Baseline Sensitivity and Resistance Detection of Stemphylium lycopersici to Pydiflumetofen
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolates and Culture Conditions
2.2. Fungicides and Reagents
2.3. Establishment of Baseline Sensitivity and Determination of Resistance Level
2.4. Amplification and Sequence Analysis of Sdhs Genes from S. lycopersici
2.5. Biological Fitness Assay for Different Sensitive Types of S. lycopersici to Pydiflumetofen
3. Results
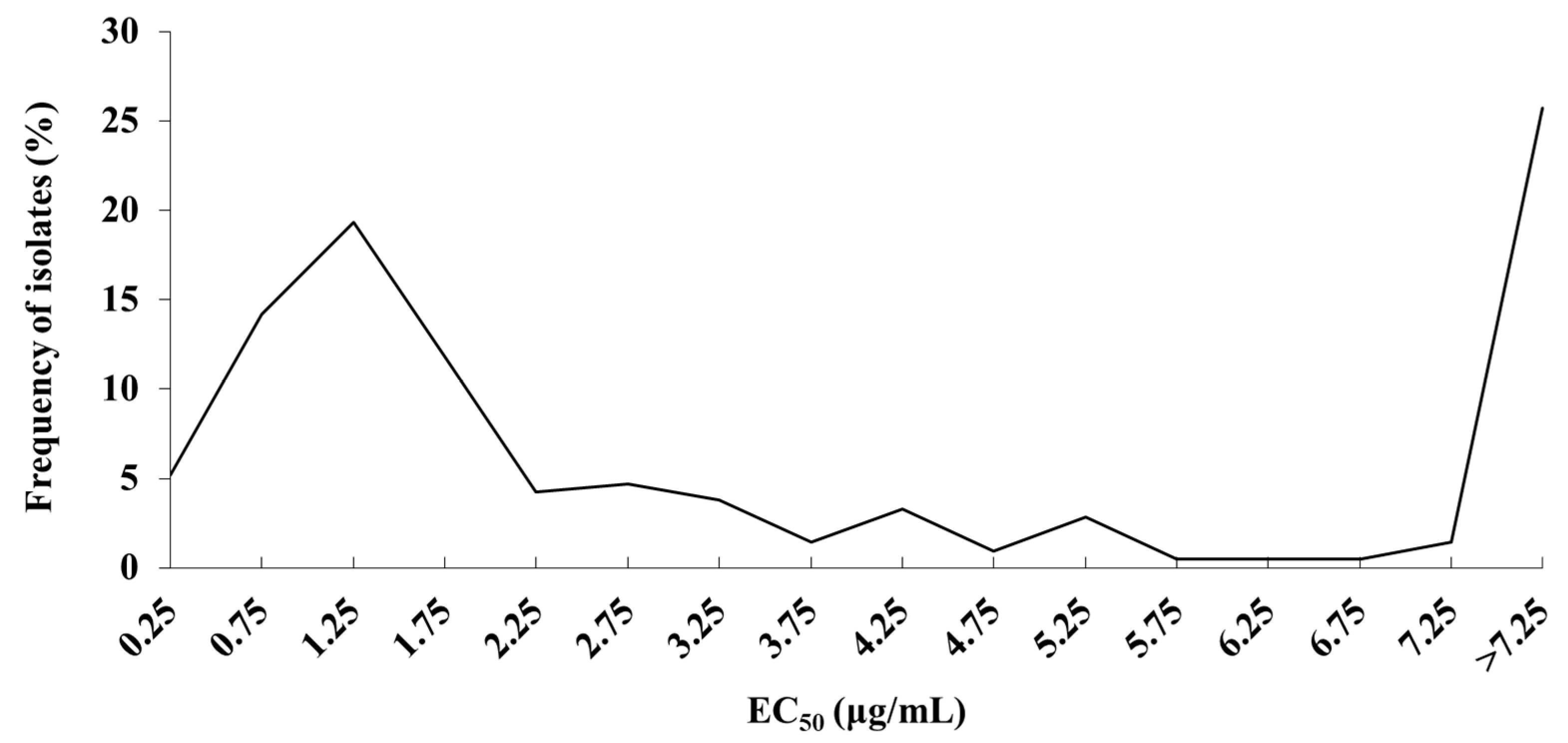
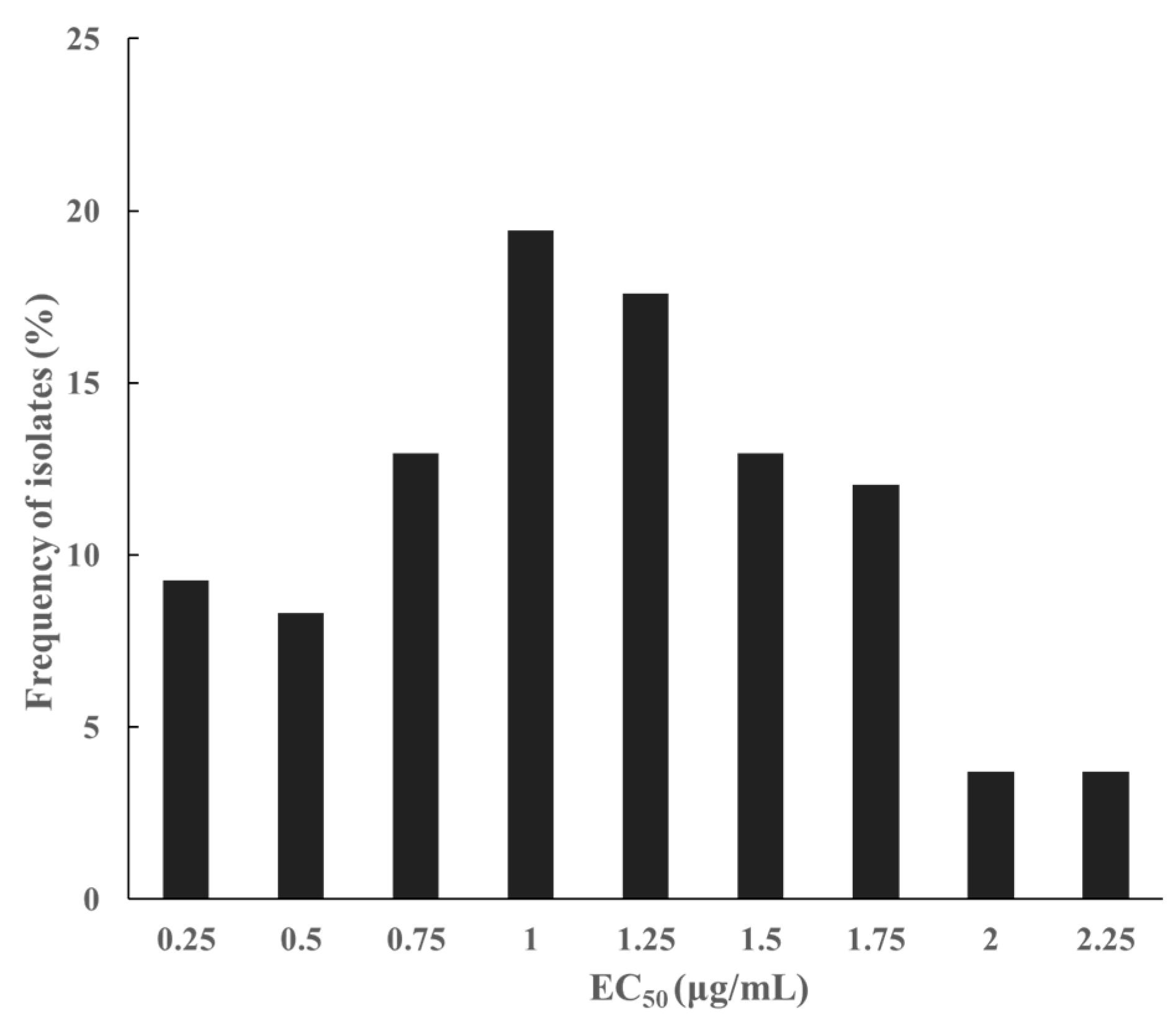
3.1. Sensitive Baseline of S. lycopersici to Pydiflumetofen
3.2. Sensitivity of S. lycopersici to Pydiflumetofen in Hebei Province
3.3. Mutation Analysis of Sdh Amino Acid of S. lycopersici
3.4. Biological Fitness of Pydiflumetofen-Resistant Isolates of S. lycopersici
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, Y.; Wang, T.; Liu, M.; Nie, Z.; Yang, H.; Zhao, T.; Xu, X.; Jiang, J.; Li, J. Virus-induced gene silencing of SlPKY1 attenuates defense responses against gray leaf spot in tomato. Sci. Hortic. 2020, 264, 109149. [Google Scholar] [CrossRef]
- Zheng, L.; Lv, R.; Huang, J.; Jiang, D.; Liu, X.; Hsiang, T. Integrated control of garlic leaf blight caused by Stemphylium solani in China. Can. J. Plant Pathol. 2010, 32, 135–145. [Google Scholar] [CrossRef]
- Chai, A.; Du, G.; Shi, Y.; Xie, X. Leaf spot on sweet potato (Ipomoea batatas) caused by Stemphylium solani, a new disease in China. J. Phytopatho. 2015, 163, 1046–1049. [Google Scholar] [CrossRef]
- Xie, X.W.; Zhang, Z.X.; Wang, Y.Y.; Shi, Y.X.; Chai, A.L.; Du, G.F.; Li, B.J. First report of Stemphylium solani causing leaf spot on wild eggplant in China. Can. J. Plant Patho. 2016, 38, 517–521. [Google Scholar] [CrossRef]
- Liu, H.; Wang, H.; Liao, X.; Gao, B.; Lu, X.; Sun, D.; Gong, W.; Zhong, J.; Zhu, H.; Pan, X.; et al. Mycoviral gene integration converts a plant pathogenic fungus into a biocontrol agent. Proc. Natl. Acad. Sci. USA 2022, 119, e2214096119. [Google Scholar] [CrossRef]
