Chemical Composition, Antioxidant and Anti-Inflammatory Activity of Shiitake Mushrooms (Lentinus edodes)
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrument and Materials
2.2. Reagents and Chemicals
2.3. Fungal Material
2.4. Large-Scale Fermentation of Strain LE-M
2.5. The Isolation of Chemical Components
2.6. Antioxidant Activity Assays
2.7. Measurement of Nitrite Production and TNF-α
2.8. MTT Assay
3. Results
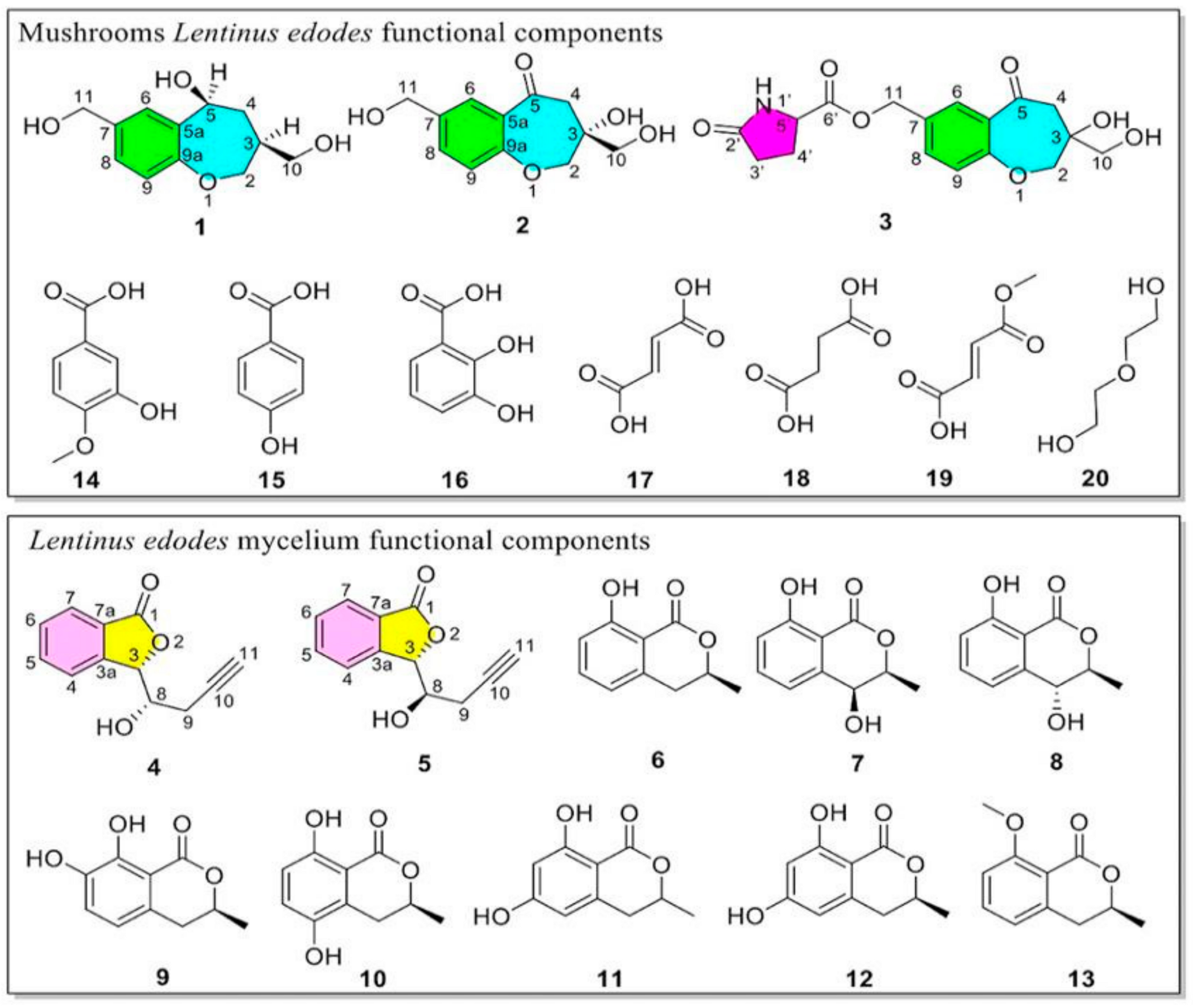
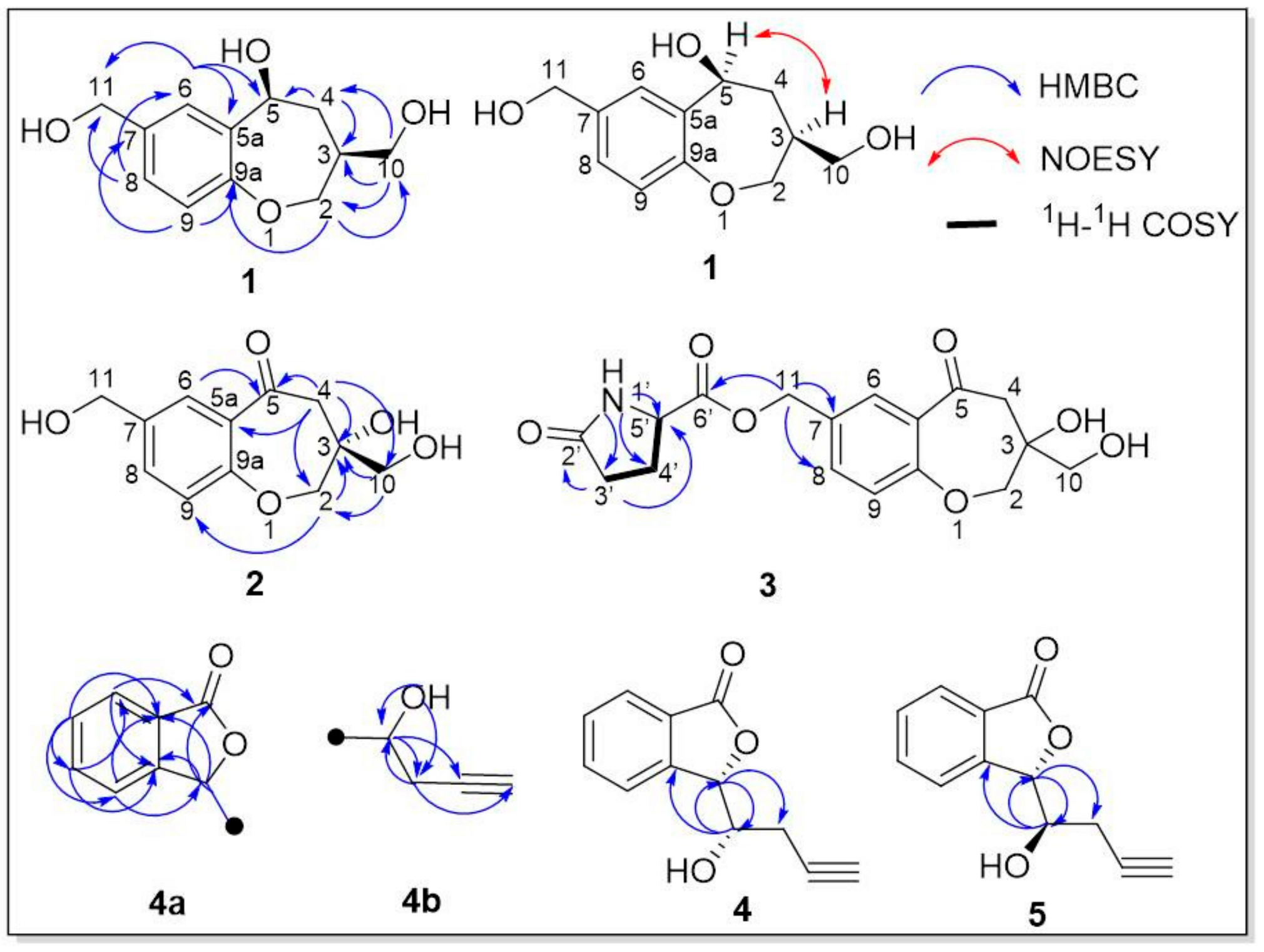
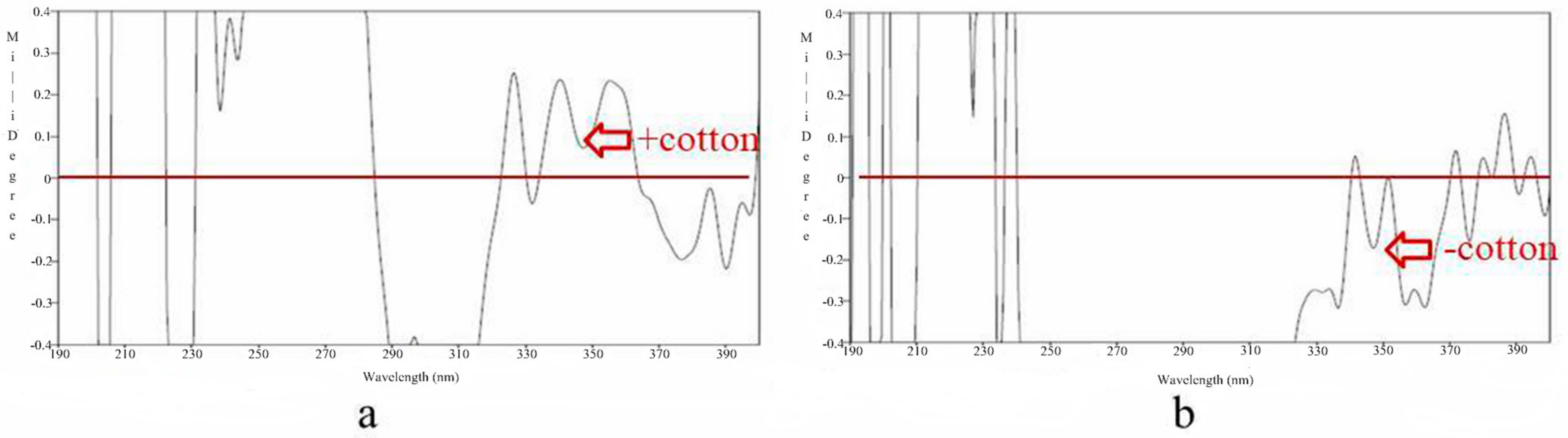
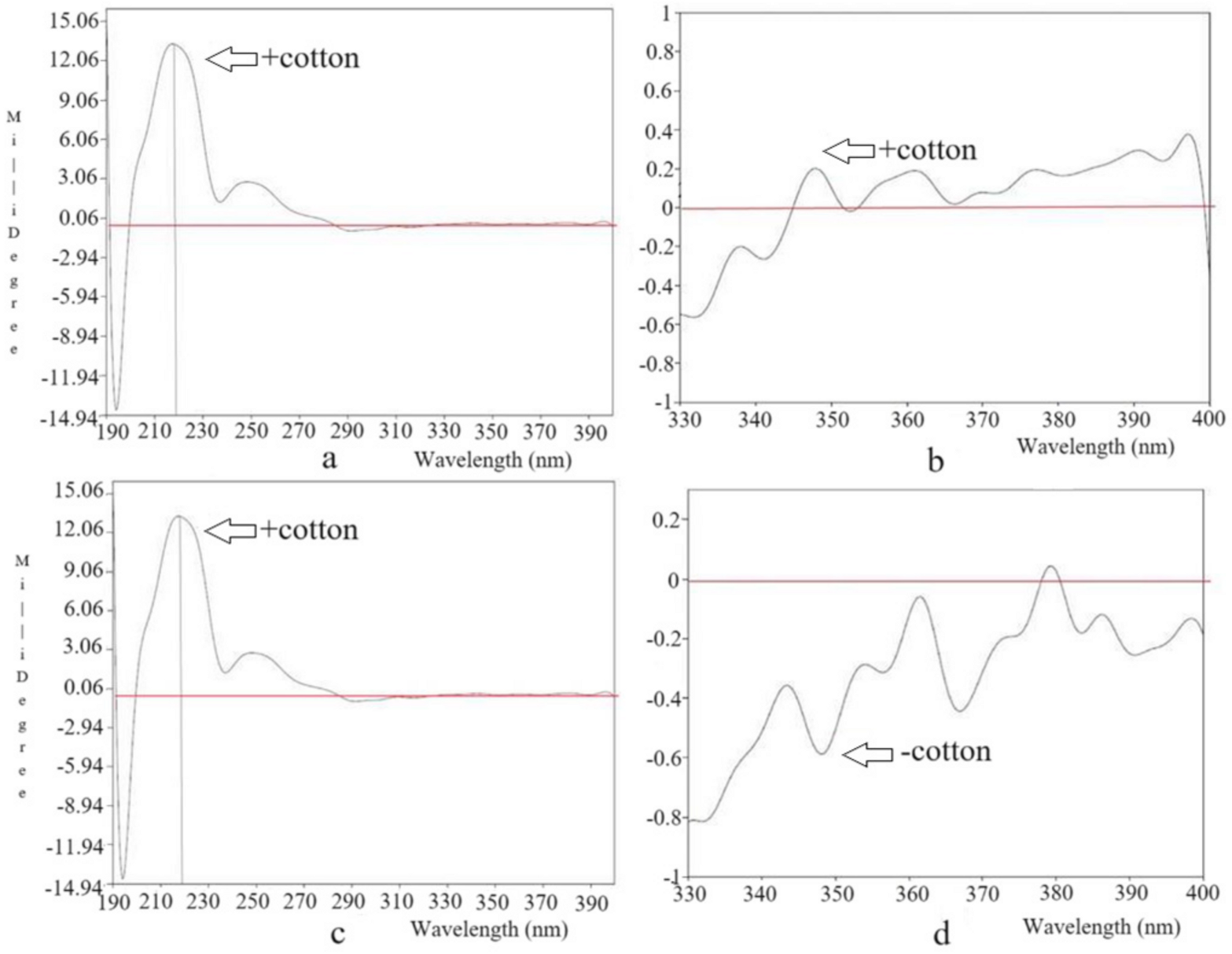
3.1. Structure Elucidation of Components
3.2. Antioxidant and TNF-α and NO Inhibitory Activities of the Components
3.3. Chemical Composition Comparison
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, X.; Shi, Y.; Shi, D.; Tu, Y.; Liu, L. Biological Activities of Secondary Metabolites from the Edible-Medicinal Macrofungi. J. Fungi 2024, 10, 144. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Li, H.; Liu, Y.; Li, C.; Fang, Z.; Hu, B.; Li, X.; Zeng, Z.; Liu, Y. Changes in flavor and biological activities of Lentinula edodes hydrolysates after Maillard reaction. Food Chem. 2024, 431, 137138. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Kou, X.; Liu, C.; Wang, Y.; Yu, Y.; Ma, J.; Liu, Y.; Xue, Z. Effect of nanopackaging on the quality of edible mushrooms and its action mechanism: A review. Food Chem. 2023, 407, 135099. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.L. Edible and Medicinal Macrofungi. J. Fungi 2023, 9, 908. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Cui, X.; Song, Z.; Kong, W.; Kang, Y.; Kong, W.; Ng, T.B. Coating shiitake mushrooms (Lentinus edodes) with a polysaccharide from Oudemansiella radicata improves product quality and flavor during postharvest storage. Food Chem. 2021, 352, 129357. [Google Scholar] [CrossRef] [PubMed]
