 December 2023). [CrossRef]
- Senger, M.; Moresco, E.; Henrique Briega, A.; Harakava, R.; Mantovanello Lucon, C.M. The Agronomic efficiency of the inoculant FT10 (Trichoderma asperelloides) on four lettuce varieties. Comun. Sci. 2022, 13, e3750. [Google Scholar] [CrossRef]
- Atero-Calvo, S.; Izquierdo Ramos, M.J.; García-Huertas, C.; Rodríguez-Alcántara, M.; Navarro Morillo, I.; Navarro-León, E. An Evaluation of the Effectivity of the Green Leaves Biostimulant on Lettuce Growth, Nutritional Quality, and Mineral Element Efficiencies under Optimal Growth Conditions. Plants 2024, 13, 917. [Google Scholar] [CrossRef]
- Topolovec-Pintarić, S.; Kovaček, A.M.; Malev, O.; Kušan, I.; Matočec, N.; Pošta, A.; Pole, L.; Mešić, A. Biological Control of Sclerotinia sclerotiorum on Greenhouse Lettuce Using Trichoderma koningiopsis. Agg. Microbiol. Res. 2025, 16, 35. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.; Druzhinina, I.S. In honor of John Bissett: Authoritative guidelines on molecular identification of Trichoderma. Fungal Divers. 2021, 107, 1–69. [Google Scholar] [CrossRef]
- Lefort, V.; Longueville, J.E.; Gascuel, O. SMS: Smart Model Selection in PhyML. Mol. Biol. Evol. 2017, 34, 2422–2424. [Google Scholar] [CrossRef]
- Jaklitsch, W.M.; Põldmaa, K.; Samuels, G.J. Reconsideration of Protocrea (Hypocreales, Hypocreaceae). Mycologia 2008, 10, 962–984. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.-F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogeny. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef]
- Dodd, S.L.; Lieckfeldt, E.; Samuels, G.J. Hypocrea atroviridis sp. nov., the teleomorph of Trichoderma atroviride. Mycologia 2003, 95, 27–40. [Google Scholar] [CrossRef]
- Jaklitsch, W.M. European species of Hypocrea Part I. The green-spored species. Stud. Mycol. 2009, 63, 1–91. [Google Scholar] [CrossRef] [PubMed]
- Jaklitsch, W.M.; Samuels, G.J.; Ismaiel, A.; Voglmayr, H. Disentangling the Trichoderma viridescens complex. Persoonia-Mol. Phylogeny Evol. Fungi 2013, 31, 112–146. [Google Scholar] [CrossRef]
- Zheng, H.; Qiao, M.; Lv, Y.; Du, X.; Zhang, K.-Q.; Yu, Z. New Species of Trichoderma Isolated as Endophytes and Saprobes from Southwest China. J. Fungi 2021, 7, 467. [Google Scholar] [CrossRef]
- Ding, M.Y.; Chen, W.; Ma, X.C.; Lv, B.W.; Jiang, S.Q.; Yu, Y.N.; Rahimi, M.J.; Gao, R.W.; Zhao, Z.; Cai, F.; et al. Emerging salt marshes as a source of Trichoderma arenarium sp. nov. and other fungal bioeffectors for biosaline agriculture. J. Appl. Microbiol. 2021, 130, 179–195. [Google Scholar] [CrossRef]
- Jaklitsch, W.M.; Voglmayr, H. Biodiversity of Trichoderma (Hypocreaceae) in Southern Europe and Macaronesia. Stud. Mycol. 2015, 80, 1–87. [Google Scholar] [CrossRef]
- Samuels, G.J.; Dodd, S.L.; Lu, B.-S.; Petrini, O.; Schroers, H.-J.; Druzhinina, I.S. The Trichoderma koningii aggregate species. Stud. Mycol. 2006, 56, 67–133. [Google Scholar] [CrossRef] [PubMed]
- Tomah, A.A.; Abd Alamera, I.S.; Li, B.; Zhanga, J.Z. A new species of Trichoderma and gliotoxin role: A new observation in enhancing biocontrol potential of Trichoderma virens against Phytophthora capsici on chili pepper. Biol. Control 2020, 145, 104261. [Google Scholar] [CrossRef]