- Yang, S.; Wu, R. Pathogen identification and bioligical characteristics of pathogens of tomato leaf spot and fruit spot disease. Hubei Agric. Sci. 2019, 58, 104–107. [Google Scholar]
- Yang, H.; Shen, F.; Wang, H.; Zhao, T.; Zhang, H.; Jiang, J.; Xu, X.; Li, J. Functional analysis of SlERF01 gene in the disease resistance to S. lycopersici. BMC Plant Biol. 2020, 20, 376. [Google Scholar] [CrossRef]
- Liu, A.M.; Sun, J.D.; Tao, X.Z.; Qu, Q.W.; Xu, D.M. The occurrence and integrated control of tomato gray leaf spot in protected field. China Plant Prot. 2004, 24, 23–24. (In Chinese) [Google Scholar]
- Li, C.; Xu, Z.; Wang, S.; Qi, J.; Zhang, B.; Zhao, X.; Chen, B.; Li, L. Antifungal activity and control efficacy of pyraclostrobin and other fungicides against tomato gray leaf spot. Shandong Agri. Sci. 2012, 44, 103–105. (In Chinese) [Google Scholar]
- Avenot, H.F.; Michailides, T.J. Progress in understanding molecular mechanisms and evolution of resistance to succinate dehydrogenase inhibiting (SDHI) fungicides in phytopathogenic fungi. Crop Protect. 2010, 29, 643–651. [Google Scholar] [CrossRef]
- Sierotzki, H.; Scalliet, G. A review of current knowledge of resistance aspects for the next-generation succinate dehydrogenase inhibitor fungicides. Phytopathology 2013, 103, 880–887. [Google Scholar] [CrossRef]
- Zuccolo, M.; Kunova, A.; Musso, L.; Forlani, F.; Pinto, A.; Vistoli, G.; Gervasoni, S.; Cortesi, P.; Dallavalle, S. Dual-active antifungal agents containing strobilurin and SDHI-based pharmacophores. Sci. Rep. 2019, 9, 11377. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; He, L.; Zhu, J.; Cheng, J.; Li, B.; Liu, F.; Mu, W. The relationship between features enabling SDHI fungicide binding to the Sc-Sdh complex and its inhibitory activity against Sclerotinia sclerotiorum. Pest Manag. Sci. 2020, 76, 2799–2808. [Google Scholar] [CrossRef]
