The Mechanism of Fraxetin as a Sustainable Fungicide for Larch Shoot Blight: Lipid Peroxidation and Oxidative Stress in Neofusicoccum laricinum
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Metabolomics Determination and Fraxetin Content Determination
2.3. Evaluation of Fraxetin’s Fungicidal Activity and Its Efficacy in Larch Shoot Blight Control
2.4. Physiological Analysis of Fraxetin’s Antifungal Mechanism
2.5. Multi-Omics Mechanism Investigation of Fraxetin’s Antifungal Action
2.6. Data Analysis
3. Results
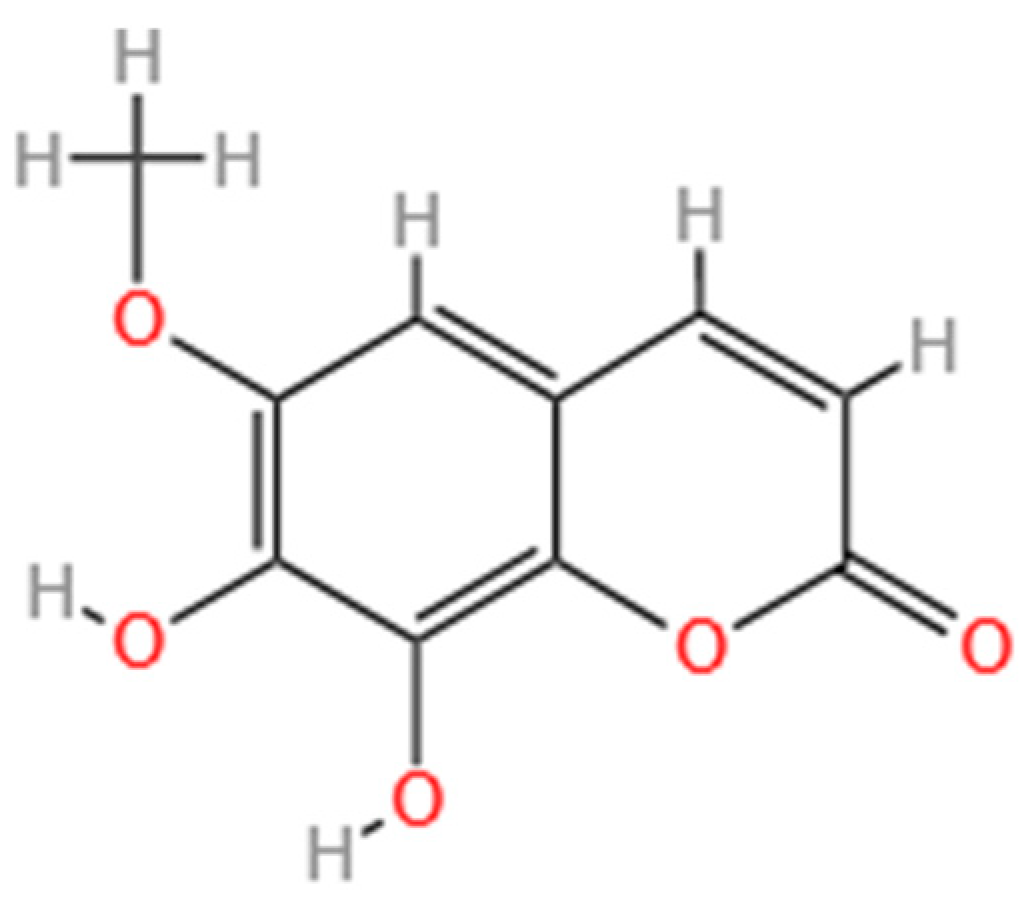
3.1. Fraxetin Functions as a Key Phytoalexin Mediating Disease Resistance in Larch
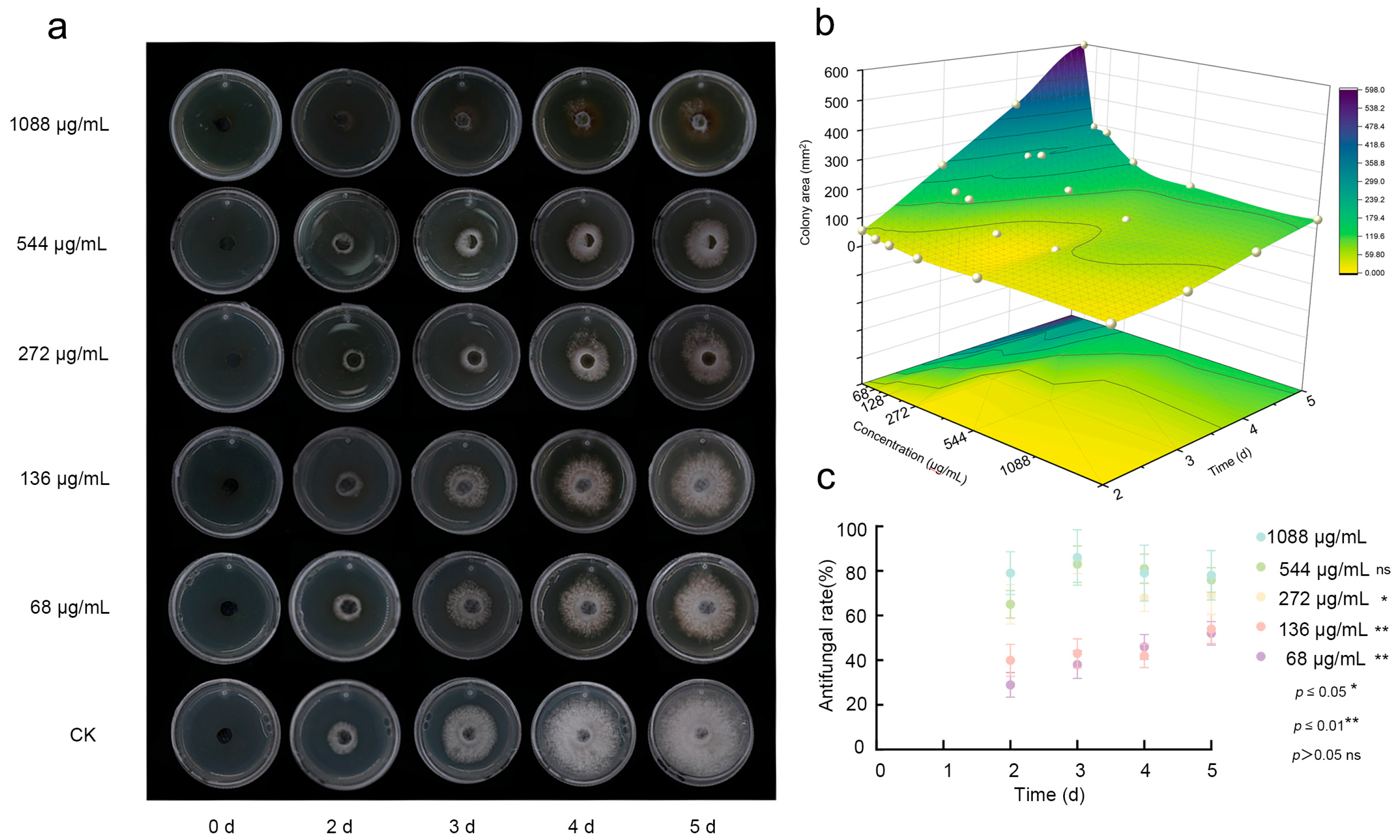
3.2. Dose- and Time-Dependent Antifungal Activity of Fraxetin Against N. laricinum
3.3. Efficacy of Exogenous Fraxetin in Controlling Larch Shoot Blight
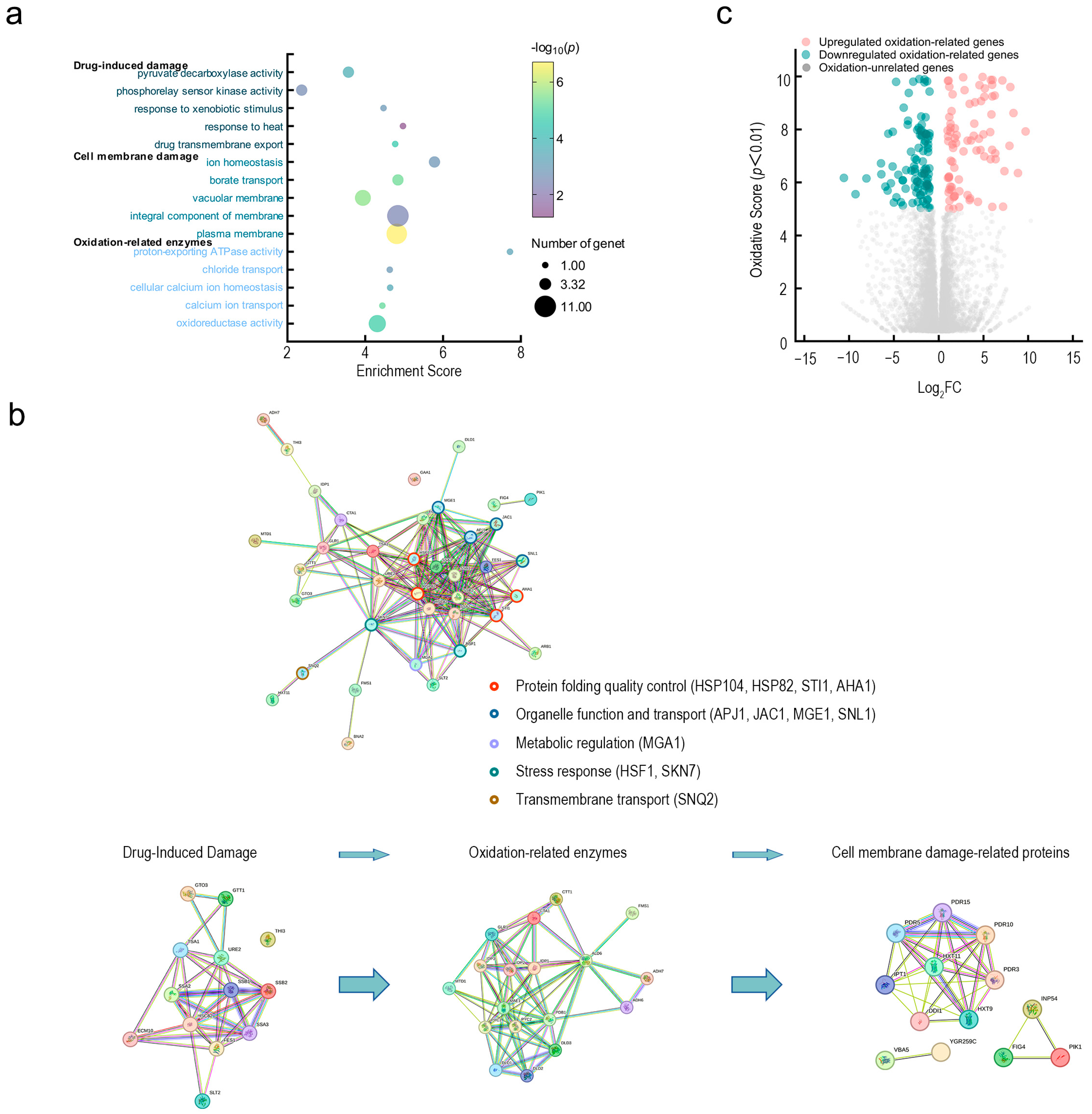
3.4. Transcriptomic Insights into Membrane Damage and Oxidative Stress Modulation in N. laricinum by Fraxetin
3.5. Dose- and Time-Dependent Antifungal Mechanisms of Fraxetin: Oxidative Stress Cascade and Membrane Damage in N. laricinum
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Hattori, Y.; Ando, Y.; Nakashima, C. Taxonomical re-examination of the genus Neofusicoccum in Japan. Mycoscience 2021, 62, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Bruda, E.A.; Xia, R.; Zhang, R.; Wang, H.; Yu, Q.; Hu, M.; Wang, F. Evaluation on the efficacy of Farrerol in inhibiting shoot blight of larch (Neofusicoccum laricinum). Plants 2024, 13, 3004. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, N.J.; Bass, C.; Dixon, A.; Neve, P. The evolutionary origins of pesticide resistance. Biol. Rev. 2019, 94, 135–155. [Google Scholar] [CrossRef]
- DeFalco, T.A.; Zipfel, C. Molecular mechanisms of early plant pattern-triggered immune signaling. Mol. Cell 2021, 81, 3449–3467. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Staskawicz, B.J.; Dangl, J.L. The plant immune system: From discovery to deployment. Cell 2024, 187, 2095–2116. [Google Scholar] [CrossRef]
- Mishra, S.; Srivastava, A.; Singh, A.; Pandey, G.C.; Srivastava, G. An overview of symbiotic and pathogenic interactions at the fungi-plant interface under environmental constraints. Front. Fungal. Biol. 2024, 5, 1363460. [Google Scholar] [CrossRef] [PubMed]
