Identification of Secondary Metabolites by UHPLC-ESI-HRMS/MS in Antifungal Strain Trichoderma harzianum (LBAT-53)
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains
2.2. Fermentation Process
2.3. Antifungal In Vitro Assay
2.4. Extraction of Secondary Metabolites
2.5. Chemical Screening
2.6. Cleanup of the Sample
2.7. UHPLC-ESI-HRMS/MS Conditions and Data Analysis
2.8. Statistical Analysis
3. Results
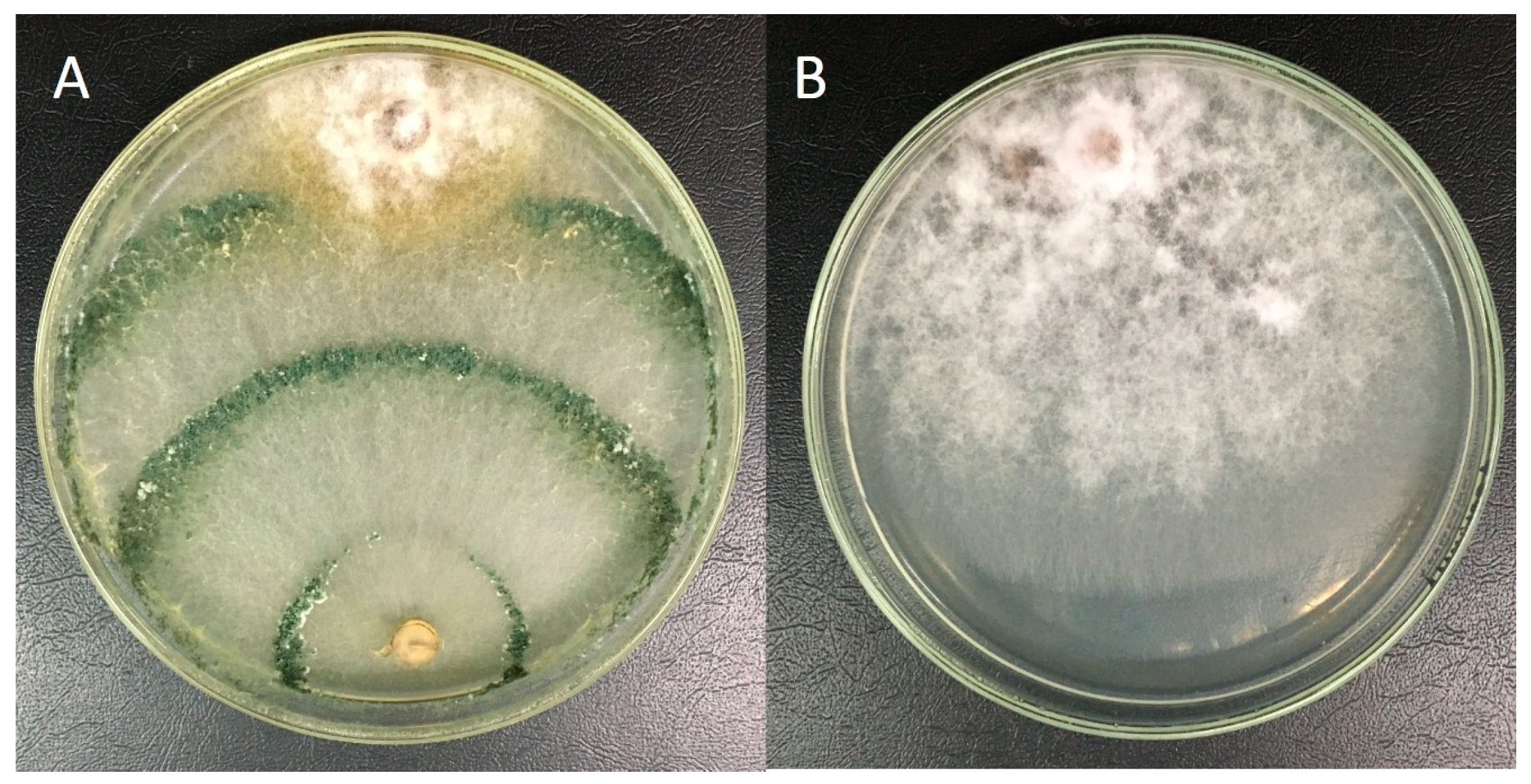