- Łysakowska, P.; Sobota, A.; Wirkijowska, A. Medicinal Mushrooms: Their Bioactive Components, Nutritional Value and Application in Functional Food Production-A Review. Molecules 2023, 28, 5393. [Google Scholar] [CrossRef] [PubMed]
- Sheng, K.; Wang, C.; Chen, B.; Kang, M.; Wang, M.; Liu, K.; Wang, M. Recent advances in polysaccharides from Lentinus edodes (Berk.): Isolation, structures and bioactivities. Food Chem. 2021, 358, 129883. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Lai, H.; Liang, J.; Cheng, L.; He, L.; Wang, H.; Teng, Q.; Cai, W.; Wang, R.; Zhu, L.; et al. Optimization Co-Culture of Monascus purpureus and Saccharomyces cerevisiae on Selenium-Enriched Lentinus edodes for Increased Monacolin K Production. J. Fungi 2024, 10, 503. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Ganesan, K.; Xu, B. Unlocking the Power: New Insights into the Anti-Aging Properties of Mushrooms. J. Fungi 2024, 10, 215. [Google Scholar] [CrossRef]
- Yu, C.; Dong, Q.; Chen, M.; Zhao, R.; Zha, L.; Zhao, Y.; Zhang, M.; Zhang, B.; Ma, A. The Effect of Mushroom Dietary Fiber on the Gut Microbiota and Related Health Benefits: A Review. J. Fungi 2023, 9, 1028. [Google Scholar] [CrossRef]
- Hu, X.; Cheng, D.; Zhang, Y.; Li, P.; Wu, X.; Fu, J. Fermented Lentinus edodes extract containing α-glucan ameliorates concanavalin A-induced autoimmune hepatitis in mice. Food Sci. Hum. Wellness 2024, 13, 2102–2115. [Google Scholar] [CrossRef]
- Sayari, M.; Pradeep, S.N. Extraction of chitin-glucan complex from shiitake (Lentinula edodes) fruiting bodies using natural deep eutectic solvents and its prebiotic potentialInt. J. Biol. Macromol. 2024, 273, 133046. [Google Scholar] [CrossRef]
