A Protein with Unknown Function, Ps495620, Is Critical for the Sporulation and Oospore Production of Phytophthora sojae
Abstract
1. Introduction
2. Materials and Methods
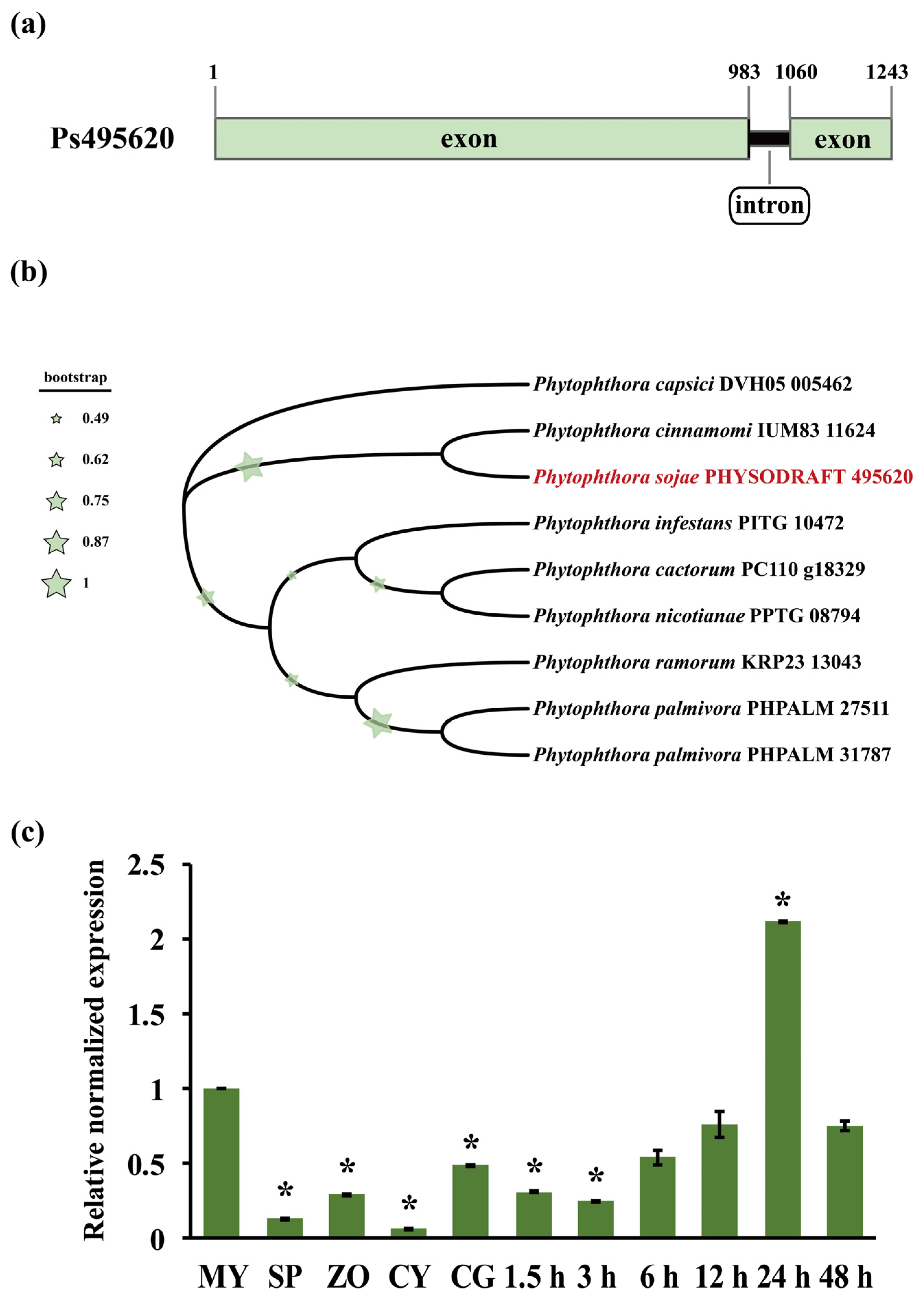
2.1. Sequence and Phylogenetic Analyses of Ps495620
2.2. Phytophthora sojae Strains and Culture Conditions
2.3. RNA Extraction and Gene Expression Analysis of Ps495620
2.4. Plasmid Construction
2.5. Phytophthora sojae Transformation
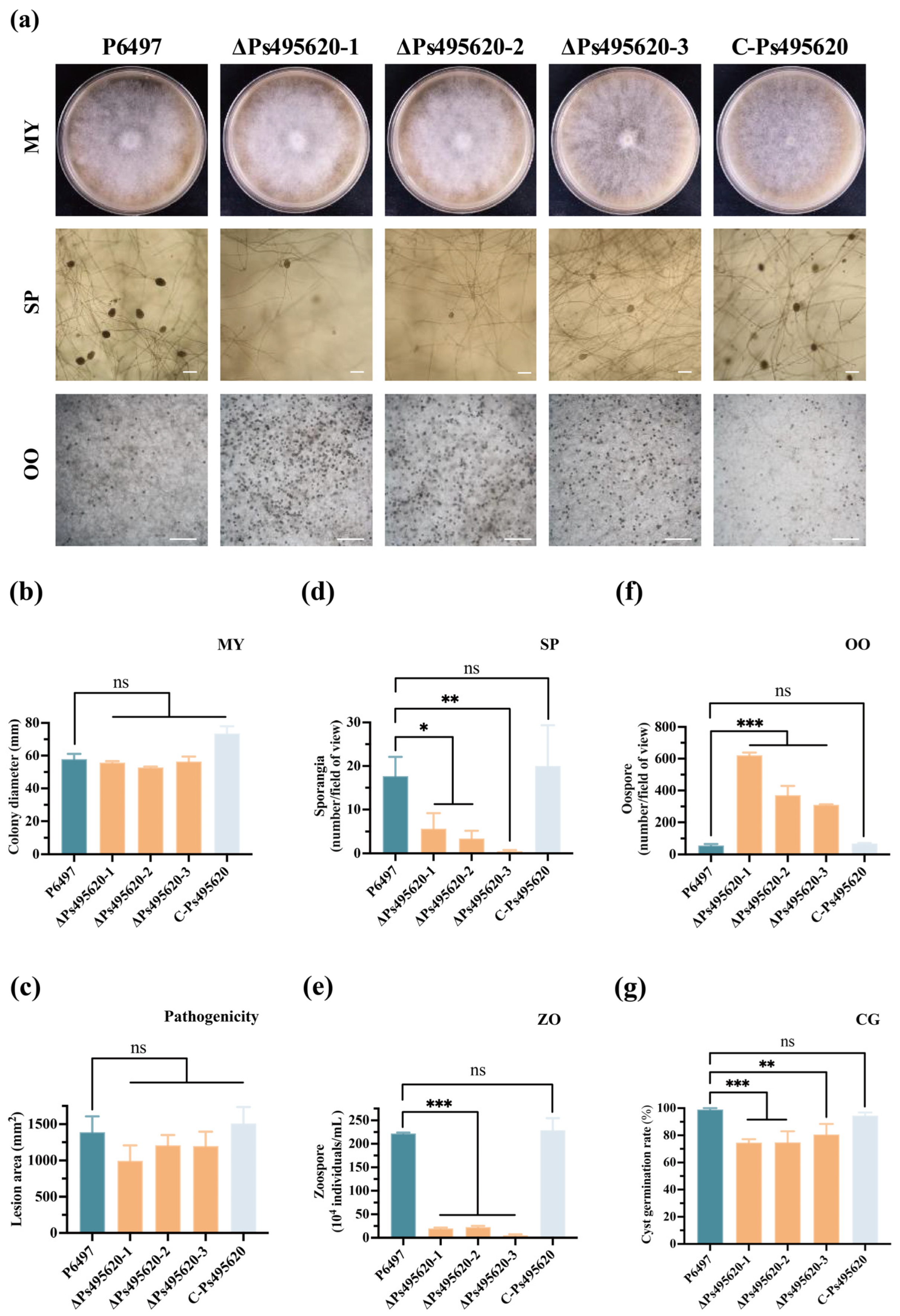
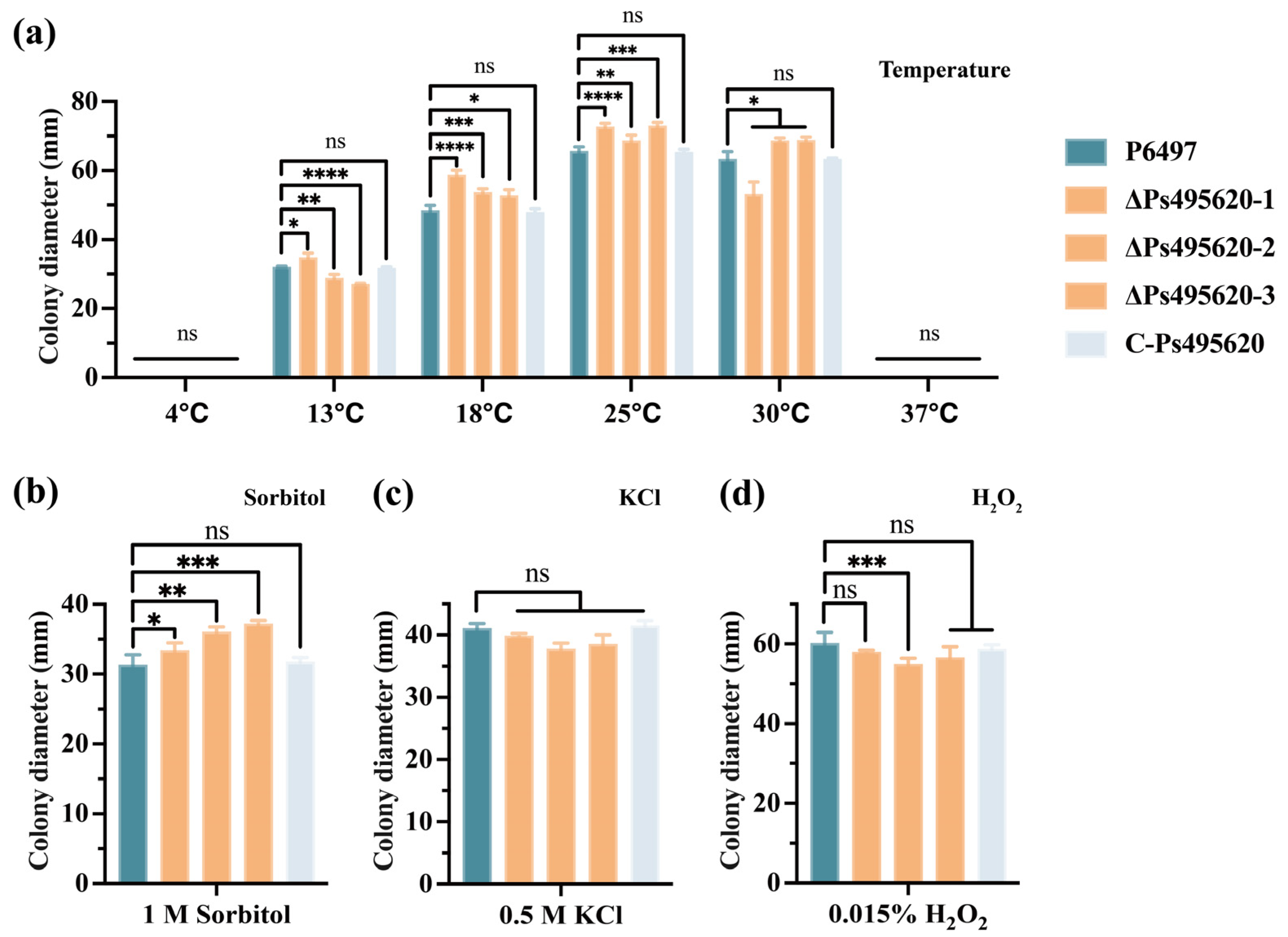
2.6. Phenotype Analysis of Transformants and P. sojae
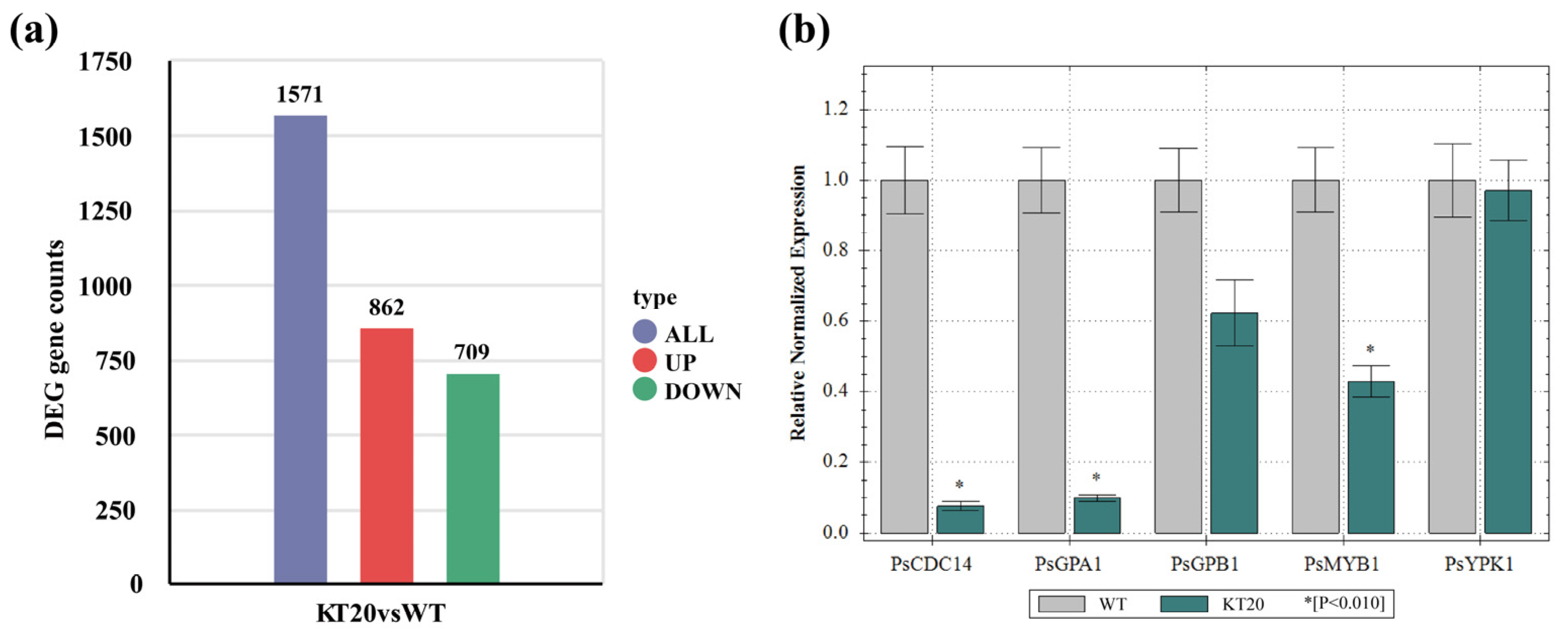
2.7. Transcriptome Sequencing and Analysis
3. Results
3.1. Sequence and Expression Profile Analyses of Ps495620
3.2. Ps495620 Plays a Critical Role in Sporangium Formation and Oospore Production
3.3. Ps495620 Is Crucial for the Stress Response in P. sojae
3.4. Transcriptome Analysis of Ps495620 Knockout Transformants at the Sporangia Stage
3.5. Differential Gene Expression Analysis of Ps495620 Knockout Transformant (KT20) and P6497 (WT) Strains at the Sporangia Stage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamoun, S.; Furzer, O.; Jones, J.D.G.; Judelson, H.S.; Ali, G.S.; Dalio, R.J.D.; Roy, S.G.; Schena, L.; Zambounis, A.; Panabières, F.; et al. The Top 10 Oomycete Pathogens in Molecular Plant Pathology. Mol. Plant Pathol. 2015, 16, 413–434. [Google Scholar] [CrossRef]
- Tyler, B.M. Molecular Basis of Recognition Between Phytophthora Pathogens and Their Hosts. Annu. Rev. Phytopathol. 2002, 40, 137–167. [Google Scholar] [CrossRef]