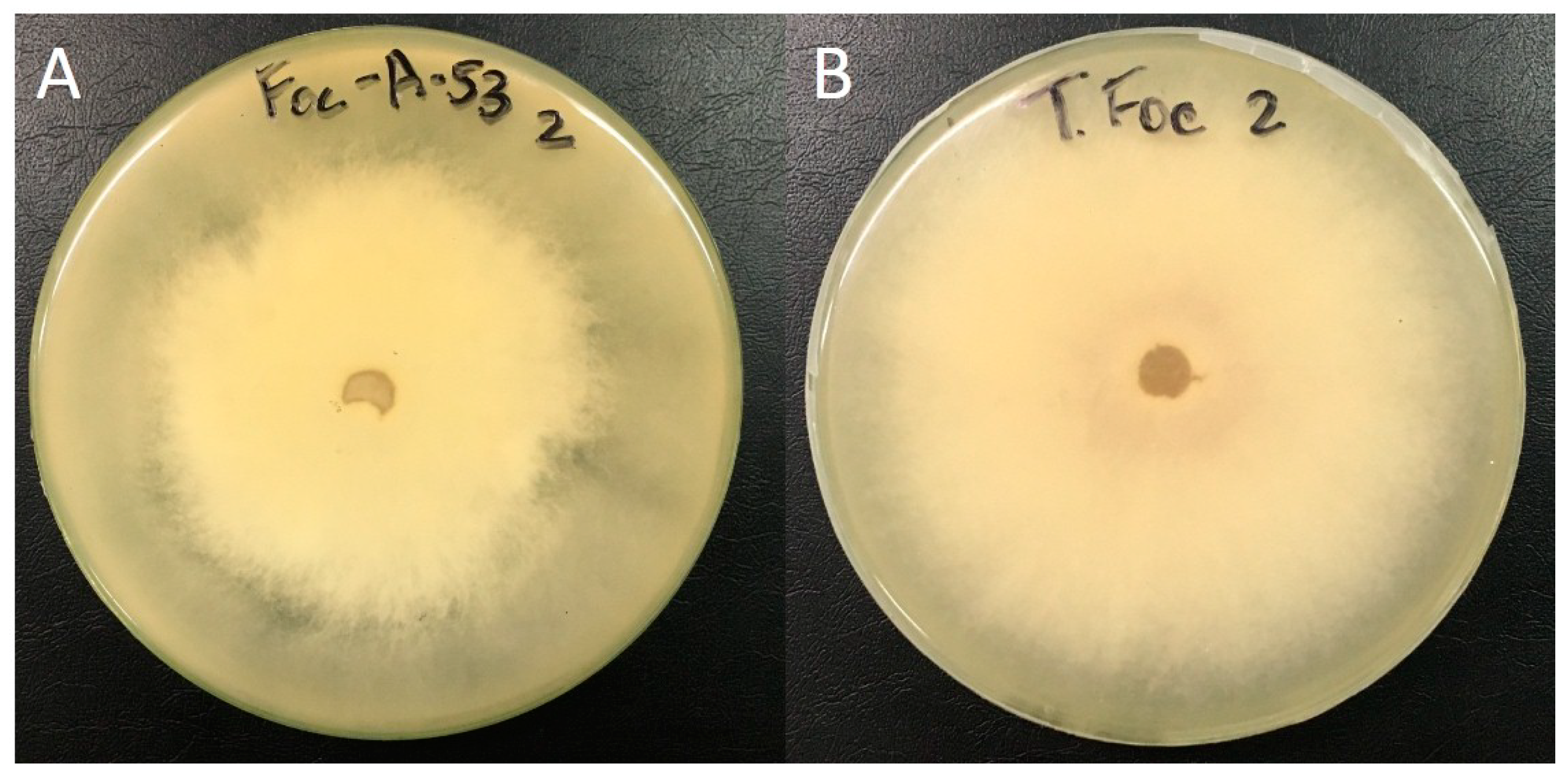
3.1. Antifungal In Vitro Assay
3.2. Chemical Screening
3.3. UHPLC-ESI-MS/MS
3.3.1. Anthraquinone Identification
3.3.2. Other Phenolic Compounds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO Global Agriculture towards 2050. Available online: https://www.fao.org/fileadmin/templates/wsfs/docs/Issues_papers/HLEF2050_Global_Agriculture.pdf (accessed on 14 November 2023).
