Harnessing Genetic Resistance in Maize and Integrated Rust Management Strategies to Combat Southern Corn Rust
Abstract
1. Introduction
2. Discovery and Distribution of Southern Corn Rust
3. Biological Characteristics and Symptoms
3.1. Pathogen Taxonomy and Host Range
3.2. Physiological Races
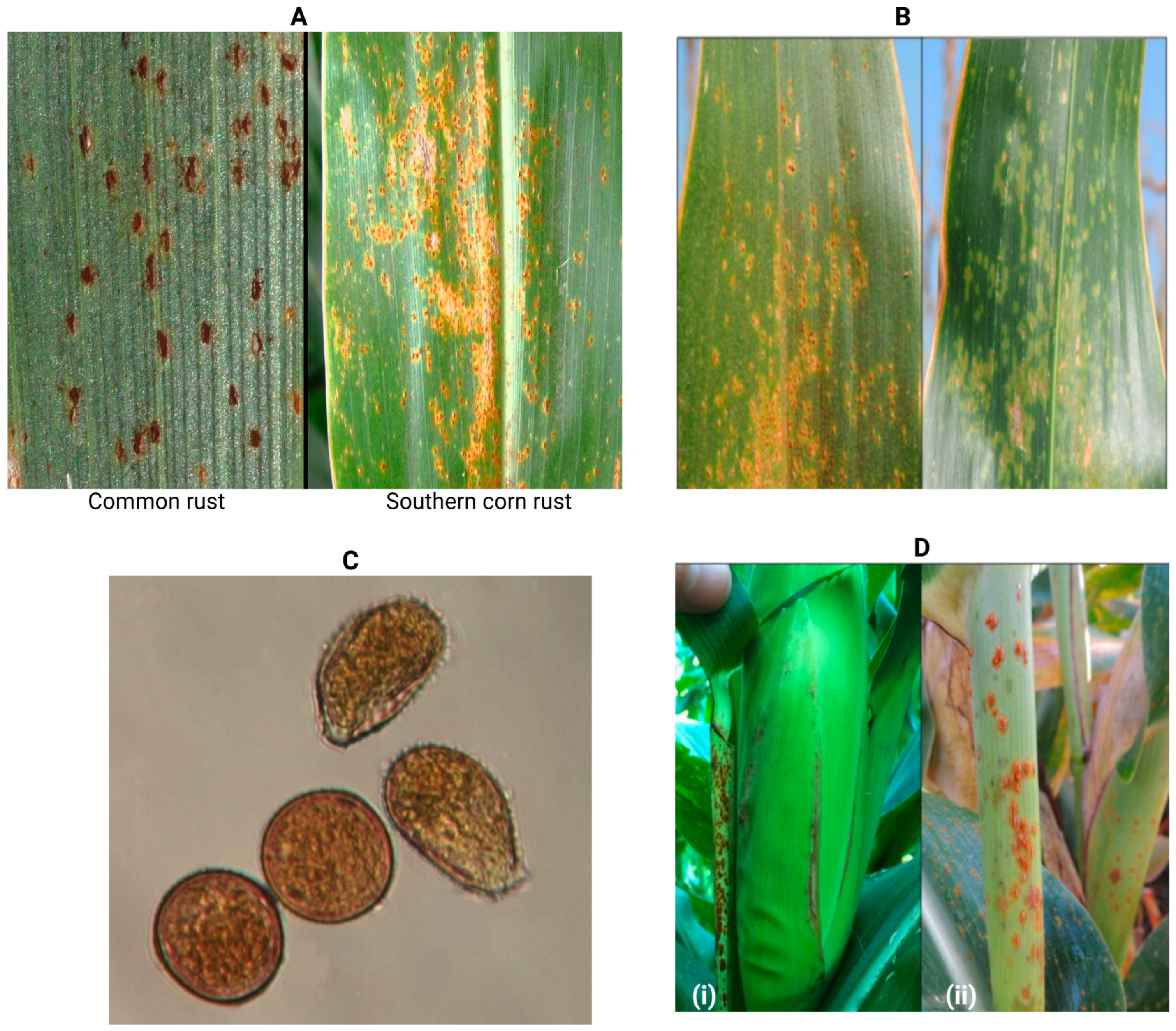
3.3. Signs and Symptoms
4. P. polysora Life Cycle
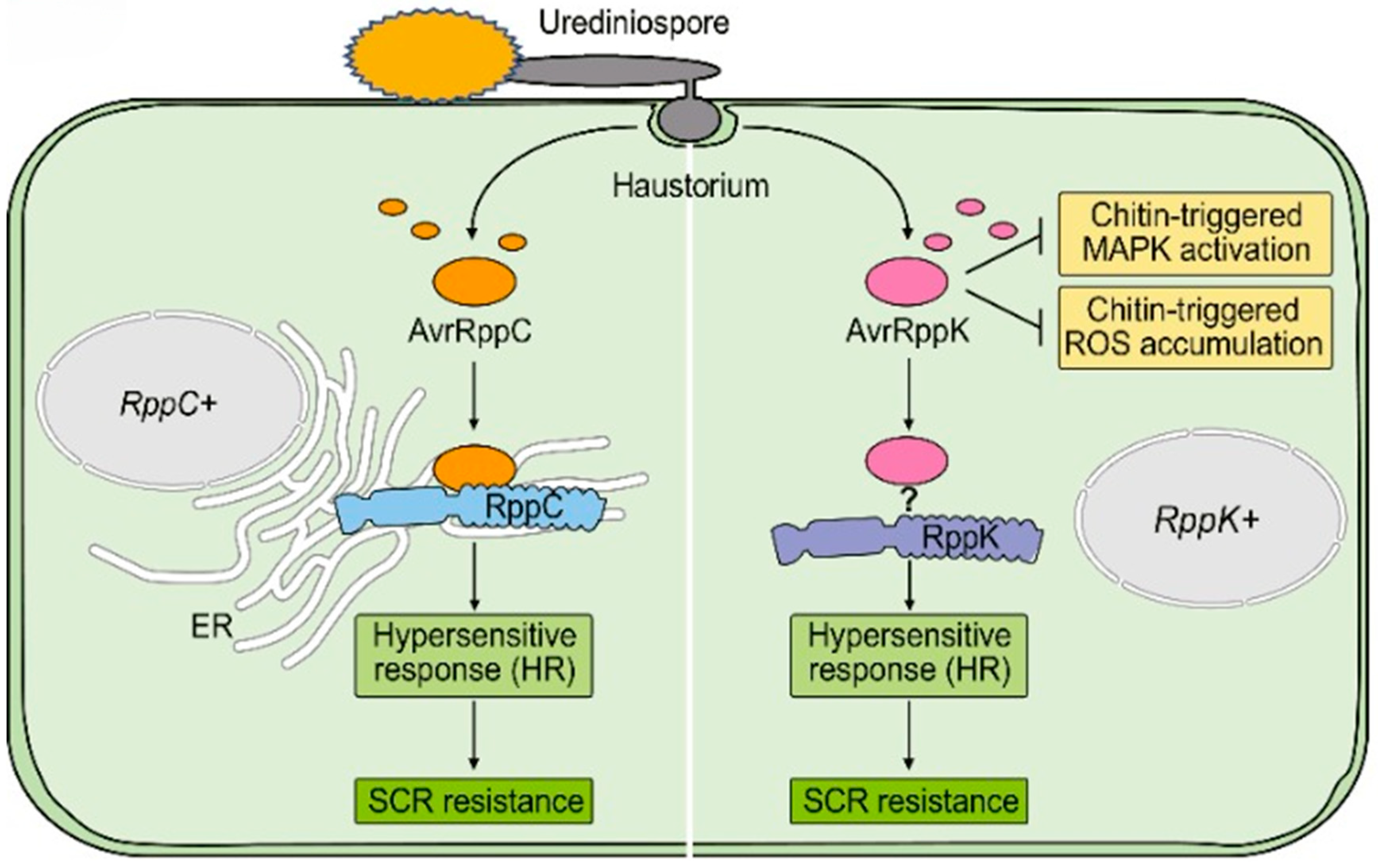
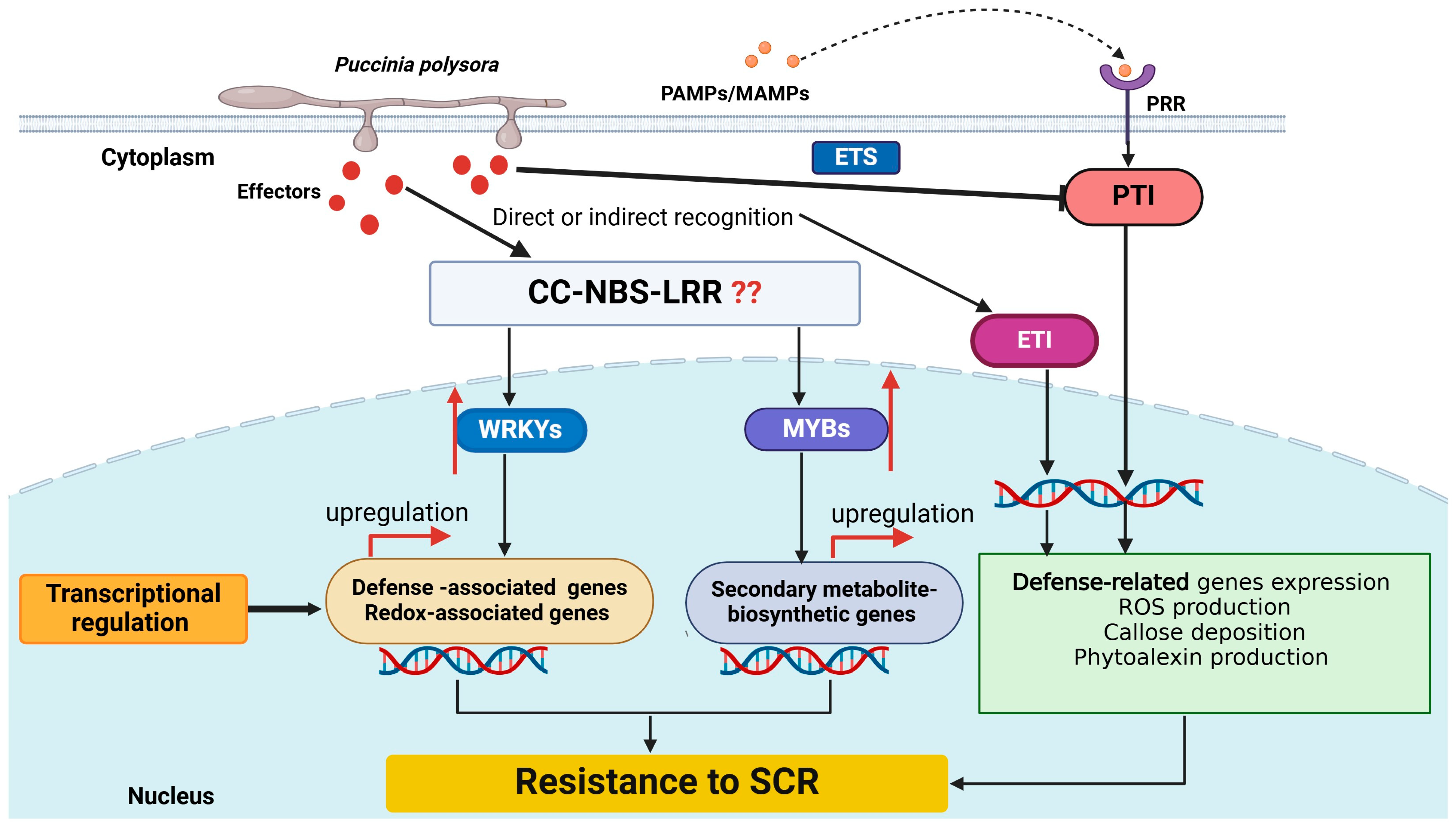
5. Molecular Interactions Between Puccinia polysora and Maize
6. Genetic Resistance of Maize to Southern Corn Rust
| Gene | Maize Variety | Chromosome | R-Gene Product | Reference |
|---|---|---|---|---|
| Rpp1 | AFRO.29 | Nd | [54] | |
| Rpp2 | AFRO.24 (SLP 20-4A) | Nd | [54] | |
| Rpp3-Rpp8 | Nd | [17] | ||
| Rpp9 | PT186208 | Nd | [88] | |
| Rpp10 | AFRO.761 | Nd | [89] | |
| Rpp11 | AFRO.600 | Nd | [89] | |
| RppP25 | P25 | 10S | Nd | [90] |
| RppQ | Qi319 | 10S | Nd | [22] |
| RppD | W2D | 10S | Nd | [78] |
| RppC | CML470 | 10S | CC-NBS-LRR ptotein | [77] |
| Rpp12 | Jiku12 | 10S | Nd | [91] |
| RppS | SCML205 | 10S | Nd | [23] |
| RppS313 | S313 × PHW52 | 10S | Nd | [79] |
| RppM | Kangxiujing2416 (Jing2416k) | 10S | CC-NBS-LRR ptotein | [8] |
| RppCML496 | CML496 | 10S | Nd | [80] |
| RppK | K22 × DAN340 | 10S | CC-NBS-LRR ptotein | [29] |
| RppSLN | N531_R | 10S | NBS-LRR ptotein | [92] |
7. The Network of Maize Resistance to Puccinia polysora Infection
8. Transcriptional Regulation of Maize Resistance to Puccinia polysora
9. Economic Impact of SCR
10. SCR Integrated Management
10.1. Deployment of Resistant Cultivars
10.2. Scouting
10.3. Chemical Control
10.4. Disease Prediction
11. Limitations and Future Perspectives
12. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, N.; Lin, B.; Wang, H.; Li, X.; Yang, F.; Ding, X.; Yan, J.; Chu, Z. Natural variation in ZmFBL41 confers banded leaf and sheath blight resistance in maize. Nat. Genet. 2019, 51, 1540–1548. [Google Scholar] [CrossRef] [PubMed]
- Tay, W.T.; Meagher, R.L.; Czepak, C.; Groot, A.T. Spodoptera frugiperda: Ecology, evolution, and management options of an invasive species. Annu. Rev. Entomol. 2023, 68, 299–317. [Google Scholar] [CrossRef] [PubMed]
- Rhind, D.; Waterston, J.M.; Deighton, F.C. Occurrence of Puccinia polysora Underw. in West Africa. Nature 1952, 169, 631. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, C.; Cao, P.; Wu, S.; Sun, J.; Jin, D.; Wang, B. Characterization and fine mapping of RppQ, a resistance gene to southern corn rust in maize. Mol. Genet. Genom. 2007, 278, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Dolezal, W.; Tiwari, K.; Kemerait, R.; Kichler, J.; Sapp, P.; Pataky, J. An unusual occurrence of southern rust, caused by Rpp9-virulent Puccinia polysora, on corn in Southwestern Georgia. Plant Dis. 2009, 93, 676. [Google Scholar] [CrossRef] [PubMed]