- Fang, Y.; Cui, L.; Gu, B.; Arredondo, F.; Tyler, B.M. Efficient Genome Editing in the Oomycete Phytophthora sojae Using CRISPR/Cas9. Curr. Protoc. Microbiol. 2017, 44, 21A.1.1–21A.1.26. [Google Scholar] [CrossRef]
- Judelson, H.S. Sexual Reproduction in Oomycetes: Biology, Diversity, and Contributions to Fitness. In Oomycete Genetics and Genomics; Lamour, K., Kamoun, S., Eds.; Wiley: Hoboken, NJ, USA, 2009; pp. 121–138. ISBN 978-0-470-25567-4. [Google Scholar]
- Wang, Y.; Dou, D.; Wang, X.; Li, A.; Sheng, Y.; Hua, C.; Cheng, B.; Chen, X.; Zheng, X.; Wang, Y. The PsCZF1 Gene Encoding a C2H2 Zinc Finger Protein Is Required for Growth, Development and Pathogenesis in Phytophthora sojae. Microb. Pathog. 2009, 47, 78–86. [Google Scholar] [CrossRef]
- Guo, T.; Wang, X.-W.; Shan, K.; Sun, W.; Guo, L.-Y. The Loricrin-like Protein (LLP) of Phytophthora infestans Is Required for Oospore Formation and Plant Infection. Front. Plant Sci. 2017, 8, 142. [Google Scholar] [CrossRef]
- Pei, Y.; Ji, P.; Si, J.; Zhao, H.; Zhang, S.; Xu, R.; Qiao, H.; Duan, W.; Shen, D.; Yin, Z.; et al. A Phytophthora Receptor-like Kinase Regulates Oospore Development and Can Activate Pattern-Triggered Plant Immunity. Nat. Commun. 2023, 14, 4593. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Kim, K.S.; Roy, S.; Judelson, H.S. A Motif within a Complex Promoter from the Oomycete Phytophthora infestans Determines Transcription during an Intermediate Stage of Sporulation. Fungal Genet. Biol. 2009, 46, 400–409. [Google Scholar] [CrossRef]
- Cvitanich, C.; Judelson, H.S. A Gene Expressed during Sexual and Asexual Sporulation in Phytophthora infestans Is a Member of the Puf Family of Translational Regulators. Eukaryot. Cell 2003, 2, 465–473. [Google Scholar] [CrossRef]