- Zhao, R.; Chen, K.Y.; Mao, L.J.; Zhang, C.L. Eleven new species of Trichoderma (Hypocreaceae, Hypocreales) from China. Mycology 2024, 16, 180–209. [Google Scholar] [CrossRef]
- Holmes, K.A.; Schroers, H.J.; Thomas, S.E.; Evans, H.C.; Samuels, G.J. Taxonomy and biocontrol potential of a new species of Trichoderma from the Amazon basin of South America. Mycol. Progress. 2004, 3, 199–210. [Google Scholar] [CrossRef]
- Montoya, Q.V.; Meirelles, L.A.; Chaverri, P.; Rodrigues, A. Unraveling Trichoderma species in the attine ant environment: Description of three new taxa. Antonie Leeuwenhoek 2016, 109, 633–651. [Google Scholar] [CrossRef]
- Tyurin, I.V. A new modification of the volumetric method for determining humus using chromic acid. Eurasian Soil. Sci. 1931, 6, 36–47. [Google Scholar]
- Oreshkin, N.G. Extraction of available phosphorus by the Egner-Riehm-Domingo method. Agrokhimiya 1980, 8, 135–138. [Google Scholar]
- Croatian Meteorological and Hydrological Service. Available online: https://meteo.hr/klima_e.php?section=klima_podaci¶m=k1&Grad=zagreb_maksimir (accessed on 10 April 2025).
- AOAC. Official Methods of Analysis of AOAC International, 22nd ed.; Oxford University Press: New York, NY, USA, 2023. [Google Scholar] [CrossRef]
- Shivanand, P.; Taha, H.; Yakop, F.H. Isolation of Fungi from Various Habitats and their Possible Bioremediation. Curr. Sci. 2019, 116, 733–740. [Google Scholar] [CrossRef]
- Porras, M.; Barrau, C.; Santos, B.; Arroyo, F.T.; Blanco, C.; Romero, F. Effects of temperature on in vitro response of Trichoderma strains against strawberry pathogen Rhizoctonia solani Kühn. Plant Prot. Sci. 2002, 38, 620–622. [Google Scholar] [CrossRef]
- Royse, D.J.; Ries, S.M. The influence of fungi isolated from peach twigs on the pathogenicity of Cytospora cincta. Phytopathology 1978, 68, 603–607. [Google Scholar] [CrossRef]
- SAS®/STAT, version 9.4; SAS Institute Inc.: Cary, NC, USA, 2013. Available online: http://documentation.sas.com/doc/en/pgmsascdc/9.4_3.3/statug/titlepage.htm (accessed on 28 March 2025).
- Jambhulkar, P.P.; Singh, B.; Raja, M.; Ismaiel, A.; Lakshman, D.K.; Tomar, M.; Sharma, P. Genetic diversity and antagonistic properties of Trichoderma strains from the crop rhizospheres in southern Rajasthan, India. Sci. Rep. 2024, 14, 8610. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.J.; Zhao, J.; Liu, Y.; Wang, S.X.; Zheng, S.Y.; Qin, W.T. Diversity of Trichoderma species contaminating substrates of Lentinula edodes in China and their interaction evaluation. Front. Microbiol. 2024, 14, 1288585. [Google Scholar] [CrossRef] [PubMed]
- El Sobky, M.A.; Eissa, R.A.; Abdel Lateif, K.S.; Fahmi, A.I.; El Zanaty, A.M.; Hassan, M.M.; Elsharkawy, M.M. Genetic diversity assessment of Trichoderma spp. isolated from various Egyptian locations using its gene sequencing marker, rep PCR, and their cellulolytic activity. Egypt. J. Biol. Pest Control 2024, 34, 24. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K. Beneficial effects of Trichoderma secondary metabolites on crops. Phytother. Res. 2020, 34, 2835–2842. [Google Scholar] [CrossRef]
- Poštić, D.; Štrbanović, R.; Tabaković, M.; Popović, T.; Ćirić, A.; Banjac, N.; Trkulja, N.; Stanisavljević, R. Germination and the Initial Seedling Growth of Lettuce, Celeriac and Wheat Cultivars after Micronutrient and a Biological Application Pre-Sowing Seed Treatment. Plants 2021, 10, 1913. [Google Scholar] [CrossRef]