- Gyun, S.P.; Juhyun, S.; Seongwoo, H.; Judy, G.; Jae-Wook, O. Anticarcinogenic potential of the mushroom polysaccharide lentinan on gastric and colon cancer cells: Antiproliferative, antitumorigenic, Mu-2-related death-inducing gene, MUDENG ramifications. J. Ind. Eng. Chem. 2024, 135, 122–130. [Google Scholar] [CrossRef]
- Barcan, A.S.; Barcan, R.A.; Vamanu, E. Therapeutic Potential of Fungal Polysaccharides in Gut Microbiota Regulation: Implications for Diabetes, Neurodegeneration, and Oncology. J. Fungi 2024, 10, 394. [Google Scholar] [CrossRef] [PubMed]
- Masashi, M.; Ken-ichiro, M. Anti-inflammatory and immunomodulatory properties of polysaccharides in mushrooms. Curr. Opin. Biotech. 2024, 86, 103076. [Google Scholar] [CrossRef]
- Sánchez, C. Reactive oxygen species and antioxidant properties from mushrooms. Synth. Syst. Biotechnol. 2016, 24, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Berger, R.G.; Bordewick, S.; Krahe, N.-K.; Ersoy, F. Mycelium vs. Fruiting Bodies of Edible Fungi-A Comparison of Metabolites. Microorganisms 2022, 10, 1379. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, L.-H.; Tong, L.-L.; Yuan, L.; Ren, B.; Guo, D.-S. Comparative metabolic profiling of mycelia, fermentation broth, spore powder and fruiting bodies of Ophiocordyceps gracilis by LC–MS/MS. Phytochem. Anal. 2023, 34, 984–996. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhao, S.; Li, Z.; Pan, J.; Zhou, Y.; Hu, Q.; Zou, Y. Identification of altered metabolic functional components using metabolomics to analyze the different ages of fruiting bodies of Sanghuangporus vaninii cultivated on cut log substrates. Front. Nutr. 2023, 10, 1197998. [Google Scholar] [CrossRef]