- Chen, L.; Shen, D.; Sun, N.; Xu, J.; Wang, W.; Dou, D. Phytophthora sojae TatD Nuclease Positively Regulates Sporulation and Negatively Regulates Pathogenesis. Mol. Plant-Microbe Interact. 2014, 27, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Ye, W.; Wu, J.; Xuan, M.; Li, Y.; Gao, J.; Wang, Y.; Wang, Y.; Dong, S.; Wang, Y. The MADS-Box Transcription Factor PsMAD1 Is Involved in Zoosporogenesis and Pathogenesis of Phytophthora sojae. Front. Microbiol. 2018, 9, 2259. [Google Scholar] [CrossRef]
- Hua, C.; Wang, Y.; Zheng, X.; Dou, D.; Zhang, Z.; Govers, F.; Wang, Y. A Phytophthora sojae G-Protein α Subunit Is Involved in Chemotaxis to Soybean Isoflavones. Eukaryot. Cell 2008, 7, 2133–2140. [Google Scholar] [CrossRef] [PubMed]
- Nabi, R.B.S.; Tayade, R.; Imran, Q.M.; Hussain, A.; Shahid, M.; Yun, B.-W. Functional Insight of Nitric-Oxide Induced DUF Genes in Arabidopsis thaliana. Front. Plant Sci. 2020, 11, 1041. [Google Scholar] [CrossRef]
- Bateman, A.; Coggill, P.; Finn, R.D. DUFs: Families in Search of Function. Acta Crystallograph. Sect. F Struct. Biol. Cryst. Commun. 2010, 66, 1148–1152. [Google Scholar] [CrossRef] [PubMed]
- Blasco, L.; Bleriot, I.; de Aledo, M.G.; Fernández-García, L.; Pacios, O.; Oliveira, H.; López, M.; Ortiz-Cartagena, C.; Fernández-Cuenca, F.; Pascual, Á.; et al. Development of an Anti-Acinetobacter Baumannii Biofilm Phage Cocktail: Genomic Adaptation to the Host. Antimicrob. Agents Chemother. 2022, 66, e01923-21. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-Y.; Sun, J.-L.; Tian, Y.-Y.; Liu, J.-X. The FtsH-Inactive Protein FtsHi5 Is Required for Chloroplast Development and Protein Accumulation in Chloroplasts at Low Ambient Temperature in Arabidopsis. Front. Plant Sci. 2022, 12, 830390. [Google Scholar] [CrossRef]