- Banjac, N.; Stanisavljević, R.; Dimkić, I.; Velijević, N.; Soković, M.; Cirić, A. Trichoderma harzianum IS005–12 promotes germination, seedling growth and seedborne fungi suppression in Italian ryegrass forage. Plant Soil. Environ. 2021, 67, 130–136. [Google Scholar] [CrossRef]
- Rouphael, Y.; Carillo, P.; Colla, G.; Fiorentino, N.; Sabatino, L.; El-Nakhel, C.; Giordano, M.; Pannico, A.; Cirillo, V.; Shabani, E.; et al. Appraisal of Combined Applications of Trichoderma virens and a Biopolymer-Based Biostimulant on Lettuce Agronomical, Physiological, and Qualitative Properties under Variable N Regimes. Agronomy 2020, 10, 196. [Google Scholar] [CrossRef]
- Fiorentino, N.; Ventorino, V.; Woo, S.L.; Pepe, O.; De Rosa, A.; Gioia, L.; Romano, I.; Lombardi, N.; Napolitano, M.; Colla, G.; et al. Trichoderma-Based Biostimulants Modulate Rhizosphere Microbial Populations and Improve N Uptake Efficiency, Yield, and Nutritional Quality of Leafy Vegetables. Front. Plant Sci. 2018, 9, 743. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. Available online: https://www.nature.com/articles/nrmicro797 (accessed on 14 December 2023). [CrossRef] [PubMed]
- Hoque, M.M.; Ajwa, H.; Othman, M.; Smith, R.; Cahn, M. Yield and postharvest quality of lettuce in response to nitrogen, phosphorus, and potassium fertilizers. HortScience 2010, 45, 1539–1544. Available online: https://journals.ashs.org/view/journals/hortsci/45/10/article-p1539.xml (accessed on 14 December 2023). [CrossRef]
- Simko, L. Genetic variation in response to N, P, or K deprivation in baby leaf lettuce. Horticulturae 2020, 6, 15. [Google Scholar] [CrossRef]
- Inkham, C.; Panjama, K.; Seehanam, P.; Ruamrungsri, S. Effect of nitrogen, potassium and calcium concentrations on growth, yield and nutritional quality of green oak lettuce. Acta Hortic. 2021, 1312, 409–416. Available online: https://stri.cmu.ac.th/rpm2/files/publication/227.pdf (accessed on 14 December 2023). [CrossRef]
- de Lima, D.P.; Pinto Júnior, E.S.; de Menezes, A.V.; de Souza, D.A.; de São José, V.P.B.; da Silva, B.P.; de Almeida, A.Q.; de Carvalho, I.M.M. Chemical composition, minerals concentration, total phenolic compounds, flavonoids content and antioxidant capacity in organic and conventional vegetables. Food Res. Int. 2024, 175, 113684. [Google Scholar] [CrossRef] [PubMed]
- Howell, C.R. Mechanisms Employed by Trichoderma Species in the Biological Control of Plant Diseases: The History and Evolution of Current Concepts. Plant Dis. 2003, 87, 4–10. [Google Scholar] [CrossRef]
- Poveda, J.; Eugui, D. Combined use of Trichoderma and beneficial bacteria (mainly Bacillus and Pseudomonas): Development of microbial synergistic bio-inoculants in sustainable agriculture. Biol. Control 2022, 176, 105100. Available online: https://www.sciencedirect.com/science/article/pii/S1049964422002651 (accessed on 14 December 2023). [CrossRef]
- Sharma, P.; Jambhulkar, P.P.; Raja, M.; Sain, S.K.; Javeria, S. Trichoderma spp. in consortium and their rhizospheric interactions. In Trichoderma: Host Pathogen Interactions and Applications, 1st ed.; Sharma, A.K., Sharma, P., Eds.; Springer: Singapore, 2020; pp. 267–292. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).