- González, E.; Rodríguez, S. ‘INIVIT PB-12’. Nuevo cultivar de plátano (Musa spp.) tipo burro en entidades productivas del país. Agric. Trop. 2018, 4, 120–126. [Google Scholar]
- Fusarium Wilt. 2019. Available online: https://fusariumwilt.org/index.php/es/el-problema/ (accessed on 24 January 2024).
- Niwas, R.; Chand, G.; Nath Gupta, R. Fusarium Wilt: A Destructive Disease of Banana and Their Sustainable Management. In Fusarium: An Overview of the Genus; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Rupp, S.; Weber, R.W.; Rieger, D.; Detzel, P.; Hahn, M. Spread of Botrytis cinerea Strains with Multiple Fungicide Resistance in German Horticulture. Front. Microbiol. 2017, 7, 2075. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, S.; García, C.; Santamaria, O. Metabolites Produced by Fungi against Fungal Phytopathogens: Review, Implementation and Perspectives. Plants 2022, 11, 81. [Google Scholar] [CrossRef] [PubMed]
- Bubici, G.; Kaushal, M.; Prigigallo, M.I.; Gomez-Lama Cabanás, C.; Mercado-Blanco, J. Biological control agents against Fusarium Wilt of banana. Front. Microbiol. 2019, 10, 616. [Google Scholar] [CrossRef] [PubMed]
- Zin, N.A.; Badaluddin, N.A. Biological functions of Trichoderma spp. for agriculture applications. Ann. Agric. Sci. 2020, 65, 168–178. [Google Scholar] [CrossRef]
- Bhandari, S.; Pandey, K.R.; Joshi, Y.R.; Lamichhane, S.K. An overview of multifaceted role of Trichoderma spp. for sustainable agriculture. Arch. Agric. Environ. Sci. 2021, 6, 72–79. [Google Scholar] [CrossRef]
- Sood, M.; Kapoor, D.; Kumar, V.; Sheteiwy, M.D.; Ramakrishnan, M.; Landi, M.; Araniti, F.; Sharma, A. Trichoderma: The “Secrets” of a Multitalented Biocontrol Agent. Plants 2020, 9, 762. [Google Scholar] [CrossRef] [PubMed]
- Küçük, C.; Kivanc, M. Isolation of Trichoderma spp. and Determination of Their Antifungal, Biochemical and Physiological Features. Turk. J. Biol. 2003, 27, 247–253. [Google Scholar]
- Infante, D.; González, N.; Reyes, Y. Mecanismos deacción de Trichoderma frente a hongos fitopatógenos. Rev. Prot. Veg. 2009, 24, 14–21. [Google Scholar]
- Sánchez-García, B.M.; Espinosa Huerta, E.; Villordo-Pineda, E.; Rodríguez-Guerra, R.; Mora Avilés, M.A. Identificación molecular y evaluación antagónica in vitro de cepas nativas de Trichoderma spp. sobre hongos fitopatógenos de raíz en frijol (Phaseolus vulgaris L.) cv. Montcalm. Agrociencia 2017, 51, 63–79. [Google Scholar]
- Sridharan, A.P.; Sugitha, T.; Karthikeyan, G.; Sivakumar, U. Comprehensive profiling of the VOCs of Trichoderma longibrachiatum EF5 while interacting with Sclerotium rolfsii and Macrophomina phaseolina. Microbiol. Res. 2020, 236, 126436–126449. [Google Scholar] [CrossRef]
- Gutiérrez-Rivas, R.E. Evaluación In Vitro e In Vivo de la Capacidad Antagónica de Trichoderma harzianum para el Control de Rhizoctonia spp. Bachelor’s Thesis, Universidad Nacional de Tumbes, Tumbes, Peru, 2022. [Google Scholar]