- Chen, J.; Ying, L.; Zeng, L.; Li, C.; Jia, Y.; Yang, H.; Yang, G. The Novel Compound Heterozygous Rare Variants May Impact Positively Selected Regions of TUBGCP6, a Microcephaly Associated Gene. Front. Ecol. Evol. 2022, 10, 1059477. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, F.; Luo, J.; Qiao, M.; Zeng, W.; Li, J.; Xu, W. The DUF288 Domain Containing Proteins GhSTLs Participate in Cotton Fiber Cellulose Synthesis and Impact on Fiber Elongation. Plant Sci. 2022, 316, 111168. [Google Scholar] [CrossRef] [PubMed]
- Sakata, M.; Takano-Kai, N.; Miyazaki, Y.; Kanamori, H.; Wu, J.; Matsumoto, T.; Doi, K.; Yasui, H.; Yoshimura, A.; Yamagata, Y. Domain Unknown Function DUF1668-Containing Genes in Multiple Lineages Are Responsible for F1 Pollen Sterility in Rice. Front. Plant Sci. 2021, 11, 632420. [Google Scholar] [CrossRef]
- Zhong, H.; Zhang, H.; Guo, R.; Wang, Q.; Huang, X.; Liao, J.; Li, Y.; Huang, Y.; Wang, Z. Characterization and Functional Divergence of a Novel DUF668 Gene Family in Rice Based on Comprehensive Expression Patterns. Genes 2019, 10, 980. [Google Scholar] [CrossRef]
- Jayaraman, K.; Sevanthi, A.M.; Raman, K.V.; Jiwani, G.; Solanke, A.U.; Mandal, P.K.; Mohapatra, T. Overexpression of a DUF740 Family Gene (LOC_Os04g59420) Imparts Enhanced Climate Resilience through Multiple Stress Tolerance in Rice. Front. Plant Sci. 2023, 13, 947312. [Google Scholar] [CrossRef]