- Xu, X.; Dong, Y.; Yang, J.; Wang, L.; Ma, L.; Song, F.; Ma, X. Secondary Metabolites from Marine-Derived Fungus Penicillium rubens BTBU20213035. J. Fungi 2024, 10, 424. [Google Scholar] [CrossRef]
- Jiyoon, P.; Jiseong, K.; Sunghoon, H.; Daehyun, O.; Young, E.; Sang-Jip, N.; Hyeung-geun, P.; Min, J.L.; Dong-Chan, O. Sadopeptins A and B, Sulfoxide- and Piperidone-Containing Cyclic Heptapeptides with Proteasome Inhibitory Activity from a Streptomyces sp. J. Nat. Prod. 2023, 86, 612–620. [Google Scholar] [CrossRef]
- Abulaizi, A.; Wang, R.; Xiong, Z.; Zhang, S.; Li, Y.; Ge, H.; Guo, Z. Secondary Metabolites with Agricultural Antagonistic Potential from Aspergillus sp. ITBBc1, a Coral-Associated Marine Fungus. Mar. Drugs 2024, 22, 270. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.Z.; Alhebsi, M.S.R.; Ghnimi, S.; Kamal-Eldin, A. Inability of total antioxidant activity assays to accurately assess the phenolic compounds of date palm fruit (Phoenix dactylifera L.). NFS J. 2021, 22, 32–40. [Google Scholar] [CrossRef]
- Luo, W.; Tweedie, D.; Beedie, S.L.; Vargesson, N.; Figg, W.D.; Greig, N.H.; Scerba, M.T. Design, synthesis and biological assessment of N-adamantyl, substituted adamantyl and noradamantyl phthalimidines for nitrite, TNF-α and angiogenesis inhibitory activities. Bioorg. Med. Chem. 2018, 26, 1547–1559. [Google Scholar] [CrossRef] [PubMed]
- Ayub, S.; Verma, J.; Das, N. Effect of endosulfan and malathion on lipid peroxidation, nitrite and TNF-α release by rat peritoneal macrophages. Int. Immunopharmacol. 2003, 3, 1819–1828. [Google Scholar] [CrossRef] [PubMed]
- Reveglia, P.; Masi, M.; Evidente, A. Melleins—Intriguing Natural Compounds. Biomolecules 2020, 10, 772. [Google Scholar] [CrossRef] [PubMed]