- Ttacca, B.L.; Yataco, R.J.Y.; Farfán, A.A.; Calderón, L.M.; Cantoral, J.C.A. Antibiosis y micoparasitismo de hongos endófitos sobre el agente causal del moho gris del arándano (Botrytis cinerea). Bioagro 2022, 34, 209–222. [Google Scholar] [CrossRef]
- Karlsson, M.; Atanasova, L.; Jensen, D.F.; Zeilinger, S. Necrotrophic mycoparasites and their genomes. Microbiol. Spec. 2017, 5, 1–21. [Google Scholar] [CrossRef]
- Joo, J.H.; Hussein, K.A. Biological Control and Plant Growth Promotion Properties of Volatile Organic Compound-Producing Antagonistic Trichoderma spp. Front. Plant Sci. 2022, 13, 897668. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Hernández, E.O.; Castillo, F.D.H.; Herrera, R.R.; Fuentes, S.E.V.; Drouaillet, B.E.; Santillán, J.A.L. Actividad antagónica de Trichoderma spp. sobre Rhizoctonia solani in vitro. Investig. Cienc. Univ. Autón. Aguascalientes 2016, 65, 5–11. [Google Scholar] [CrossRef]
- Saldan, N.C.; Almeida, R.T.; Avíncola, A.; Porto, C.; Galuch, M.B.; Magon, T.F.; Oliveira, C.C. Development of an analytical method for identification of Aspergillus flavus based on chemical markers using HPLC-MS. Food Chem. 2018, 241, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.J.; Choi, J.N.; Kim, J.; Park, S.B.; Yeo, S.H.; Choi, J.H.; Lee, C.H. GC-MS based metabolite profiling of rice koji fermentation by various fungi. Biosci. Biotechnol. Biochem. 2010, 74, 2267–2272. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, M.M.; Nicoletti, R.; Salvatore, F.; Naviglio, D.; Andolfi, A. GC–MS approaches for the screening of metabolites produced by marine-derived Aspergillus. Mar. Chem. 2018, 206, 19–33. [Google Scholar] [CrossRef]
- García, T.H.; da Rocha, C.Q.; Dias, M.J.; Pino, L.L.; del Barrio, G.; Roque, A.; Pérez, C.E.; Dos Santos, L.C.; Spengler, I.; Vilegas, W. Comparison of the qualitative chemical composition of extracts from Ageratina havanensis collected in two different phenological stages by FIA-ESI-IT-MSn and UPLC/ESI-MSn:Antiviral activity. Nat. Prod. Commun. 2017, 12, 31–34. [Google Scholar] [CrossRef]
- Fernández, O.; Elósegui, O. Alternativas de producción de Trichoderma en Cuba. Fitosanidad 2006, 10, 147. [Google Scholar]
- Stefanova, M. Introducción y eficacia técnica del biocontrol de fitopatógenos con Trichoderma spp. en Cuba. Fitosanidad 2007, 11, 75–79. [Google Scholar]
- Calero, A.; Quintero, E.; Pérez, Y. Utilización de diferentes bioproductos en la producción de frijol común (Phaseolus vulgaris L.). Agrotec. Cuba 2017, 41, 17–24. [Google Scholar]
- Pérez Torres, E.J.; Bernal Cabrera, A.; Milanés Virreles, P.; Leiva Mora, M.; Sierra Reyes, Y.; Cupull Santana, R. Actividad antagónica de Trichoderma harzianum Rifai sobre el agente causal del tizón del arroz (Pyricularia grisea Sacc.). Centro Agrícola 2017, 44, 13–19. [Google Scholar]