- Zaynab, M.; Fatima, M.; Abbas, S.; Sharif, Y.; Umair, M.; Zafar, M.H.; Bahadar, K. Role of secondary metabolites in plant defense against pathogens. Microb. Pathog. 2018, 124, 198–202. [Google Scholar] [CrossRef]
- Pu, X.; Kitaoka, N.; Rodríguez-López, C.E.; Chen, S. Editorial: Plant secondary metabolite biosynthesis. Front. Plant. Sci. 2024, 15, 1477551. [Google Scholar] [CrossRef]
- Ahuja, I.; Kissen, R.; Bones, A.M. Phytoalexins in defense against pathogens. Trends Plant Sci. 2012, 17, 73–90. [Google Scholar] [CrossRef]
- Zhao, Q.; Dixon, R.A. Transcriptional networks for lignin biosynthesis: More complex than we thought? Trends Plant Sci. 2011, 16, 227–233. [Google Scholar] [CrossRef]
- Matern, U.; Kneusel, R. Phenolic compounds in plant disease resistance. Phytoparasitica 1988, 16, 153–170. [Google Scholar] [CrossRef]
- González-López, J.A.; Pérez-Mondragón, A.A.; Cuevas-Suárez, C.E.; Trejo-Carbajal, N.; Herrera-González, A.M. Evaluation of dental composites resins formulated with non-toxic monomers derived from catechol. J. Mech. Behav. Biomed. Mater. 2020, 104, 103613. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Jia, H.; Yang, Z.; Wang, S.; Liu, Y.; Ma, H.; Liang, X.; Wang, B.; Zhu, M.; Meng, Y.; et al. Sufficient coumarin accumulation improves apple resistance to Cytospora mali under high-potassium status. Plant Physiol. 2023, 192, 1396–1419. [Google Scholar] [CrossRef]
- Mazimba, O. Umbelliferone: Sources, chemistry and bioactivities review. Bull. Fac. Pharm. Cairo Univ. 2017, 55, 223–232. [Google Scholar] [CrossRef]
- Ojala, T.; Remes, S.; Haansuu, P.; Vuorela, H.; Hiltunen, R.; Haahtela, K.; Vuorela, P. Antimicrobial activity of some coumarin containing herbal plants growing in Finland. J. Ethnopharmacol. 2000, 73, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Guan, D.; Valls, M.; Ding, W. Sustainable natural bioresources in crop protection: Antimicrobial hydroxycoumarins induce membrane depolarization-associated changes in the transcriptome of Ralstonia solanacearum. Pest Manag. Sci. 2021, 77, 5170–5185. [Google Scholar] [CrossRef]