- Brewbaker, J.L.; Kim, S.K.; So, Y.S.; Logroño, M.; Moon, H.G.; Ming, R.; Lu, X.W.; Josue, A.D. General Resistance in maize to southern rust (Puccinia polysora Underw.). Crop Sci. 2011, 51, 1393–1409. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, R.; Shi, Z.; Zhao, Y.; Su, A.; Wang, Y.; Xing, J.; Ge, J.; Li, C.; Wang, X.; et al. Identification and fine mapping of RppM, a southern corn rust resistance gene in maize. Front Plant Sci. 2020, 11, 1057. [Google Scholar] [CrossRef]
- Wang, X.M.; Liu, J.; Guo, Y.Y.; Duan, C.X.; Zhu, Z.D.; Sun, S.L.; Yang, Z.H. Multiorigins of initial infection sources of Puccinia polysora causing southern rust of maize in China. J. Maize Sci. 2020, 28, 1–14. [Google Scholar]
- Sun, Q.; Li, L.; Guo, F.; Zhang, K.; Dong, J.; Luo, Y.; Ma, Z. Southern corn rust caused by Puccinia polysora Underw: A review. Phytopathol. Res. 2021, 3, 25. [Google Scholar] [CrossRef]
- Ma, Z.; Sun, Q.; Li, L.; Zhang, K.; Gao, J.; Dong, J. Research progresses of southern corn rust in China: A review. Acta Phytophyl. Sin. 2022, 49, 276–282. [Google Scholar]
- Guo, Y.Y.; Chen, M.G.; Sun, S.L.; Wu, X.F.; Jiang, K.; Zhu, Z.D.; Li, H.J.; He, Y.Q.; Wang, X.M. Genetic diversity of Puccinia polysora Underw. China Sci. Agric. Sin. 2013, 46, 4523–4533. [Google Scholar] [CrossRef]
- Cammack, R.H. Studies on Puccinia polysora underw: II. A consideration of the method of introduction of P. polysora into Africa. Trans. Br. Mycol. Soc. 1959, 42, 27–32. [Google Scholar] [CrossRef]
- Futrell, M.C.; Hooker, A.L.; Gene, S.E. Resistance in maize to corn rust, controlled by a single dominant gene. Crop Sci. 1975, 15, 597–599. [Google Scholar] [CrossRef]
- Scott, G.E.; Futrell, M.C. Big epidemic on the way? Southern corn rust. Crops Soils Mag. 1976; April–May, 16–18. [Google Scholar]
- Scott, G.E.; King, S.B. Maize yield losses caused by southern corn rust (Puccinia polysora). Crop Sci. 1980, 20, 812–814. [Google Scholar]
- Ryland, A.K.; Storey, H.H. Physiological races of Puccinia polysora Underw. Nature 1955, 176, 655–656. [Google Scholar] [CrossRef]
- Robert, A.L. Host ranges and races of the corn rusts. Phytopathology 1962, 52, 1010–1012. [Google Scholar]
- Ullstrup, A.J. Inheritance and linkage of a gene determining resistance in maize to an American race of Fuccinia polysora. Phytopathology 1965, 55, 425–428. [Google Scholar]
- Storey, H.H.; Howland, A.K. Resistance in maize to a third East African race of Puccinia polysora Underw. Ann. Appl. Biol. 1967, 60, 297–303. [Google Scholar] [CrossRef]
- Casela, C.R.; Ferreira, A.S. Variability in isolates of Puccinia polysora in Brazil. Fitopatol. Bras. 2002, 27, 414–416. [Google Scholar] [CrossRef]