- Pérez, L.; Porras, A. Impacto potencial del cambio climático sobre las plagas de bananos y plátanos en Cuba. Fitosanidad 2015, 19, 201–221. [Google Scholar]
- Samaniego, L.M.; Harouna, M.; Odalys Corbea, O.; Rondón, A.J.; Placeres, I. Aislamiento, identificación y evaluación de cepas autóctonas de Trichoderma spp. antagonistas de patógenos del suelo. Rev. Prot. Veg. 2018, 33, 1–11. [Google Scholar]
- Bell, D.K.; Wells, H.D.; Markham, C.R. In vitro antagonism of Trichoderma species against six fungal plant pathogens. Phytopathology 1982, 72, 379–382. [Google Scholar] [CrossRef]
- Dennis, C.; Webster, J. Antagonistic properties of species-groups of Trichoderma. II. Production of volatile antibiotics. Trans. Br. Mycol. Soc. 1971, 57, 41–48. [Google Scholar] [CrossRef]
- Hernández, G.; Ramos, B.; Sultani, H.N.; Ortiz, Y.; Spengler, I.; Castañeda, R.F.; Rivera, D.G.; Arnold, N.; Westermann, B.; Mirabal, Y. Cultural Characterization and Antagonistic Activity of Cladobotryum virescens against Some Phytopathogenic Fungi and Oomycetes. Agronomy 2023, 13, 389. [Google Scholar] [CrossRef]
- Rondina, R.V.D.; Coussio, J.D. Estudio fitoquímico de plantas medicinales argentinas (1). Rev. Investig. Agropec. (Ser. 2. Biol. Prod. Veg.) 1969, 6, 352. [Google Scholar]
- Laub, A.; Sendatzki, A.K.; Palfner, G.; Wessjohann, L.; Schmidt, J.; Arnold, N. HPTLC-DESI-HRMS based Profiling of Anthraquinones in Complex Mixtures –A Proof-Of-Concept Study using Crude Extracts of Chilean Mushrooms. Foods 2020, 9, 156. [Google Scholar] [CrossRef]
- Khan, M.R.; Mohiddin, F.A. Trichoderma: Its multifarious utility in crop improvement. In Crop Improvement trough Microbial Biotechnology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 263–291. [Google Scholar] [CrossRef]
- Pérez, E.; Bernal, A.; Milanés, P.; Sierra, Y.; Leiva, M.; Marín, S.; Monteagudo, O. Eficiencia de Trichoderma harzianum (cepa a-34) y sus filtrados en el control de tres enfermedades fúngicas foliares en arroz. Bioagro 2018, 30, 17–26. [Google Scholar]
- Ali, M.; Ali, H.; Haq, I.; Shah, I.; Naeem, M.; Samad, A.; Sher, Z.; Asmat, T.M. Evaluation of Trichoderma as a biological control against different pathogenic bacteria and fungi. Pure Appl. Biol. 2020, 9, 1223–1229. [Google Scholar] [CrossRef]
- Babychan, M.; Simon, S. Efficacy of Trichoderma spp. against Fusarium oxysporum f. sp. lycopersici. (FOL) infecting pre-and post-seedling of tomato. J. Pharmacogn. Phytochem. 2017, 6, 616–619. [Google Scholar]
- Ferreira, F.V.; Musumeci, M.A. Trichoderma as biological control agent: Scope and prospects to improve efficacy. World J. Microbiol. Biotechnol. 2021, 37, 90. [Google Scholar] [CrossRef] [PubMed]