- Leng, Z.-X.; Liu, Y.; Chen, Z.-Y.; Guo, J.; Chen, J.; Zhou, Y.-B.; Chen, M.; Ma, Y.-Z.; Xu, Z.-S.; Cui, X.-Y. Genome-Wide Analysis of the DUF4228 Family in Soybean and Functional Identification of GmDUF4228–70 in Response to Drought and Salt Stresses. Front. Plant Sci. 2021, 12, 628299. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Zhang, Y.; Yang, Y.; Qiao, Y.; Qian, Y.; Wang, J.; Wang, X.; Kang, Z.; Liu, J. The Puccinia striiformis Effector Pst11215 Manipulates Mitochondria to Suppress Host Immunity by Promoting TaVDIP1-mediated Ubiquitination of TaVDAC1. New Phytol. 2024, 244, 1961–1978. [Google Scholar] [CrossRef]
- Shang, S.; He, Y.; Hu, Q.; Fang, Y.; Cheng, S.; Zhang, C. Fusarium graminearum Effector FgEC1 Targets Wheat TaGF14b Protein to Suppress TaRBOHD-mediated ROS Production and Promote Infection. J. Integr. Plant Biol. 2024, 66, 2288–2303. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.; Qin, Y.; Du, Y.; Song, C.; Kang, Z.; Guo, J.; Guo, J. Stripe Rust Effector Pst03724 Modulates Host Immunity by Inhibiting NAD Kinase Activation by a Calmodulin. Plant Physiol. 2024, 195, 1624–1641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Du, X.; Zhou, X.; Jin, D.; Miao, J.; Liu, X. An FYVE-Domain-Containing Protein, PsFP1, Is Involved in Vegetative Growth, Oxidative Stress Response and Virulence of Phytophthora sojae. Int. J. Mol. Sci. 2021, 22, 6601. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Wang, W.; Shen, J.; Zhang, J.; Zhang, X.; Liu, X. A Patched-like Protein PsPTL Is Not Essential for the Growth and Response to Various Stresses in Phytophthora sojae. Front. Microbiol. 2021, 12, 673784. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhu, H.; Chen, J.; Ru, B.; Peng, Q.; Miao, J.; Liu, X. PsAF5 Functions as an Essential Adapter for PsPHB2-Mediated Mitophagy under ROS Stress in Phytophthora sojae. Nat. Commun. 