- Scott, G.E.; King, S.B.; Armour Jr, J.W. Inheritance of resistance to southern corn rust in maize populations 1. Crop Sci. 1984, 24, 265–267. [Google Scholar] [CrossRef]
- Chen, C.X.; Wang, Z.L.; Yang, D.E.; Ye, C.J.; Zhao, Y.B.; Jin, D.M.; Weng, M.L.; Wang, B. Molecular tagging and genetic mapping of the disease resistance gene RppQ to southern corn rust. Theor. Appl. Genet. 2004, 108, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, N.; Zhao, P.; He, Y.; Wang, S. Geographic and genetic identification of RppS, a novel locus conferring broad resistance to southern corn rust disease in China. Euphytica 2015, 205, 17–23. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Z.; Tian, L.; Ding, Y.; Zhang, J.; Zhou, J.; Liu, P.; Chen, Y.; Wu, L. Comparative proteomics combined with analyses of transgenic plants reveal ZmREM1.3 mediates maize resistance to southern corn rust. Plant Biotechnol. J. 2019, 17, 2153–2168. [Google Scholar] [CrossRef]
- Mueller, D.S.; Wise, K.A.; Sisson, A.J.; Allen, T.W.; Bergstrom, G.C.; Bissonnette, K.M.; Bradley, C.A.; Byamukama, E.; Chilvers, M.I.; Collins, A.A.; et al. Corn yield loss estimates due to diseases in the United States and Ontario, Canada, from 2016 to 2019. Plant Health Prog. 2020, 21, 238–247. [Google Scholar] [CrossRef]
- Delaney, D.E.; Webb, C.A.; Hulbert, S.H. A novel rust resistance gene in maize showing overdominance. Mol. Plant-Microbe Interac. 1998, 11, 242–245. [Google Scholar] [CrossRef]
- Periyannan, S.; Milne, R.J.; Figueroa, M.; Lagudah, E.S.; Dodds, P.N. An overview of genetic rust resistance: From broad to specific mechanisms. PLoS Pathog. 2017, 13, e1006380. [Google Scholar] [CrossRef]
- Deng, C.; Leonard, A.; Cahill, J.; Lv, M.; Li, Y.; Thatcher, S.; Li, X.; Zhao, X.; Du, W.; Li, Z.; et al. The RppC-AvrRppC NLR-effector interaction mediates the resistance to southern corn rust in maize. Mol. Plant 2022, 15, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, B.; Ding, J.; Wang, H.; Deng, C.; Wang, J.; Yang, Q.; Pi, Q.; Zhang, R.; Zhai, H.; et al. Cloning southern corn rust resistant gene RppK and its cognate gene AvrRppK from Puccinia polysora. Nat. Commun. 2022, 13, 4392. [Google Scholar] [CrossRef] [PubMed]
- Underwood, L.M. Some New Fungi, Chiefly from Alabama. Bull. Torrey Bot. Club 1897, 24, 81–86. [Google Scholar] [CrossRef]
- Orian, G. Occurrence of Puccinia polysora Underwood in the Indian Ocean Area. Nature 1954, 173, 505. [Google Scholar] [CrossRef]
- Cummins, G.B. Identity and distribution of three rusts of corn. Phytopathology 1941, 31, 856–857. [Google Scholar]
- Krattiger, A.; Kulisek, E.S.; Casela, C. Diagnosing maize diseases with proprietary biotechnology applications transferred from pioneer Hi-Bred international to Brazil and Latin America. In Diagnosing Maize Diseases in Latin America; ISAAA Briefs No. 9; Casela, C., Renfro, B., Krattiger, A.F., Eds.; The International Service for the Acquisition of Agri-biotech Applications ISAAA: Ithaca, NY, USA, 1998; pp. 1–4. [Google Scholar]