- Khaledi, N.; Taheri, P. Biocontrol mechanisms of Trichoderma harzianum against soybean charcoal rot caused by Macrophomina phaseolina. J. Plant Prot. Res. 2016, 56, 21–31. [Google Scholar] [CrossRef]
- Martínez-Coca, B.; Infante, D.; Caraballo, W.; Duarte-Leal, Y.; Echevarría-Hernández, A. Antagonism of strains of Trichoderma asperellum Samuels, Lieckfeldt & Nirenberg against isolates of Fusarium spp. from chickpea. Rev. Prot. Veg. 2018, 3, 1–13. [Google Scholar]
- Sierra, A.; Hernández, A.A.; Díaz, J.A.; Carr, A. Control in vitro con Trichoderma spp. de hongos fitopatógenos de vitroplantas de piña (Ananas comosus L.) Merr. Control in vitro with Trichoderma spp. of phytopathogenus of pineapple vitroplants (Pineapples comosus (L). Merr. Centro Agrícola 2007, 34, 19–23. [Google Scholar]
- Caballero, A.J.; Pocasangre, L.E.; Casanoves, F.; Avelin, J.; Tapia, A.C.; Ortiz, J.L. Uso de aislamientos endofíticos de Trichoderma spp., para el biocontrol del Fusarium oxysporum f. sp. cubense (Mal de Panamá) raza 1 en vitroplantas de banano del cultivar Gros Michel (AAA) en condiciones de invernadero. Univ. Rev. Cient. 2013, 4, 71–82. [Google Scholar]
- Hernández-Melchor, D.J.; Ferrera-Cerrato, R.; López-Pérez, P.A.; Ferrera-Rodríguez, M.R.; García-Ávila, C.d.l.J.; Alarcón, A. Qualitative and quantitative enzimatic profile of native Trichoderma strains and biocontrol potential against Fusarium oxysporum f.sp. cubense RACE 1. J. Microbiol. Biotech. Food Sci. 2022, 11, e3264. [Google Scholar] [CrossRef]
- Lakhdari, W.; Benyahia, I.; Bouhenna, M.M.; Bendif, H.; Khelafi, H.; Bachir, H.; Ladjal, A.; Hammi, H.; Mouhoubi, D.; Khelil, H.; et al. Exploration and Evaluation of Secondary Metabolites from Trichoderma harzianum: GC-MS Analysis, Phytochemical Profiling, Antifungal and Antioxidant Activity Assessment. Molecules 2023, 28, 5025. [Google Scholar] [CrossRef]
- Camba, W.E.; Petroche, D.J.; Cortez, L.A.; Mariscal, W.E. Tamizaje fitoquímico, fenoles totales y actividad antioxidante de Citrus aurantium. RECIAMUC 2022, 6, 470–479. [Google Scholar] [CrossRef]
- Zhao, D.L.; Zhang, X.F.; Huang, R.H.; Wang, D.; Wang, X.Q.; Li, Y.Q.; Zheng, C.J.; Zhang, P.; Zhang, C.S. Antifungal nafuredin and epithiodiketopiperazine derivatives from the mangrove-derived fungus Trichoderma harzianum D13. Front. Microbiol. 2020, 11, 1495. [Google Scholar] [CrossRef]
- Ni, M.; Wu, Q.; Wang, G.S.; Liu, Q.Q.; Yu, M.X.; Tang, J. Analysis of metabolic changes in Trichoderma asperellum TJ01 at different fermentation time-points by LC-QQQ-MS. J. Environ. Sci. Health Part B 2019, 54, 20–26. [Google Scholar] [CrossRef]
- Liu, S.Y.; Lo, C.T.; Shibu, M.A.; Leu, Y.L.; Jen, B.Y.; Peng, K.C. Study on the Anthraquinones Separated from the Cultivation of Trichoderma harzianum Strain Th-R16 and Their Biological Activity. J. Agric. Food Chem. 2009, 57, 7288–7292. [Google Scholar] [CrossRef] [PubMed]