- Avenot, H.; Luna, M.; Michailides, T. Phenotypic and molecular characterization of resistance to the SDHI fungicide fluopyram in populations of Alternaria alternata from pistachio orchards in California. Crop Prot. 2019, 124, 104838. [Google Scholar] [CrossRef]
- Neves, D.; Bradley, C. Baseline sensitivity of Cercospora zeae-maydis to pydiflumetofen, a new succinate dehydrogenase inhibitor fungicide. Crop Prot. 2019, 119, 177–179. [Google Scholar] [CrossRef]
- Wu, X.; Dong, F.; Xu, J.; Liu, X.; Wu, X.; Zheng, Y. Enantioselective separation and dissipation of pydiflumetofen enantiomers in grape and soil by supercritical fluid chromatography–tandem mass spectrometry. J. Sep. Sci. 2020, 43, 2217–2227. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Si, N.G.; Sun, Q.; Zhao, J. The efficacy of several fungicides against cucumber downy mildew and wheat powdery mildew. Agrochemistry 2018, 57, 918–920. [Google Scholar]
- Huang, X.P.; Luo, J.; Song, Y.F.; Mei, W.; Liu, M.F. Bioactivity, physiological characteristics and efficacy of the SDHI fungicide pydiflumetofen against Sclerotinia sclerotiorum. Pestic. Biochem. Physiol. 2019, 160, 70–78. [Google Scholar] [CrossRef]
- Duan, Y.; Xiu, Q.; Li, H.; Li, T.; Wang, J.; Zhou, M. Pharmacological characteristics and control efficacy of a novel SDHI fungicide pydiflumetofen against Sclerotinia sclerotiorum. Plant Dis. 2018, 103, 77–82. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Zhang, H.; Wang, Z.; Xu, H. The research progress in and perspective of potential fungicides: Succinate dehydrogenase inhibitors. Bioorgan. Med. Chemi. 2021, 50, 116476. [Google Scholar] [CrossRef]
- Ayer, K.; Villani, S.; Choi, M.-W.; Cox, K. Characterization of the VisdhC and VisdhD Genes in Venturia inaequalis, and sensitivity to fluxapyroxad, pydiflumetofen, inpyrfluxam, and benzovindiflupyr. Plant Dis. 2018, 103, 1092–1100. [Google Scholar] [CrossRef]