- Nattrass, R.M. Occurrence of Puccinia polysora Underw. in East Africa. Nature 1953, 171, 527. [Google Scholar] [CrossRef]
- Reyes, G.M. An epidemic outbreak of the maize rust in eastern and central Visayas, Philippines. Philipp. J. Agric. 1953, 18, 115–128. [Google Scholar]
- Payak, M.M. Interception of Puccina polysora Southern Rust of Maize in India; National Bureau of Plant Genetic Resources, Indian Agricultural Research Institute: New Delhi, India, 1992; pp. 1–5. [Google Scholar]
- Raid, R.N.; Pennypacker, S.P.; Stevenson, R.E. Characterization of Puccinia polysora epidemics in Pennsylvania and Maryland. Phytopathology 1988, 78, 579–585. [Google Scholar] [CrossRef]
- Pont, W. Maize diseases are common in North Queensland. Qld. Agric. J. 1963, 89, 357–365. [Google Scholar]
- Barker, S.J. Testing of Maize hybrids resistant to Puccinia polysora on the Atherton Tableland, Queensland. Qld. J. Agric. Anim. Sci. 1969, 26, 319–327. [Google Scholar]
- Unartngam, J.; Janruang, P.; To-anan, C. Genetic diversity of Puccinia polysora in Thailand based on inter simple sequence repeat (ISSR) markers analysis. Int. J. Agric. Technol. 2011, 7, 1125–1137. [Google Scholar]
- Nishi, K.; Kawase, A.; Namiki, F.; Hirayae, K. Seasonal prevalence of southern rust (Puccinia polysora) of corn in Kumamoto prefecture. Kyushu Plant Prot. Res. 1998, 44, 9–11. [Google Scholar] [CrossRef]
- Hirayae, K.; Kawase, A.; Umeda, Y.; Nakatani, D.; Yamaguchi, T.; Nishi, K. Genetic variation of southern rust fungus of corn in Japan. Kyushu Plant Prot. Res. 1998, 44, 12–14. [Google Scholar] [CrossRef][Green Version]
- Duan, D.-R.; He, H.Z. Description of a rust Puccinia polysora on corn in Hainan Island. Mycosystema 1984, 3, 125–126. [Google Scholar]
- Agarwal, P.C.; Khetarpal, R.; Payak, M.M. Polysora rust of maize caused by Puccinia polysora and its spread in Karnataka. Indian J. Agric. Sci. 2001, 71, 275–276. [Google Scholar]
- Liu, Y.Y.; Wang, J. Southern corn rust occurred in Hebei Province in 1998. Plant Prot. 1999, 25, 53. (In Chinese) [Google Scholar]
- Ren, Z.T.; Ma, Y.; Ren, Z.Z.; Li, H.X.; Li, H.Z. The emergence of southern corn rust and its prevention-control countermeasure. J. Maize Sci. 2005, 13, 124–126. [Google Scholar]
- Liu, X.F.; Xu, J.Y.; Gu, Y.L.; Sun, Q.Y.; Yuan, W.Y.; Ma, Z.H. Occurrence of Puccinia polysora Causing Southern Corn Rust in the Northeast Huanghuaihai Region of China. Plant Dis. 2018, 102, 826. [Google Scholar] [CrossRef]
- Byamukama, E. Southern Rust Developing Late in Corn. 2020. Available online: https://extension.sdstate.edu/southern-rust-developing-late-corn (accessed on 24 December 2024).