- Yang, L.; Wei, Z.; Li, S.; Xiao, R.; Xu, Q.; Ran, Y.; Ding, W. Plant secondary metabolite, daphnetin reduces extracellular polysaccharides production and virulence factors of Ralstonia solanacearum. Pestic. Biochem. Physiol. 2021, 179, 104948. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Y.; He, X.; Xiao, Q.; Han, S.; Jia, Z.; Li, S.; Ding, W. Discovery of a novel plant-derived agent against Ralstonia solanacearum by targeting the bacterial division protein FtsZ. Pestic. Biochem. Physiol. 2021, 177, 104892. [Google Scholar] [CrossRef]
- Ha, N.M.; Son, N.T. Health benefits of fraxetin: From chemistry to medicine. Arch. Pharm. 2024, 357, e2400092. [Google Scholar] [CrossRef]
- Wang, H.; Zou, D.; Xie, K.; Xie, M. Antibacterial mechanism of fraxetin against Staphylococcus aureus. Mol. Med. Rep. 2014, 10, 2341–2345. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.N.; Yao, Z.L.; Yang, D.; Ke, J.; Wu, Q.L.; Li, J.K.; Zhou, X.D. Chemical constituents from Fraxinus hupehensis and their antifungal and herbicidal activities. Biomolecules 2020, 10, 74. [Google Scholar] [CrossRef] [PubMed]
- Wang, H. Study on the Effect of Vanillin Inhibiting Neofusicoccum laricinum by Destroying Cell Membrane. Master’s Thesis, Northeast Forestry University, Harbin, China, 2024. [Google Scholar]
- Zhang, R.; Chen, X.; Xia, R.; Wang, J.; Chen, J.; Zhang, S.; Wang, F. Molecular mechanism underlying the resistance and susceptibility of Larch to Larch Shoot Blight. Tree Health 2025, 2, 1–9. [Google Scholar]
- Escamez, S.; Bollhöner, B.; Tuominen, H. Quick histochemical staining methods to detect cell death in xylem elements of plant tissues. Methods Mol. Biol. 2017, 1544, 27–36. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, J.; Lai, L.; Sun, Q.; Yi, S.; Jiang, L.; Zheng, Y. Evaluating physiological changes of grass and semishrub species with seasonality for understanding the process of shrub encroachment in semiarid grasslands. Funct. Plant Biol. 2020, 47, 628–638. [Google Scholar] [CrossRef]
- Gutteridge, J.M.; Halliwell, B. The measurement and mechanism of lipid peroxidation in biological systems. Trends Biochem. Sci. 1990, 15, 129–135. [Google Scholar] [CrossRef]