2024, 15, 1967. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhai, C.; Hua, C.; Qiu, M.; Hao, Y.; Nie, P.; Ye, W.; Wang, Y. PsHint1, Associated with the G-Protein α Subunit PsGPA1, Is Required for the Chemotaxis and Pathogenicity of Phytophthora sojae: A Gα-Binding Protein Is Required for Chemotaxis. Mol. Plant Pathol. 2016, 17, 272–285. [Google Scholar] [CrossRef]
- Qiu, M.; Li, Y.; Zhang, X.; Xuan, M.; Zhang, B.; Ye, W.; Zheng, X.; Govers, F.; Wang, Y. G Protein α Subunit Suppresses Sporangium Formation through a Serine/Threonine Protein Kinase in Phytophthora sojae. PLOS Pathog. 2020, 16, e1008138. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Yang, X.; Dong, S.; Sheng, Y.; Wang, Y.; Zheng, X. Transient Silencing Mediated by in Vitro Synthesized Double-Stranded RNA Indicates That PsCdc14 Is Required for Sporangial Development in a Soybean Root Rot Pathogen. Sci. China Life Sci. 2011, 54, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lu, J.; Tao, K.; Ye, W.; Li, A.; Liu, X.; Kong, L.; Dong, S.; Zheng, X.; Wang, Y. A Myb Transcription Factor of Phytophthora sojae, Regulated by MAP Kinase PsSAK1, Is Required for Zoospore Development. PLoS ONE 2012, 7, e40246. [Google Scholar] [CrossRef] [PubMed]
- Biemans-Oldehinkel, E.; Doeven, M.K.; Poolman, B. ABC Transporter Architecture and Regulatory Roles of Accessory Domains. FEBS Lett. 2006, 580, 1023–1035. [Google Scholar] [CrossRef]
- Higgins, C.F. ABC Transporters: From Microorganisms to Man. Annu. Rev. Cell Biol. 1992, 8, 67–113. [Google Scholar] [CrossRef] [PubMed]