- Halvorson, J.; Kim, Y.; Gill, U.; Friskop, A. First report of the southern corn rust pathogen Puccinia polysora on Zea mays in North Dakota. Can. J. Plant Pathol. 2021, 43, S352–S357. [Google Scholar] [CrossRef]
- Babadoost, M. Common and Southern Rust of Sweet Corn; University of Illinois Extension: Urbana, IL, USA, 1991; RPD No. 965; Available online: https://ipm.illinois.edu/diseases/rpds/965.pdf (accessed on 17 August 2024).
- Guerra, F.A.; Brücher, E.; De Rossi, R.L.; Plazas, M.C.; Guerra, G.D.; Ducasse, D.A. First report of Oxalis conorrhiza as alternate host of Puccinia sorghi, causal agent of common rust of maize. Plant Dis. 2015, 100, 519. [Google Scholar] [CrossRef]
- Hennen, J.F.; Figueiredo, M.B.; de Carvalho Jr, A.A.; Hennen, P.G. Catalogue of the Species of Plant Rust Fungi (Uredinales) of Brazil; Instituto de Pesquisas Jardim Botânico do Rio de Janeiro: Rio de Janeiro, Brazil, 2005; Available online: https://www.yumpu.com/en/document/view/11842663/catalogue-of-the-species-of-plant-rust-fungi-jardim-botanico-do-rio- (accessed on 20 August 2024).
- Storey, H.H.; Howland, A.K. Resistance in maize to the tropical American rust fungus, Puccinia polysora Underw. Heredity 1957, 11, 289–301. [Google Scholar] [CrossRef]
- Storey, H.H.; Rowland, A.K. Resistance in maize to the tropical American rust fungus, Puccinia polysora. II. Linkage of genes Rpp1 and Rpp2. Heredity 1959, 13, 61–65. [Google Scholar] [CrossRef][Green Version]
- Jackson-Ziems, T.A. Rust Diseases of Corn in Nebraska. G1680. NebGuide; University of Nebraska Extension: Greeley, NE, USA, 2014; Available online: https://extensionpublications.unl.edu/assets/pdf/g1680.pdf (accessed on 1 November 2024).
- Bradley, C.; Allen, T.; Faske, T.; Isakeit, T.; Jackson-Ziems, T.; Mehl, K.; Mueller, D.; Sisson, A.; Tenuta, A.; Weems, J.; et al. An overview of southern rust. Crop Prot. Netw. 2019, CPN-2009. [Google Scholar] [CrossRef]
- Ahumada, D. Corn Rusts: Common and Southern Rust; NC State Extension Publications: Raleigh, NC, USA, 2023; Available online: https://content.ces.ncsu.edu/corn-rusts-common-and-southern-rust (accessed on 18 September 2024).
- Crouch, J.A.; Szabo, L.J. Real-Time PCR detection and discrimination of the southern and common corn rust pathogens Puccinia polysora and Puccinia sorghi. Plant Dis. 2011, 95, 624–632. [Google Scholar] [CrossRef]
- Huang, L.Q.; Zhang, K.Y.; Dong, J.Y.; Sun, Q.Y.; Li, L.F.; Ma, Z.H. A fast method for distinguishing southern rust pathogen Puccinia polysora from common rust pathogen Puccinia sorghi. J. Plant Prot. 2020, 47, 1385–1386. (In Chinese) [Google Scholar]
- Wise, K. Diseases of Corn. Common and Southern Rusts. BP-82-W; Purdue University Extension: West Lafayette, IN, USA, 2010; Available online: https://www.extension.purdue.edu/extmedia/BP/BP-82-W.pdf (accessed on 22 November 2024).
- Sun, Q.; Liu, J.; Zhang, K.; Huang, C.; Li, L.; Dong, J.; Luo, Y.; Ma, Z. De novo transcriptome assembly, polymorphic SSR markers development and population genetics analyses for southern corn rust (Puccinia polysora). Sci. Rep. 2021, 11, 18029. [Google Scholar] [CrossRef]
- Mueller, D.S.; Wise, K.A.; Sisson, A.J.; Allen, T.W.; Bergstrom, G.C.; Bosley, D.B.; Bradley, C.A.; Broders, K.D.; Byamukama, E.; Chilvers, M.I.; et al. Corn yield loss estimates due to diseases in the United States and Ontario, Canada from 2012 to 2015. Plant Health Prog. 2016, 17, 211–222. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Ngou, B.P.M.; Ding, P.; Xin, X.-F. PTI-ETI crosstalk: An integrative view of plant immunity. Curr. Opin. Plant Biol. 2021, 62, 102030. [Google Scholar] [CrossRef]
- Wang, J.; Hu, M.; Wang, J.; Qi, J.; Han, Z.; Wang, G.; Qi, Y.; Wang, H.-W.; Zhou, J.-M.; Chai, J. Reconstitution and structure of a plant NLR resistosome conferring immunity. Science 2019, 364, eaav5870. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, X.; Dai, L.; Wang, G. Recent progress in elucidating the structure, function and evolution of disease resistance genes in plants. J. Genet. Genom. 2007, 34, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Zhou, S.; Liu, W. Gene-for-gene-mediated resistance to southern corn rust in maize. Trends Plant Sci. 2023, 28, 255–258. [Google Scholar] [CrossRef]
- Parlevliet, J.E. Components of resistance that reduce the rate of epidemic development. Annu. Rev. Phytopathol. 1979, 17, 203–222. [Google Scholar] [CrossRef]
- Nelson, R.R. Genetics of horizontal resistance to plant diseases. Annu. Rev. Phytopathol. 1978, 16, 359–378. [Google Scholar] [CrossRef]
- Robertson, D.S. Understanding the relationship between qualitative and quantitative genetics. In Development and Application of Molecular Markers to Problems in Plant Genetics; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1989; pp. 81–87. [Google Scholar]