- Luo, J.; Wang, Y.; Tang, S.; Liang, J.; Lin, W.; Luo, L. Isolation and identification of algicidal compound from Streptomyces and algicidal mechanism to Microcystis aeruginosa. PLoS ONE 2013, 8, e76444. [Google Scholar] [CrossRef]
- Minami, M.; Yoshikawa, H. A simplified assay method of superoxide dismutase activity for clinical use. Clin. Chim. Acta 1979, 92, 337–342. [Google Scholar] [CrossRef]
- Spitz, D.R.; Oberley, L.W. An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal. Biochem. 1989, 179, 8–18. [Google Scholar] [CrossRef]
- Balaha, M.; Ahmed, N.; Geddawy, A.; Kandeel, S. Fraxetin prevented sodium fluoride-induced chronic pancreatitis in rats: Role of anti-inflammatory, antioxidant, antifibrotic and anti-apoptotic activities. Int. Immunopharmacol. 2021, 93, 107372. [Google Scholar] [CrossRef] [PubMed]
- Azzi, A. Molecular mechanism of alpha-tocopherol action. Free Radic. Biol. Med. 2007, 43, 16–21. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.X.; Yang, T.M.; Li, C.S.; Zhang, L.H.; Du, D.N.; Wang, R.X.; Wang, J.; Wei, M.; Ba, X.Q. Linking oxidative DNA lesion 8-OxoG to tumor development and progression. Yi Chuan 2022, 44, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yang, P.; Ji, Y.; Lu, J. The role of Natural Organic Matter in the degradation of phenolic pollutants by sulfate radical oxidation: Radical scavenging vs reduction. Environ. Sci. Technol. 2025, 59, 3325–3335. [Google Scholar] [CrossRef]
- Narasimhan, K.K.S.; Devarajan, A.; Karan, G.; Sundaram, S.; Wang, Q.; van Groen, T.; del Monte, F.; Rajasekaran, N.S. Reductive stress promotes protein aggregation and impairs neurogenesis. Redox Biol. 2020, 37, 101739. [Google Scholar] [CrossRef]
- Zhang, H.; Limphong, P.; Pieper, J.; Liu, Q.; Rodesch, C.K.; Christians, E.; Benjamin, I.J. Glutathione-dependent reductive stress triggers mitochondrial oxidation and cytotoxicity. FASEB J. 2012, 26, 1442–1451. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.B.; Wang, F.Y.; Lu, Y.; Yang, L.M.; Long, N.; Wang, S.S.; Xie, Z.; Levine, M.; Zou, T.; Sessler, J.L.; et al. Cu(II) complex that synergistically potentiates cytotoxicity and an antitumor immune response by targeting cellular redox homeostasis. Proc. Natl. Acad. Sci. USA 2024, 121, e2404668121. [Google Scholar] [CrossRef]