- Naman, D.; Mireille, F.; Laurent, D.; Basil, D.; Sameer, A.S.M. In silico studies on Epicoccum spp. Secondary metabolites as potential drugs for mucormycosis. Results Chem. 2024, 7, 101420. [Google Scholar] [CrossRef]
- Lazić, V.; Klaus, A.; Kozarski, M.; Doroški, A.; Tosti, T.; Simić, S.; Vunduk, J. The Effect of Green Extraction Technologies on the Chemical Composition of Medicinal Chaga Mushroom Extracts. J. Fungi 2024, 10, 225. [Google Scholar] [CrossRef] [PubMed]
- Baptista, F.; Campos, J.; Costa-Silva, V.; Pinto, A.R.; Saavedra, M.J.; Ferreira, L.M.; Rodrigues, M.; Barros, A.N. Nutraceutical Potential of Lentinula edodes’ Spent Mushroom Substrate: A Comprehensive Study on Phenolic Composition, Antioxidant Activity, and Antibacterial Effects. J. Fungi 2023, 9, 1200. [Google Scholar] [CrossRef]
- Wu, F.; Wang, H.; Chen, Q.; Pang, X.; Jing, H.; Yin, L.; Zhang, X. Lignin Promotes Mycelial Growth and Accumulation of Polyphenols and Ergosterol in Lentinula edodes. J. Fungi 2023, 9, 237. [Google Scholar] [CrossRef]
- Ma, Z.L.; Yu, Z.P.; Zheng, Y.Y.; Han, N.; Zhang, Y.H.; Song, S.Y.; Mao, J.Q.; Li, J.J.; Yao, G.S.; Wang, C.Y. Bioactive Alpha-Pyrone and Phenolic Glucosides from the Marine-Derived Metarhizium sp. P2100. J. Fungi 2023, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Frelek, J.; Szczepek, W.J. [Rh2(OCOCF3)4] as an auxiliary chromophore in chiroptical studies on steroidal alcohols. Tetrahe Dron Asymmetry 1999, 10, 1507–1520. [Google Scholar] [CrossRef]
- Frelek, J.; Jagodziński, J.; Meyer-Figge, H. Chiroptical properties of binuclear rhodium com-plexes of lanostane alcohols. Chirality 2001, 13, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Liu, S.; Guo, L. Antifungal metabolites from the plant endophytic fungus Pestalotiopsis foedan. J. Nat. Prod. 2008, 71, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Desisa, B.; Muleta, D.; Dejene, T.; Jida, M.; Goshu, A.; Martin-Pinto, P. Substrate Optimization for Shiitake (Lentinula edodes (Berk.) Pegler) Mushroom Production in Ethiopia. J. Fungi 2023, 9, 811. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.X.; Shi, L.E.; Jiang, Z.B.; Bai, X.L.; Ying, R.F. Calcium Enrichment in Edible Mushrooms: A Review. J. Fungi 2023, 9, 338. [Google Scholar] [CrossRef] [PubMed]
- Amara, A.A.; El-Baky, N.A. Fungi as a Source of Edible Proteins and Animal Feed. J. Fungi 2023, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, F.; Chopra, H.; Baig, A.A.; Avula, S.K.; Kumari, S.; Mohanta, T.K.; Saravanan, M.; Mishra, A.K.; Sharma, N.; Mohanta, Y.K. Edible Mushrooms as Novel Myco-Therapeutics: Effects on Lipid Level, Obesity and BMI. J. Fungi 2022, 8, 211. [Google Scholar] [CrossRef] [PubMed]
- Al-Obaidi, J.R.; Jambari, N.N.; Ahmad-Kamil, E.I. Mycopharmaceuticals and Nutraceuticals: Promising Agents to Improve Human Well-Being and Life Quality. J. Fungi 2021, 7, 503. [Google Scholar] [CrossRef]
- Niego, A.G.; Rapior, S.; Thongklang, N.; Raspé, O.; Jaidee, W.; Lumyong, S.; Hyde, K.D. Macrofungi as a Nutraceutical Source: Promising Bioactive Compounds and Market Value. J. Fungi 2021, 7, 397. [Google Scholar] [CrossRef]
| NO | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δc | δH (m.; J in Hz) | δc | δH (m.; J in Hz) | δc | δH (m.; J in Hz) | |
| 1 | - | - | - | - | - | - |
| 2 | 75.0 | 3.17 (1H, d, 11.0) 4.31 (1H, dd, 11.0, 3.8) | 80.7 | 4.17 (1H, d, 12.0) 3.84 (1H, d, 12.0) | 80.1 | 4.19 (1H, m) 3.87 (1H, d, 12.0) |
| 3 | 42.5 | 2.15 (1H, m) | 76.7 | - | 76.7 | - |
| 4 | 39.6 | 2.02 (1H, d, 12.0) 1.28 (1H, q, 12.0) | 52.5 | 2.92 (1H, d, 12.0) 2.76 (1H, d, 12.0) | 52.5 | 2.93 (1H, d, 12.0) 2.77 (1H, d, 12.0) |