- Zheng, H.; Chen, J.; Mu, C.; Makumbi, D.; Xu, Y.; Mahuku, G. Combined linkage and association mapping reveal QTL for host plant resistance to common rust (Puccinia sorghi) in tropical maize. BMC Plant Biol. 2018, 18, 310. [Google Scholar] [CrossRef] [PubMed]
- Hulbert, S.H. Structure and evolution of the rp1 complex conferring rust resistance in maize. Annu. Rev. Phytopathol. 1997, 35, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Holland, J.B.; Uhr, D.V.; Jeffers, D.; Goodman, M.M. Inheritance of resistance to southern corn rust in tropical-by-corn-belt maize populations. Theor. Appl. Genet. 1998, 96, 232–241. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, G.; Wu, X.; Li, N.; Shi, D.; Zhang, D.; Ji, C.; Xu, M.; Wang, S. Fine mapping of RppP25, a southern rust resistance gene in maize. J. Integr. Plant Biol. 2013, 55, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Wanlayaporn, K.; Authrapun, J.; Vanavichit, A.; Tragoonrung, S. QTL mapping for partial resistance to southern corn rust using RILs of tropical sweet corn. Am. J. Plant Sci. 2013, 4, 879–889. [Google Scholar] [CrossRef]
- Lu, L.; Xu, Z.; Sun, S.; Du, Q.; Zhu, Z.; Weng, J.; Duan, C. Discovery and fine mapping of qSCR6.01, a novel major QTL conferring southern rust resistance in maize. Plant Dis. 2020, 104, 1918–1924. [Google Scholar] [CrossRef]
- Yao, G.-Q.; Chan, J.; Cao, B.; Cui, L.-G.; Dou, S.-L.; Han, Z.-J.; Liu, T.-S.; Li, C.-L.; Wang, L.-M. Mapping the maize southern rust resistance gene in inbred line CML470. J. Plant Genet. Resour. 2013, 14, 518–522. (In Chinese) [Google Scholar]
- Zhang, Y.; Xu, L.; Zhang, D.-F.; Dai, J.-R.; Wang, S.-C. Mapping of southern corn rust-resistant genes in the W2D inbred line of maize (Zea mays L.). Mol. Breed. 2010, 25, 433–439. [Google Scholar] [CrossRef]
- Wang, B.; Qin, J.; Shi, C.; Zheng, J.; Qin, Y.; Huang, A. QTL mapping and genetic analysis of a gene with high resistance to southern corn rust. Sci. Agric. Sin. 2019, 52, 2033–2041. [Google Scholar] [CrossRef]
- Lv, M.; Deng, C.; Li, X.; Zhao, X.; Li, H.; Li, Z.; Tian, Z.; Leonard, A.; Jaqueth, J.; Li, B. Identification and fine-mapping of RppCML496, a major QTL for resistance to Puccinia polysora in maize. Plant Genome 2021, 14, e20062. [Google Scholar] [CrossRef]
- Karasov, T.L.; Chae, E.; Herman, J.J.; Bergelson, J. Mechanisms to mitigate the trade-off between growth and defense. Plant Cell 2017, 29, 666–680. [Google Scholar] [CrossRef] [PubMed]
- de Souza Camacho, L.R.; Coan, M.M.D.; Scapim, C.A.; Barth Pinto, R.J.; Tessmann, D.J.; Contreras-Soto, R.I. A genome-wide association study for partial resistance to southern corn rust in tropical maize. Plant Breed. 2019, 138, 770–780. [Google Scholar] [CrossRef]
- Chen, W.J.; Lu, L.; Li, W.; Zhang, X.; Sun, S.; Zhu, Z.; Wang, X.M.; Duan, C.X. QTL mapping for resistance to southern corn rust in maize. J. Plant Genet. Resour. 2019, 20, 521–529. [Google Scholar]
- Deng, C.; Li, H.; Li, Z.; Tian, Z.; Chen, J.; Chen, G.; Zhang, X.; Ding, J.; Chang, Y. New QTL for resistance to Puccinia polysora Underw in maize. J. Appl. Genet. 2019, 60, 147–150. [Google Scholar] [CrossRef]
- Meng, R.; Lv, Z.; Yan, J.; Chen, G.; Zhao, F.; Zeng, L.; Xu, B. Development of spectral disease indices for southern corn rust detection and severity classification. Remote Sens. 2020, 12, 3233. [Google Scholar] [CrossRef]
- Deng, C.; Lv, M.; Li, X.; Zhao, X.; Li, H.; Li, Z.; Tian, Z.; Leonard, A.; Jaqueth, J.; Li, B.; et al. Identification and fine mapping of qSCR4.01, a novel major QTL for resistance to Puccinia polysora in Maize. Plant Dis. 2020, 104, 1944–1948. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, X.; Hou, C.; Hu, M.; Du, W.; Sun, P.; Dai, Z.; Wang, X.; Chen, R.; Gao, F.; et al. qSCR4.05 and qSCR4.08, two QTLs on chromosome 4 are associated with resistance to southern corn rust in maize. Physiol. Mol. Plant Pathol. 2024, 134, 102420. [Google Scholar] [CrossRef]
- Scheffer, R.P.; Ullstrup, A.J. A host-specific toxic metabolite from Heiminthosporium carbonum. Phytopathology 1965, 55, 1037–1038. [Google Scholar]
- Storey, H.H.; Howland, A.K. Inheritance of resistance in maize to the virus of streak disease in East Africa. Ann. Appl. Biol. 1967, 59, 429–436. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, S.; Dai, J.; Huang, L.; Cao, H. Studies of genetic analysis and SSR linked marker location of gene resistance to southern rust in inbred line P25 of maize. Acta Genet. Sin. 2003, 30, 706–710. [Google Scholar]
- Zhang, X.L. Study on the Resistance of Maize to Northern Corn Leaf Blight and Southern Corn Rust. Ph.D. Thesis, Graduate School of Chinese Academy of Agricultural Science, Beijing, China, 2013. Available online: https://www.dissertationtopic.net/down/1784956 (accessed on 15 September 2024).