- Zhai, X.; Yang, R.; Chu, Q.; Guo, Z.; Hou, P.; Li, X.; Bai, C.; Lu, Z.; Qiao, L.; Fu, Y.; et al. AMPK-regulated glycerol excretion maintains metabolic crosstalk between reductive and energetic stress. Nat. Cell Biol. 2025, 27, 141–153. [Google Scholar] [CrossRef]
- Yan, J.; Feng, Z.; Xiao, Y.; Zhou, M.; Zhao, X.; Lin, X.; Shi, W.; Busch, W.; Li, B. ANAC044 orchestrates mitochondrial stress signaling to trigger iron-induced stem cell death in root meristems. Proc. Natl. Acad. Sci. USA 2025, 122, e2411579122. [Google Scholar] [CrossRef]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxidative Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef]
- Payton, F.; Bose, R.; Alworth, W.L.; Kumar, A.P.; Ghosh, R. 4-Methylcatechol-induced oxidative stress induces intrinsic apoptotic pathway in metastatic melanoma cells. Biochem. Pharmacol. 2011, 81, 1211–1218. [Google Scholar] [CrossRef]
- Roussaki, M.; Zelianaios, K.; Kavetsou, E.; Hamilakis, S.; Hadjipavlou-Litina, D.; Kontogiorgis, C.; Liargkova, T.; Detsi, A. Structural modifications of coumarin derivatives: Determination of antioxidant and lipoxygenase (LOX) inhibitory activity. Bioorganic Med. Chem. 2014, 22, 6586–6594. [Google Scholar] [CrossRef] [PubMed]
- Kalyanram, P.; Ma, H.; Marshall, S.; Goudreau, C.; Cartaya, A.; Zimmermann, T.; Stadler, I.; Nangia, S.; Gupta, A. Interaction of amphiphilic coumarin with DPPC/DPPS lipid bilayer: Effects of concentration and alkyl tail length. Phys. Chem. Chem. Phys. 2020, 22, 15197–15207. [Google Scholar] [CrossRef] [PubMed]
- Campos, F.M.; Couto, J.A.; Figueiredo, A.R.; Tóth, I.V.; Rangel, A.O.; Hogg, T.A. Cell membrane damage induced by phenolic acids on wine lactic acid bacteria. Int. J. Food Microbiol. 2009, 135, 144–151. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Glorieux, C.; Calderon, P.B. Catalase, a remarkable enzyme: Targeting the oldest antioxidant enzyme to find a new cancer treatment approach. Biol. Chem. 2017, 398, 1095–1108. [Google Scholar] [CrossRef]