- Chen, F.; Liu, X.; Chen, S.; Schnabel, E.; Schnabel, G. Characterization of Monilinia fructicola isolates resistant to both propiconazole and boscalid. Plant Dis. 2012, 97, 645–651. [Google Scholar] [CrossRef]
- Avenot, H.F.; van den Biggelaar, H.; Morgan, D.; Moral, J.; Joosten, M.; Michailides, T. Sensitivities of baseline isolates and boscalid-resistant mutants of Alternaria alternata from pistachio to fluopyram, penthiopyrad and fluxapyroxad. Plant Dis. 2013, 98, 197–205. [Google Scholar] [CrossRef]
- Sun, H.; Cui, J.; Tian, B.; Cao, S.; Zhang, X.; Chen, H. Resistance risk assessment for Fusarium graminearum to pydiflumetofen, a new succinate dehydrogenase inhibitor. Pest Manag. Sci. 2020, 76, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yang, Y.; Wang, X.; Chen, Z.; Ruan, X.; Chang, Z.; Huang, Z.; Gao, W.; Zhang, C.; Liu, X. Pathogenic diversity and fungicide sensitivity of soybean root rot oomycetes in Heilongjiang Province, China. Pest Manag. Sci. 2025, 81, 5270–5280. [Google Scholar] [CrossRef] [PubMed]
- Bi, L.; Xu, J.; Wang, Q.; Li, T.; Wang, J.; Zhou, M.; Duan, Y. Baseline sensitivity and resistance risk assessment of Stemphylium solani to fluxapyroxad. Crop Prot. 2022, 156, 105944. [Google Scholar] [CrossRef]
- Hou, Y.; Mao, X.; Wang, J.; Zhan, S.; Zhou, M. Sensitivity of Fusarium asiaticum to a novel succinate dehydrogenase inhibitor fungicide pydiflumetofen. Crop Prot. 2017, 96, 237–244. [Google Scholar] [CrossRef]
- Liu, X.; Sun, Y.; Liu, C.; Liu, B.; Li, T.; Chen, X.; Chen, Y. Various amino acid substitutions in succinate dehydrogenase complex regulating differential resistance to pydiflumetofen in Magnaporthe oryzae. Pestic. Biochem. Physiol. 2024, 203, 105990. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Bai, Y.; Cheng, X.; Li, H.; Chen, X.; Chen, Y. Study on mechanisms of resistance to SDHI fungicide pydiflumetofen in Fusarium fujikuroi. Agric. Food Chem. 2023, 71, 14330–14341. [Google Scholar] [CrossRef]