- Ciechanover, A. Proteolysis: From the Lysosome to Ubiquitin and the Proteasome. Nat. Rev. Mol. Cell Biol. 2005, 6, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Kouzarides, T. Chromatin Modifications and Their Function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef]
- Mattick, J.S.; Makunin, I.V. Non-Coding RNA. Hum. Mol. Genet. 2006, 15, R17–R29. [Google Scholar] [CrossRef]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug Resistance in Cancer: Role of ATP–Dependent Transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K. Characterization of Berberine Transport into Coptis Japonica Cells and the Involvement of ABC Protein. J. Exp. Bot. 2002, 53, 1879–1886. [Google Scholar] [CrossRef]
- Geisler, M.; Murphy, A.S. The ABC of Auxin Transport: The Role of P-glycoproteins in Plant Development. FEBS Lett. 2006, 580, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-P.; Li, Z.-S.; Drozdowicz, Y.M.; Hörtensteiner, S.; Martinoia, E.; Rea, P.A. AtMRP2, an Arabidopsis ATP Binding Cassette Transporter Able to Transport Glutathione S-Conjugates and Chlorophyll Catabolites: Functional Comparisons with AtMRP1. Plant Cell 1998, 10, 267–282. [Google Scholar] [CrossRef]
- Tusnády, G.E.; Sarkadi, B.; Simon, I.; Váradi, A. Membrane Topology of Human ABC Proteins. FEBS Lett. 2006, 580, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Rust, S.; Rosier, M.; Funke, H.; Real, J.; Amoura, Z.; Piette, J.-C.; Deleuze, J.-F.; Brewer, H.B.; Duverger, N.; Denèfle, P.; et al. Tangier Disease Is Caused by Mutations in the Gene Encoding ATP-Binding Cassette Transporter 1. Nat. Genet. 1999, 22, 352–355. [Google Scholar] [CrossRef]
- Shulenin, S.; Nogee, L.M.; Annilo, T.; Wert, S.E.; Whitsett, J.A.; Dean, M. ABCA3 Gene Mutations in Newborns with Fatal Surfactant Deficiency. N. Engl. J. Med. 2004, 350, 1296–1303. [Google Scholar] [CrossRef] [PubMed]
