Glycation of Nail Proteins as a Risk Factor for Onychomycosis. Comment on Gupta et al. Diabetic Foot and Fungal Infections: Etiology and Management from a Dermatologic Perspective. J. Fungi 2024, 10, 577
Author Contributions
Funding
Conflicts of Interest
References
- Gupta, A.K.; Shemer, A.; Economopoulos, V.; Talukder, M. Diabetic Foot and Fungal Infections: Etiology and Management from a Dermatologic Perspective. J. Fungi 2024, 10, 577. [Google Scholar] [CrossRef] [PubMed]
- Kishabongo, A.S.; Katchunga, P.; Van Aken, E.H.; Speeckaert, R.; Lagniau, S.; Coopman, R.; Speeckaert, M.M.; Delanghe, J.R. Glycation of Nail Proteins: From Basic Biochemical Findings to a Representative Marker for Diabetic Glycation-Associated Target Organ Damage. PLoS ONE 2015, 10, e0120112. [Google Scholar] [CrossRef] [PubMed]
- Jurgeleviciene, I.; Stanislovaitiene, D.; Tatarunas, V.; Jurgelevicius, M.; Zaliuniene, D. Assessment of Absorption of Glycated Nail Proteins in Patients with Diabetes Mellitus and Diabetic Retinopathy. Medicina 2020, 56, 658. [Google Scholar] [CrossRef]
- Kumari, A.; Tripathi, A.H.; Gautam, P.; Gahtori, R.; Pande, A.; Singh, Y.; Madan, T.; Upadhyay, S.K. Adhesins in the Virulence of Opportunistic Fungal Pathogens of Human. Mycology 2021, 12, 296–324. [Google Scholar] [CrossRef] [PubMed]
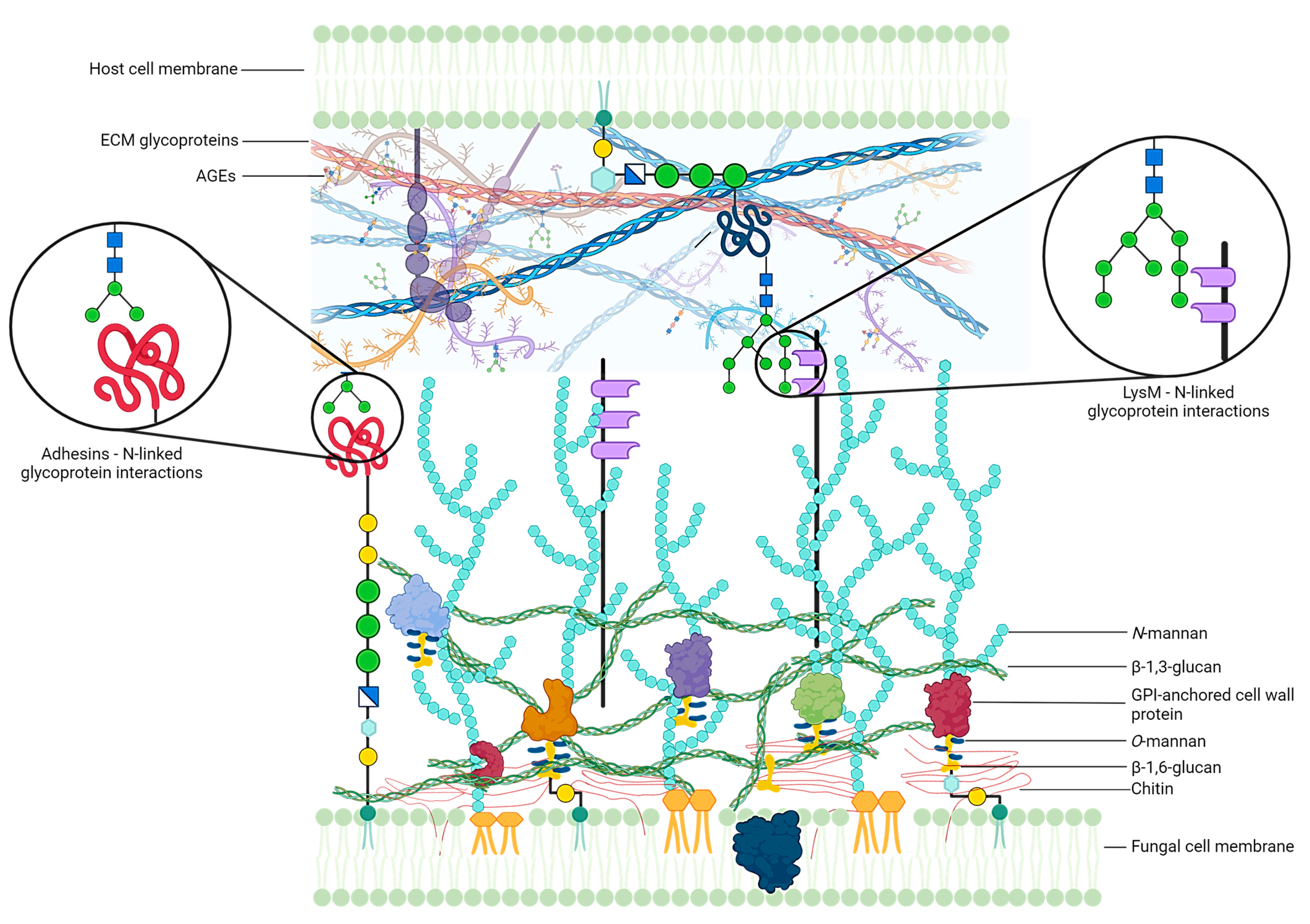
- Gómez-Gaviria, M.; Vargas-Macías, A.P.; García-Carnero, L.C.; Martínez-Duncker, I.; Mora-Montes, H.M. Role of Protein Glycosylation in Interactions of Medically Relevant Fungi with the Host. J. Fungi 2021, 7, 875. [Google Scholar] [CrossRef]
- Esquenazi, D.; Alviano, C.S.; de Souza, W.; Rozental, S. The Influence of Surface Carbohydrates during in Vitro Infection of Mammalian Cells by the Dermatophyte Trichophyton Rubrum. Res. Microbiol. 2004, 155, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Kar, B.; Patel, P.; Free, S.J. Trichophyton Rubrum LysM Proteins Bind to Fungal Cell Wall Chitin and to the N-Linked Oligosaccharides Present on Human Skin Glycoproteins. PLoS ONE 2019, 14, e0215034. [Google Scholar] [CrossRef]