- De Mattos-Shipley, K.M.; Simpson, T.J. The ‘emodin family’ of fungal natural products–amalgamating a century of research with recent genomics-based advances. Nat. Prod. Rep. 2023, 40, 174–201. [Google Scholar] [CrossRef]
- Malik, M.S.; Alsantali, R.I.; Jassas, R.S.; Alsimaree, A.A.; Syed, R.; Alsharif, M.A.; Kalpana, K.; Morad, M.; Althagafi, I.I.; Ahmed, S.A. Journey of anthraquinones as anticancer agents a systematic review of recent literature. RSC Adv. 2021, 11, 35806–35827. [Google Scholar] [CrossRef]
- Sebak, M.; Molham, F.; Greco, C.; Tammam, M.A.; Sobehd, M.; El-Demerdash, A. Chemical diversity, medicinal potentialities, biosynthesis, and pharmacokinetics of anthraquinones and their congeners derived from marine fungi: A comprehensive update. RSC Adv. 2022, 12, 24887. [Google Scholar] [CrossRef]
- Fouillaud, M.; Venkatachalam, M.; Girard-Valenciennes, E.; Caro, Y.; Dufossé, L. Anthraquinones and Derivatives from Marine-Derived Fungi: Structural Diversity and Selected Biological Activities. Mar. Drugs 2016, 14, 1–64. [Google Scholar] [CrossRef] [PubMed]
- Prateeksha; Aslam, M.Y.; Singh, B.N.; Sudheer, S.; Kharwar, R.N.; Siddiqui, S.; Abdel-Azeem, A.M.; Fernandes Fraceto, L.; Dashora, K.; Gupta, V.K. Chrysophanol: A Natural Anthraquinone with Multifaceted Biotherapeutic Potential. Biomolecules 2019, 9, 68. [Google Scholar] [CrossRef]
- Liu, S.Y.; Liao, C.K.; Lo, C.T.; Yang, H.H.; Lin, K.C.; Peng, K.C. Chrysophanol is involved in the biofertilization and biocontrol activities of Trichoderma. Physiol. Mol. Plant Pathol. 2016, 96, 1–7. [Google Scholar] [CrossRef]
- Guo, R.; Li, G.; Zhang, Z.; Peng, X. Structures and Biological Activities of Secondary Metabolites from Trichoderma harzianum. Mar. Drugs 2022, 20, 701. [Google Scholar] [CrossRef]
- Schmidt, J. Negative ion electrospray high-resolution tandem mass spectrometry of polyphenols. J. Mass. Spectrom. 2015, 51, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Leng, L.; Liu, Y.; Gao, H.; Yang, W.; Chen, S.; Liu, A. Identification and quantification of target metabolites combined with transcriptome of two rheum species focused on anthraquinone and flavonoids biosynthesis. Sci. Rep. 2020, 10, 20241. [Google Scholar] [CrossRef] [PubMed]
- Unver, T. Isorhamnetin as a promising natural bioactive flavonoid: In vitro assessment of its antifungal property. Int. J. Agric. Environ. Food Sci. 2024, 8, 54–61. [Google Scholar] [CrossRef]
- Zabka, M.; Pavela, R. Antifungal efficacy of some natural phenolic compounds against significant pathogenic and toxinogenic filamentous fungi. Chemosphere 2013, 93, 1051–1056. [Google Scholar] [CrossRef]
- Ansari, M.A.; Anurag, A.; Fatima, Z.; Hameed, S. Natural phenolic compounds: A potential antifungal agent. Microb. Pathog. Strateg. Combat. Sci. Thenol. Educ. 2013, 1, 1189–1195. [Google Scholar]