| 5 | 68.7 | 4.72 (1H, d, 11.0) | 196.6 | - | 196.3 | - |
| 5a | 138.7 | - | 127.6 | - | 127.9 | - |
| 6 | 124.7 | 7.48 (1H, s) | 127.3 | 7.62 (1H, d, 2.3) | 129.6 | 7.68 (1H, d, 2.3) |
| 7 | 137.6 | - | 137.3 | - | 130.5 | - |
| 8 | 125.9 | 7.06 (1H, dd, 8.5, 2.0) | 132.7 | 7.40 (1H, dd, 8.5, 2.3) | 134.4 | 7.50 (1H, dd, 8.3, 2.3) |
| 9 | 120.5 | 6.85 (1H, d, 8.1) | 120.7 | 7.05 (1H, d, 8.5) | 121.2 | 7.12 (1H, d, 8.3) |
| 9a | 156.5 | - | 162.3 | - | 163.2 | - |
| 10 | 62.8 | 3.31 (1H, m) 3.23 (1H, m) | 67.1 | 3.37 (2H, m) | 67.0 | 3.40 (2H, m) |
| 11 | 63.4 | 4.43 (2H, s) | 62.5 | 4.44 (2H, s) | 66.0 | 5.11 (2H, d, 12.0) |
| 2′ | 177.4 | - | ||||
| 3′ | 29.3 | 2.10 (2H, m) | ||||
| 4′ | 25.0 | 2.33 (1H, m) 1.95 (1H, m) | ||||
| 5′ | 55.2 | 4.20 (1H, m) | ||||
| 6′ | 173.2 | - | ||||
| 4 | 5 | |||
|---|---|---|---|---|
| NO | δC | δH (m.; J in Hz) | δC | δH (m.; J in Hz) |
| 1 | 170.4 | - | 170.1 | - |
| 2 | - | - | - | - |
| 3 | 82.4 | 5.67 (1H, d, 2.0) | 82.4 | 5.77 (1H, d, 5.6) |
| 4 | 123.3 | 7.72 (1H, d, 6.9) | 124.3 | 7.73 (1H, d, 6.9) |
| 5 | 134.4 | 7.77(1H, td, 7.1, 1.0) | 134.4 | 7.77 (1H, td, 7.1, 1.0) |
| 6 | 129.5 | 7.58 (1H, t, 7.6) | 129.7 | 7.61 (1H, t, 7.6) |
| 7 | 125.0 | 7.81 (1H, d, 7.6) | 125.2 | 7.83 (1H, d, 7.6) |
| 3a | 148.3 | - | 147.6 | - |
| 7a | 126.6 | - | 126.4 | - |
| 8 | 69.3 | 4.19 (1H, qd, 7.1, 2.0) | 70.1 | 3.91 (1H, m, 11.6, 5.6) |
| 9 | 24.2 | 2.48 (2H, m) | 23.8 | 2.48 (2H, m) |
| 10 | 81.6 | - | 81.3 | - |
| 11 | 73.6 | 2.95 (1H, t, 2.8) | 73.6 | 2.89 (1H, t, 2.8) |
| 8-OH | - | 5.28 (1H, d, 6.4) | - | 5.53 (1H, d, 5.6) |
| Compounds | DPPH (IC50, μg/mL) |
|---|---|
| 4 | 54.3 ± 1.8 |
| 7 | 78.9 ± 1.7 |
| 8 | 69.5 ± 0.9 |
| 9 | 54.3 ± 1.8 |