- Ito, T.; Ito, N.; Saathoff, M.; Stampachiacchiere, B.; Bettermann, A.; Bulfone-Paus, S.; Takigawa, M.; Nickoloff, B.J.; Paus, R. Immunology of the Human Nail Apparatus: The Nail Matrix Is a Site of Relative Immune Privilege. J. Investig. Dermatol. 2005, 125, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Santiago, K.; Bomfim, G.F.; Criado, P.R.; Almeida, S.R. Monocyte-Derived Dendritic Cells from Patients with Dermatophytosis Restrict the Growth of Trichophyton Rubrum and Induce CD4-T Cell Activation. PLoS ONE 2014, 9, e110879. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cosio, T.; Valsecchi, I.; Gaziano, R.; Campione, E.; Botterel, F. Glycation of Nail Proteins as a Risk Factor for Onychomycosis. Comment on Gupta et al. Diabetic Foot and Fungal Infections: Etiology and Management from a Dermatologic Perspective. J. Fungi 2024, 10, 577. J. Fungi 2025, 11, 46. https://doi.org/10.3390/jof11010046
Cosio T, Valsecchi I, Gaziano R, Campione E, Botterel F. Glycation of Nail Proteins as a Risk Factor for Onychomycosis. Comment on Gupta et al. Diabetic Foot and Fungal Infections: Etiology and Management from a Dermatologic Perspective. J. Fungi 2024, 10, 577. Journal of Fungi. 2025; 11(1):46. https://doi.org/10.3390/jof11010046
Chicago/Turabian StyleCosio, Terenzio, Isabel Valsecchi, Roberta Gaziano, Elena Campione, and Françoise Botterel. 2025. "Glycation of Nail Proteins as a Risk Factor for Onychomycosis. Comment on Gupta et al. Diabetic Foot and Fungal Infections: Etiology and Management from a Dermatologic Perspective. J. Fungi 2024, 10, 577" Journal of Fungi 11, no. 1: 46. https://doi.org/10.3390/jof11010046
APA StyleCosio, T., Valsecchi, I., Gaziano, R., Campione, E., & Botterel, F. (2025). Glycation of Nail Proteins as a Risk Factor for Onychomycosis. Comment on Gupta et al. Diabetic Foot and Fungal Infections: Etiology and Management from a Dermatologic Perspective. J. Fungi 2024, 10, 577. Journal of Fungi, 11(1), 46. https://doi.org/10.3390/jof11010046