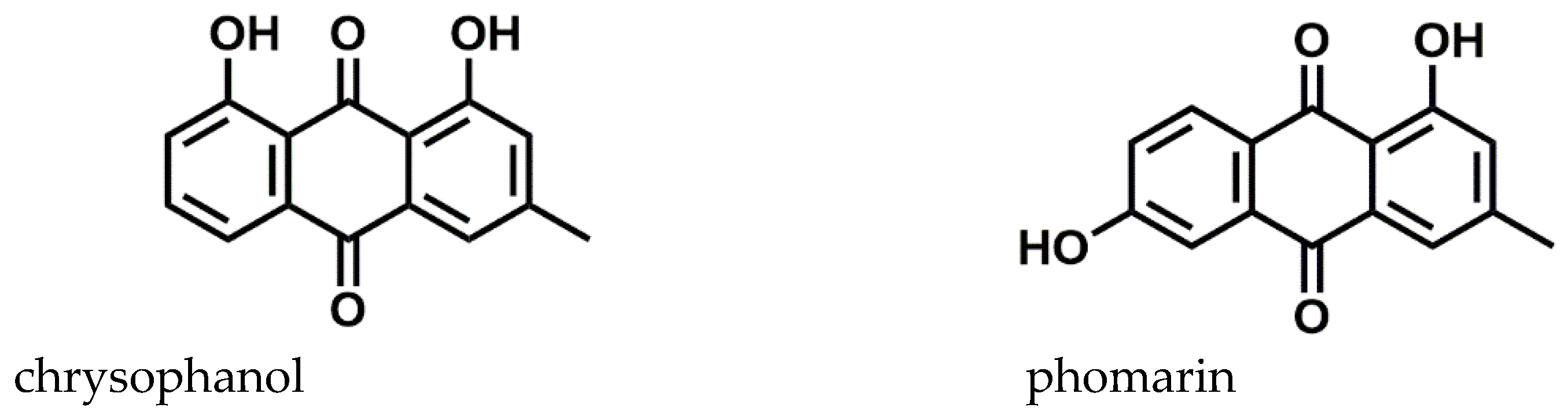




| Treatment | Percentage of Radial Growth Inhibition(%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dual Culture | Volatile Metabolites | |||||||||
| 24 h | 48 h | 72 h | 7 d | 10 d | 24 h | 48 h | 72 h | 7 d | 8 d | |
| Control PalPR7 | 0 a | 0 a | 0 a | 0 a | 0 a | 0 a | 0 a | 0 a | 0 a | 0 a |
| LBAT-53 vs. PalPR7 | 16.67 a | 10.17 b | 14.94 ab | 66.09 cd | 76.90 cd | 20.83 ab | 8.53 abc | 14.14 cd | 40.65 cd | 44.44 e |
| Family Compounds | LBAT-53 |
|---|---|
| Fraction A | |
| Phenols | +++ |
| Amine compounds | + |
| Fraction B | |
| Triterpenes/steroids | - |
| Quinones | ++ |
| Fraction C1 | |
| Alkaloids | - |
| Fraction C2 | |
| Triterpenes/steroids | - |
| Alkaloids | - |
| Cardenolides | - |
| Fraction D | |
| Flavonoids | ++ |
| Cardenolides | - |
| Alkaloids | - |
| Proanthocyanidins/catechins | - |
| Triterpenes/steroids | + |
| Fraction E | |
| Proanthocyanidins/catechins | - |
| Flavonoids | - |
| Reducing sugars | +++ |
| Fraction F | |
| Saponins | + |
| Amine compounds | +++ |
| Ionic Species | Rt (min) | Elemental Composition | [M−H]− (m/z Theoretical) | [M−H]− (m/z Experimental) | RDB | Δ ppm |
|---|---|---|---|---|---|---|
| [M−H]− | 8.32 | C15H9O4− | 253.0579 | 253.0515 | 11.5 | 0.66 |
| [M−H]− | 8.50 | C15H9O4− | 253.0579 | 253.0515 | 11.5 | 0.66 |
| [M−H]− | 6.39 | C15H9O5− | 269.0455 | 269.0459 | 11.5 | 0.57 |
| [M−H]− | 6.92 | C16H9O7− | 313.0354 | 313.0350 | 12.5 | −1.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández, G.; Ponce de la Cal, A.; Louis, Y.; Baró Robaina, Y.; Coll, Y.; Spengler, I.; Mirabal-Gallardo, Y. Identification of Secondary Metabolites by UHPLC-ESI-HRMS/MS in Antifungal Strain Trichoderma harzianum (LBAT-53). J. Fungi 2024, 10, 547. https://doi.org/10.3390/jof10080547
Hernández G, Ponce de la Cal A, Louis Y, Baró Robaina Y, Coll Y, Spengler I, Mirabal-Gallardo Y. Identification of Secondary Metabolites by UHPLC-ESI-HRMS/MS in Antifungal Strain Trichoderma harzianum (LBAT-53). Journal of Fungi. 2024; 10(8):547. https://doi.org/10.3390/jof10080547
Chicago/Turabian StyleHernández, Giselle, Amaia Ponce de la Cal, Yuset Louis, Yamilé Baró Robaina, Yamilet Coll, Iraida Spengler, and Yaneris Mirabal-Gallardo. 2024. "Identification of Secondary Metabolites by UHPLC-ESI-HRMS/MS in Antifungal Strain Trichoderma harzianum (LBAT-53)" Journal of Fungi 10, no. 8: 547. https://doi.org/10.3390/jof10080547
APA StyleHernández, G., Ponce de la Cal, A., Louis, Y., Baró Robaina, Y., Coll, Y., Spengler, I., & Mirabal-Gallardo, Y. (2024). Identification of Secondary Metabolites by UHPLC-ESI-HRMS/MS in Antifungal Strain Trichoderma harzianum (LBAT-53). Journal of Fungi, 10(8), 547. https://doi.org/10.3390/jof10080547