- Tsai, H.H.; Schmidt, W. The enigma of environmental pH sensing in plants. Nat. Plants 2021, 7, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Hammerschmidt, R. PHYTOALEXINS: What have we learned after 60 years? Annu. Rev. Phytopathol. 1999, 37, 285–306. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, S.; Xia, R.; Chen, X.; Chen, J.; Wang, F.; Li, D. From resistance mechanism to green application: Discovery of rutaevin as a key phytoalexin in Larch and cross-species resource optimization. Plants 2025, 14, 2947. [Google Scholar] [CrossRef]
- Durrant, W.E.; Dong, X. Systemic acquired resistance. Annu. Rev. Phytopathol. 2004, 42, 185–209. [Google Scholar] [CrossRef]
- Vlot, A.C.; Dempsey, D.A.; Klessig, D.F. Salicylic acid, a multifaceted hormone to combat disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef]
- Zhou, J.; Mu, Q.; Wang, X.; Zhang, J.; Yu, H.; Huang, T.; He, Y.; Dai, S.; Meng, X. Multilayered synergistic regulation of phytoalexin biosynthesis by ethylene, jasmonate, and MAPK signaling pathways in Arabidopsis. Plant Cell 2022, 34, 3066–3087. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Zhang, R.; Xia, R.; Chen, X.; Chen, J.; Yang, Y.; Mujtaba, M.; Li, D.; Wang, F. The Mechanism of Fraxetin as a Sustainable Fungicide for Larch Shoot Blight: Lipid Peroxidation and Oxidative Stress in Neofusicoccum laricinum. J. Fungi 2025, 11, 724. https://doi.org/10.3390/jof11100724
Zhang S, Zhang R, Xia R, Chen X, Chen J, Yang Y, Mujtaba M, Li D, Wang F. The Mechanism of Fraxetin as a Sustainable Fungicide for Larch Shoot Blight: Lipid Peroxidation and Oxidative Stress in Neofusicoccum laricinum. Journal of Fungi. 2025; 11(10):724. https://doi.org/10.3390/jof11100724
Chicago/Turabian StyleZhang, Shuang, Ruizhi Zhang, Rui Xia, Xinyan Chen, Jiarui Chen, Yuchun Yang, Majid Mujtaba, Danlei Li, and Feng Wang. 2025. "The Mechanism of Fraxetin as a Sustainable Fungicide for Larch Shoot Blight: Lipid Peroxidation and Oxidative Stress in Neofusicoccum laricinum" Journal of Fungi 11, no. 10: 724. https://doi.org/10.3390/jof11100724
APA StyleZhang, S., Zhang, R., Xia, R., Chen, X., Chen, J., Yang, Y., Mujtaba, M., Li, D., & Wang, F. (2025). The Mechanism of Fraxetin as a Sustainable Fungicide for Larch Shoot Blight: Lipid Peroxidation and Oxidative Stress in Neofusicoccum laricinum. Journal of Fungi, 11(10), 724. https://doi.org/10.3390/jof11100724