| Primer (5′→3′) | Sequence |
|---|---|
| SdhA-F | AAAAGTCGGGATGGCAGGTA |
| SdhA-R | TGGACCATGTGTGATCGTCT |
| SdhB-F | TGTCATAACCGAGGGAAGCT |
| SdhB-R | TCGTTGAGTGGGTGGATGAT |
| SdhC-F | ATCCACCCTCTCGACGATTC |
| SdhC-R | TTGACCTTCTCGCCATCCTT |
| SdhD-F | AAGACGACTGATACTGGGGC |
| SdhD-R | TGGCCGTACTGAGGTTGATT |
| - | Number of Isolates | Isolate/Percentage of the Total Population (%) | |||
|---|---|---|---|---|---|
| Sensitive | LR | MR | HR | ||
| Baoding | 32 | 30 (93.75) | 2 (6.25) | 0 (0.00) | 0 (0.00) |
| Chengde | 12 | 5 (41.67) | 4 (33.33) | 0 (0.00) | 3 (25.00) |
| Handan | 92 | 87 (94.57) | 5 (5.43) | 0 (0.00) | 0 (0.00) |
| Hengshui | 27 | 14 (51.85) | 7 (25.93) | 1 (3.70) | 5 (18.52) |
| Shijiazhuang | 24 | 14 (58.33) | 9 (37.50) | 1 (4.17) | 0 (0.00) |
| Tangshan | 25 | 16 (64.00) | 6 (24.00) | 1(4.17) | 2 (8.33) |
| Total | 212 | 166 (78.30) | 33 (15.57) | 3 (1.42) | 10 (4.72) |
| Name of Isolates | Region | Phenotype | Mutation Type | |||
|---|---|---|---|---|---|---|
| SdhA | SdhB | SdhC | SdhD | |||
| HSSL2-7 | Baoding | S | - | - | - | - |
| FQSL1-10 | Shijiazhuang | LR | - | - | G79R | - |
| FQSL1-14 | Shijiazhuang | LR | - | - | G79R | - |
| DXSL1-8 | Handan | LR | - | - | S135R | - |
| XTSL1-14 | Handan | LR | - | - | H134R | - |
| CYSL1-7 | Tangshan | HR | - | - | S73P | - |
| DXSL1-6 | Baoding | LR | - | - | S73P | - |
| DXSL1-10 | Hengshui | MR | - | - | S73P | - |
| DXSL2-8 | Shijiazhuang | MR | - | - | S73P | - |
| DXSL3-5 | Handan | LR | - | - | S73P | - |
| FQSL1-6 | Tangshan | LR | - | - | S73P | - |
| RYSL1-7 | Hengshui | HR | - | - | S73P | - |
| RYSL1-9 | Hengshui | LR | - | - | S73P | - |
| TSSL2-12 | Hengshui | HR | - | - | S73P | - |
| TSSL2-27 | Hengshui | LR | - | - | S73P | - |