- Wang, Y.; Ma, S.; Zhang, D.; Li, C.; Chen, L.; Tang, B.; An, Y.; Liu, X.; He, G.; Shi, Y.; et al. Identification of RppSLN from an elite landrace: A major locus conferring resistance to southern corn rust in maize (Zea mays L.). Plants 2024, 13, 3227. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Dai, Z.; Guo, Z.; Zhang, H.; Yang, J.; Gan, X.; Li, J.; Liu, Z.; Tang, J.; Gou, M. Systematic dissection of disease resistance to southern corn rust by bulked-segregant and transcriptome analysis. Crop J. 2022, 10, 426–435. [Google Scholar] [CrossRef]
- Wang, Y.; Mu, C.-H.; Li, X.-D.; Duan, C.-X.; Wang, J.-J.; Lu, X.; Li, W.-S.; Xu, Z.-N.; Sun, S.-F.; Zhang, A.; et al. Genome-wide association and transcriptome reveal genetic basis for Southern Corn Rust in maize. J. Integr. Agric. 2023, in press. [Google Scholar] [CrossRef]
- Potter, S.S. Single-cell RNA sequencing for the study of development, physiology and disease. Nat. Rev. Nephrol. 2018, 14, 479–492. [Google Scholar] [CrossRef]
- Kulkarni, A.; Anderson, A.G.; Merullo, D.P.; Konopka, G. Beyond bulk: A review of single cell transcriptomics methodologies and applications. Curr. Opin. Biotechnol. 2019, 58, 129–136. [Google Scholar] [CrossRef]
- Rich-Griffin, C.; Stechemesser, A.; Finch, J.; Lucas, E.; Ott, S.; Schäfer, P. Single-cell transcriptomics: A high-resolution avenue for plant functional genomics. Trends Plant Sci. 2020, 25, 186–197. [Google Scholar] [CrossRef]
- Yan, X.C.; Liu, Q.; Yang, Q.; Wang, K.L.; Zhai, X.Z.; Kou, M.Y.; Liu, J.L.; Li, S.T.; Deng, S.H.; Li, M.M.; et al. Single-cell transcriptomic profiling of maize cell heterogeneity and systemic immune responses against Puccinia polysora Underw. Plant Biotechnol. J. 2024, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S.; Chhetri, S.; Biswas, S. Southern rust disease of corn–a review. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 855–862. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C.M. Hormonal modulation of plant immunity. Ann. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef]
- Buscaill, P.; Rivas, S. Transcriptional control of plant defence responses. Curr. Opin. Plant Biol. 2014, 20, 35–46. [Google Scholar] [CrossRef]
- Piasecka, A.; Jedrzejczak-Rey, N.; Bednarek, P. Secondary metabolites in plant innate immunity: Conserved function of divergent chemicals. New Phytol. 2015, 206, 948–964. [Google Scholar] [CrossRef]
- Vailleau, F.; Daniel, X.; Tronchet, M.; Montillet, J.-L.; Triantaphylides, C.; Roby, D. A R2R3-MYB gene, AtMYB30, acts as a positive regulator of the hypersensitive cell death program in plants in response to pathogen attack. Proc. Natl. Acad. Sci. USA 2002, 99, 10179–10184. [Google Scholar] [CrossRef]
- Li, J.; Brader, G.; Kariola, T.; Tapio Palva, E. WRKY70 modulates the selection of signaling pathways in plant defense. Plant J. 2006, 46, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Chezem, W.R.; Memon, A.; Li, F.-S.; Weng, J.-K.; Clay, N.K. SG2-Type R2R3-MYB Transcription factor MYB15 Controls defense-induced lignification and basal immunity in Arabidopsis. Plant Cell 2017, 29, 1907–1926. [Google Scholar] [CrossRef]
- Hunt Jr, E.R.; Rock, B.N. Detection of changes in leaf water content using near-and middle-infrared reflectances. Remote Sens. Environ. 1989, 30, 43–54. [Google Scholar] [CrossRef]
- Cheng, T.; Rivard, B.; Sanchez-Azofeifa, A. Spectroscopic determination of leaf water content using continuous wavelet analysis. Remote Sens. Environ. 2011, 115, 659–670. [Google Scholar] [CrossRef]
- Bohnenkamp, D.; Kuska, M.T.; Mahlein, A.K.; Behmann, J. Hyperspectral signal decomposition and symptom detection of wheat rust disease at the leaf scale using pure fungal spore spectra as reference. Plant Pathol. 2019, 68, 1188–1195. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, Y.; Zeng, J.; Ji, G.; Liu, L.; Qiu, K.; Xu, Y. Analysis of the main occurrence characteristics and causes of the southern corn rust in China in 2015. China Plant Prot 2016, 36, 44–47. [Google Scholar]
- Liu, J.; Ma, Q.; Yu, K.; Wang, X. A report about the occurrence area of southern corn rust and the resistance of the corn cultivars in China. Crops 2009, 3, 71–75. [Google Scholar]
- Yuan, H.; Xin, X.; Li, C. Resistance comparisons to southern corn rust in different corn varieties. J. Maize Sci. 2010, 18, 107–109. [Google Scholar]
- Gao, J.; Ding, M.; Sun, Q.; Dong, J.; Wang, H.; Ma, Z. Classification of southern corn rust severity based on leaf-level hyperspectral data collected under solar illumination. Remote Sens. 2022, 14, 2551. [Google Scholar] [CrossRef]
- Mahlein, A.-K.; Kuska, M.T.; Behmann, J.; Polder, G.; Walter, A. Hyperspectral sensors and imaging technologies in phytopathology: State of the Art. Annu. Rev. Phytopathol. 2018, 56, 535–558. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, Y.; Pu, R.; Gonzalez-Moreno, P.; Yuan, L.; Wu, K.; Huang, W. Monitoring plant diseases and pests through remote sensing technology: A review. Comput. Electron. Agric. 2019, 165, 104943. [Google Scholar] [CrossRef]
- Harrelson, B.; Plumblee, M.; Mueller, J. Common and Southern Rust in Field Corn; Clemson Cooperative Extension and Land-Grant Press by Clemson Extension: Clemson, SC, USA, 2022; Available online: https://lgpress.clemson.edu/publication/common-and-southern-rust-in-field-corn/ (accessed on 25 October 2024).
- Plumblee, M.T. South Carolina Corn Production Guide; Clemson University Cooperative Extension Service: Clemson, SC, USA, 2022; Available online: https://www.clemson.edu/extension/agronomy/_files/corn-production-guide.pdf (accessed on 1 November 2024).