- Berge, K.E.; Tian, H.; Graf, G.A.; Yu, L.; Grishin, N.V.; Schultz, J.; Kwiterovich, P.; Shan, B.; Barnes, R.; Hobbs, H.H. Accumulation of Dietary Cholesterol in Sitosterolemia Caused by Mutations in Adjacent ABC Transporters. Science 2000, 290, 1771–1775. [Google Scholar] [CrossRef]
- Kennedy, M.A.; Barrera, G.C.; Nakamura, K.; Baldán, Á.; Tarr, P.; Fishbein, M.C.; Frank, J.; Francone, O.L.; Edwards, P.A. ABCG1 Has a Critical Role in Mediating Cholesterol Efflux to HDL and Preventing Cellular Lipid Accumulation. Cell Metab. 2005, 1, 121–131. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, X.; Zeng, Y.; Li, Y.; Peng, Q.; Miao, J.; Liu, X. A Protein with Unknown Function, Ps495620, Is Critical for the Sporulation and Oospore Production of Phytophthora sojae. J. Fungi 2025, 11, 12. https://doi.org/10.3390/jof11010012
Du X, Zeng Y, Li Y, Peng Q, Miao J, Liu X. A Protein with Unknown Function, Ps495620, Is Critical for the Sporulation and Oospore Production of Phytophthora sojae. Journal of Fungi. 2025; 11(1):12. https://doi.org/10.3390/jof11010012
Chicago/Turabian StyleDu, Xiaoran, Yan Zeng, Yiying Li, Qin Peng, Jianqiang Miao, and Xili Liu. 2025. "A Protein with Unknown Function, Ps495620, Is Critical for the Sporulation and Oospore Production of Phytophthora sojae" Journal of Fungi 11, no. 1: 12. https://doi.org/10.3390/jof11010012
APA StyleDu, X., Zeng, Y., Li, Y., Peng, Q., Miao, J., & Liu, X. (2025). A Protein with Unknown Function, Ps495620, Is Critical for the Sporulation and Oospore Production of Phytophthora sojae. Journal of Fungi, 11(1), 12. https://doi.org/10.3390/jof11010012