| Isolates | Phenotype | Mycelial Growth Rate (mm/d) | Lesion Size (mm) | ||
|---|---|---|---|---|---|
| 15 °C | 25 °C | 35 °C | |||
| HSSL2-7 | S | 3.48 ± 0.16 ab | 8.07 ± 0.26 ab | 5.62 ± 0.08 ab | 15.85 ± 1.07 a |
| FQSL1-10 | LR | 2.86 ± 0.08 c | 7.86 ± 0.29 b | 5.40 ± 0.15 bc | 17.98 ± 0.76 a |
| FQSL1-14 | LR | 3.14 ± 0.25 bc | 7.64 ± 0.19 b | 5.33 ± 0.08 c | 16.73 ± 0.52 a |
| DXSL1-8 | LR | 3.67 ± 0.26 a | 8.48 ± 0.16 a | 5.52 ± 0.08 abc | 14.97 ± 0.50 b |
| XTSL1-14 | LR | 3.60 ± 0.04 a | 8.10 ± 0.41 ab | 5.55 ± 0.21 abc | 14.72 ± 0.56 b |
| CYSL1-7 | HR | 3.76 ± 0.22 a | 8.48 ± 0.22 a | 5.70 ± 0.04 a | 13.33 ± 1.71 b |
| DXSL1-6 | LR | 2.88 ± 0.04 c | 8.07 ± 0.07 ab | 5.38 ± 0.08 c | 14.47 ± 0.63 b |
| RYSL1-7 | HR | 2.81 ± 0.08 c | 8.05 ± 0.16 ab | 5.40 ± 0.10 bc | 13.43 ± 0.70 b |
| TSSL2-12 | HR | 3.67 ± 0.36 a | 8.02 ± 0.18 ab | 5.55 ± 0.15 abc | 13.67 ± 0.44 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Yang, K.; Wu, J.; Bi, Q.; Lu, F.; Wang, J.; Zhao, J. Baseline Sensitivity and Resistance Detection of Stemphylium lycopersici to Pydiflumetofen. J. Fungi 2025, 11, 734. https://doi.org/10.3390/jof11100734
Liu X, Yang K, Wu J, Bi Q, Lu F, Wang J, Zhao J. Baseline Sensitivity and Resistance Detection of Stemphylium lycopersici to Pydiflumetofen. Journal of Fungi. 2025; 11(10):734. https://doi.org/10.3390/jof11100734
Chicago/Turabian StyleLiu, Xiangyu, Kexin Yang, Jie Wu, Qiuyan Bi, Fen Lu, Jiqiang Wang, and Jianjiang Zhao. 2025. "Baseline Sensitivity and Resistance Detection of Stemphylium lycopersici to Pydiflumetofen" Journal of Fungi 11, no. 10: 734. https://doi.org/10.3390/jof11100734
APA StyleLiu, X., Yang, K., Wu, J., Bi, Q., Lu, F., Wang, J., & Zhao, J. (2025). Baseline Sensitivity and Resistance Detection of Stemphylium lycopersici to Pydiflumetofen. Journal of Fungi, 11(10), 734. https://doi.org/10.3390/jof11100734