- Hollier, C.A. Southern Corn Rust: Development, Risk and Management; Department of Plant Pathology and Crop Physiology, College of Agriculture: Baton Rouge, LA, USA, 2017; Available online: https://www.lsuagcenter.com/profiles/lbenedict/articles/page1491403577615 (accessed on 1 November 2024).
- Hu, W.; Zheng, M.; Ruan, Y.; Pan, F.; Yu, B.; Yu, Y. Preliminary study on the occurrence and control of southern corn rust. Plant Prot. Technol. Ext. 2003, 23, 9–12. [Google Scholar] [CrossRef]
- Faske, T.R.; Emerson, M. Multiyear evaluation of fungicide efficacy and application timing for control of southern rust in hybrid corn in Arkansas. Plant Dis. 2021, 105, 1108–1114. [Google Scholar] [CrossRef]
- Mahlein, A.-K.; Oerke, E.-C.; Steiner, U.; Dehne, H.-W. Recent advances in sensing plant diseases for precision crop protection. Eur. J. Plant Pathol. 2012, 133, 197–209. [Google Scholar] [CrossRef]
- Isard, S.A.; Barnes, C.W.; Hambleton, S.; Ariatti, A.; Russo, J.M.; Tenuta, A.; Gay, D.A.; Szabo, L.J. Predicting soybean rust incursions into the North American continental interior using crop monitoring, spore trapping, and aerobiological modeling. Plant Dis. 2011, 95, 1346–1357. [Google Scholar] [CrossRef] [PubMed]
- Leng, W.F.; Li, G.Z.; Ma, Z.H. Distribution and molecular detection of corn rust in China. China Plant Prot. 2012, 32, 18–20. (In Chinese) [Google Scholar]
- Yang, X.; Ding, X.L.; He, Z.L.; Ma, Z.H. Determination of temperature required by southern corn rust. Plant Prot. 2015, 41, 145–147. (In Chinese) [Google Scholar]
- Ramirez-Cabral, N.Y.Z.; Kumar, L.; Shabani, F. Global risk levels for corn rusts (Puccinia sorghi and Puccinia polysora) under climate change projections. J. Phytopathol. 2017, 165, 563–574. [Google Scholar] [CrossRef]
- Yonow, T.; Hattingh, V.; de Villiers, M. CLIMEX modelling of the potential global distribution of the citrus black spot disease caused by Guignardia citricarpa and the risk posed to Europe. Crop Prot. 2013, 44, 18–28. [Google Scholar] [CrossRef]
- Kriticos, D.J.; Maywald, G.F.; Yonow, T.; Zurcher, E.J.; Herrmann, N.I.; Sutherst, R.W. Exploring the Effects of Climate on Plants, Animals and Diseases. CLIMEX. Version 4; CSIRO: Canberra, Australia, 2015; p. 184. Available online: https://www.researchgate.net/publication/309126801 (accessed on 1 November 2024).
- Jung, J.-M.; Lee, W.-H.; Jung, S. Insect distribution in response to climate change based on a model: Review of function and use of CLIMEX. Entomol. Res. 2016, 46, 223–235. [Google Scholar] [CrossRef]
- Byeon, D.; Jung, S.; Mo, C.; Lee, W.-H. Effectiveness of sensitivity analysis for parameter selection in CLIMEx modeling of Metcalfa pruinosa distribution. J. Biosyst. Eng. 2018, 43, 410–419. [Google Scholar] [CrossRef]
- Yang, L.; Li, L.; Dong, Z.; Zhu, J.; Guo, W.; Song, Y.; Cui, H.; Lv, S.; Sindhu, L.; Men, X. EIRP model driven by machine learning for predicting the occurrence risk of southern corn rust (Puccinia polysora Underw.) in northern China. Agric. For. Meteorol. 2024, 356, 110149. [Google Scholar] [CrossRef]
- Cardoso, S.D.; Gonçalves, D.; Robalo, J.I.; Almada, V.C.; Canário, A.V.M.; Oliveira, R.F. Efficient isolation of polymorphic microsatellites from high-throughput sequence data based on number of repeats. Mar. Genom. 2013, 11, 11–16. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.X.; Jiang, G.F. De novo Assembly and Characterization of the testis transcriptome and development of EST-SSR Markers in the Cockroach Periplaneta americana. Sci. Rep. 2015, 5, 11144. [Google Scholar] [CrossRef]
- Rosenkranz, R.; Borodina, T.; Lehrach, H.; Himmelbauer, H. Characterizing the mouse ES cell transcriptome with Illumina sequencing. Genomics 2008, 92, 187–194. [Google Scholar] [CrossRef][Green Version]
- Hegedus, Z.; Zakrzewska, A.; Agoston, V.C.; Ordas, A.; Rácz, P.; Mink, M.; Spaink, H.P.; Meijer, A.H. Deep sequencing of the zebrafish transcriptome response to mycobacterium infection. Mol. Immunol. 2009, 46, 2918–2930. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sniezko, R.A.; Zamany, A.; Williams, H.; Omendja, K.; Kegley, A.; Savin, D.P. Comparative transcriptomics and RNA-seq-based bulked segregant analysis reveals genomic basis underlying Cronartium ribicola vcr2 Virulence. Front. Microbiol. 2021, 12, 602812. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, J.; Wei, S.; Liu, Y.; Zhao, Z.; Shi, J. Harnessing Genetic Resistance in Maize and Integrated Rust Management Strategies to Combat Southern Corn Rust. J. Fungi 2025, 11, 41. https://doi.org/10.3390/jof11010041
Chang J, Wei S, Liu Y, Zhao Z, Shi J. Harnessing Genetic Resistance in Maize and Integrated Rust Management Strategies to Combat Southern Corn Rust. Journal of Fungi. 2025; 11(1):41. https://doi.org/10.3390/jof11010041
Chicago/Turabian StyleChang, Jiaying, Shizhi Wei, Yueyang Liu, Zhiquan Zhao, and Jie Shi. 2025. "Harnessing Genetic Resistance in Maize and Integrated Rust Management Strategies to Combat Southern Corn Rust" Journal of Fungi 11, no. 1: 41. https://doi.org/10.3390/jof11010041
APA StyleChang, J., Wei, S., Liu, Y., Zhao, Z., & Shi, J. (2025). Harnessing Genetic Resistance in Maize and Integrated Rust Management Strategies to Combat Southern Corn Rust. Journal of Fungi, 11(1), 41. https://doi.org/10.3390/jof11010041