| 10 | 48.6 ± 1.2 |
| 11 | 25.2 ± 0.5 |
| 12 | 54.3 ± 1.8 |
| 14 | 52.8 ± 1.4 |
| 15 | 48.4 ± 2.1 |
| 16 | 34.7 ± 2.5 |
| 17 | 46.2 ± 0.9 |
| 18 | >100 |
| 19 | >100 |
| 20 | 98.7 ± 3.6 |
| Trolox | 10.8 ± 0.5 |
| Compounds | NO Inhibitory Assay (IC50) μM | TNF-α Inhibitory Assay (IC50) μM |
|---|---|---|
| 4 | 34.7 ± 2.5 | >100 |
| 7 | 48.6 ± 3.4 * | >100 |
| 8 | 52.1 ± 3.6 * | >100 |
| 9 | 30.2 ± 2.5 | 94.5 ± 1.6 |
| 10 | 35.8 ± 1.7 | 75.3 ± 2.7 * |
| 11 | 28.1 ± 2.2 * | 68.1 ± 1.8 * |
| 12 | 58.4 ± 1.9 * | >100 |
| 14 | 83.1 ± 3.1 ** | 98.4 ± 1.3 |
| 15 | 79.8 ± 2.8 ** | - |
| 16 | 45.7 ± 1.2 * | 89.3 ± 1.8 |
| 17 | >100 | >100 |
| 18 | 29.9 ± 1.2 | >100 |
| 19 | 89.6 ± 1.7 ** | - |
| 20 | 91.8 ± 2.3 ** | - |
| Indomethacin | 26.8 ± 1.3 | 88.5 ± 2.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Yu, C.; Liu, Z.; Cui, X.; Guo, X.; Wang, H. Chemical Composition, Antioxidant and Anti-Inflammatory Activity of Shiitake Mushrooms (Lentinus edodes). J. Fungi 2024, 10, 552. https://doi.org/10.3390/jof10080552
Xu X, Yu C, Liu Z, Cui X, Guo X, Wang H. Chemical Composition, Antioxidant and Anti-Inflammatory Activity of Shiitake Mushrooms (Lentinus edodes). Journal of Fungi. 2024; 10(8):552. https://doi.org/10.3390/jof10080552
Chicago/Turabian StyleXu, Xiaoming, Chong Yu, Zhenyang Liu, Xiaohang Cui, Xiaohe Guo, and Haifeng Wang. 2024. "Chemical Composition, Antioxidant and Anti-Inflammatory Activity of Shiitake Mushrooms (Lentinus edodes)" Journal of Fungi 10, no. 8: 552. https://doi.org/10.3390/jof10080552
APA StyleXu, X., Yu, C., Liu, Z., Cui, X., Guo, X., & Wang, H. (2024). Chemical Composition, Antioxidant and Anti-Inflammatory Activity of Shiitake Mushrooms (Lentinus edodes). Journal of Fungi, 10(8), 552. https://doi.org/10.3390/jof10080552






