Comprehensive Review of Tinea Capitis in Adults: Epidemiology, Risk Factors, Clinical Presentations, and Management
Abstract
1. Introduction
2. Geographic Distribution of Causative Organisms
3. Epidemiology of Tinea Capitis in Adults
4. Transmission
5. Risk Factors for Tinea Capitis in Adults
6. Classification
7. Clinical Presentation and Physical Exam Findings
8. Trichoscopy
9. Differential Diagnosis
10. Diagnostic Methods
11. Treatment and Management
12. Prognosis and Sequelae of Untreated Disease
13. Antifungal Resistance
14. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gheisari, M.; Zahedi, K.; Al-Zubaidi, N. Tinea capitis caused by Trichophyton violaceum in an immunocompetent elderly patient: A case report and review of literature. Clin. Case Rep. 2023, 11, e8205. [Google Scholar] [CrossRef] [PubMed]
- Rabhi, F.; Elinkichari, D.; Mtibaa, L.; Jemli, B.; Jaber, K.; Dhaoui, R. Inflammatory Tinea Capitis Mimicking Dissecting Cellulitis in a Healthy Woman. Skin. Appendage Disord. 2023, 9, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Chiriac, A.; Diaconeasa, A.; Voicu, C.; Ivaniciuc, M.; Miulescu, R.; Chiriac, A.E.; Nenoff, P.; Wollina, U. Kerion Celsi in infants and children—A narrative review 2010–2023. Mycoses 2024, 67, e13675. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K.N.; Tay, Y.K.; Heath, C.R.; Trachtman, R.; Silverberg, N.B. Review article: Emerging issues in pediatric skin of color, part 1. Pediatr. Dermatol. 2021, 38 (Suppl. S2), 20–29. [Google Scholar] [CrossRef] [PubMed]
- Aharaz, A.; Jemec, G.B.E.; Hay, R.J.; Saunte, D.M.L. Tinea capitis asymptomatic carriers: What is the evidence behind treatment? J. Eur. Acad. Dermatol. Venereol. 2021, 35, 2199–2207. [Google Scholar] [CrossRef] [PubMed]
- Hennessee, I.P.; Benedict, K.; Dulski, T.M.; Lipner, S.R.; Gold, J.A.W. Racial disparities, risk factors, and clinical management practices for tinea capitis: An observational cohort study among US children with Medicaid. J. Am. Acad. Dermatol. 2023, 89, 1261–1264. [Google Scholar] [CrossRef]
- Zhan, P.; Liu, W. The Changing Face of Dermatophytic Infections Worldwide. Mycopathologia 2017, 182, 77–86. [Google Scholar] [CrossRef]
- Khosravi, A.R.; Shokri, H.; Vahedi, G. Factors in Etiology and Predisposition of Adult Tinea Capitis and Review of Published Literature. Mycopathologia 2016, 181, 371–378. [Google Scholar] [CrossRef]
- Mirmirani, P.; Tucker, L.Y. Epidemiologic trends in pediatric tinea capitis: A population-based study from Kaiser Permanente Northern California. J. Am. Acad. Dermatol. 2013, 69, 916–921. [Google Scholar] [CrossRef]
- Borman, A.M.; Campbell, C.K.; Fraser, M.; Johnson, E.M. Analysis of the dermatophyte species isolated in the British Isles between 1980 and 2005 and review of worldwide dermatophyte trends over the last three decades. Med. Mycol. 2007, 45, 131–141. [Google Scholar] [CrossRef]
- Zhan, P.; Li, D.; Wang, C.; Sun, J.; Geng, C.; Xiong, Z.; Seyedmousavi, S.; Liu, W.; de Hoog, G.S. Epidemiological changes in tinea capitis over the sixty years of economic growth in China. Med. Mycol. 2015, 53, 691–698. [Google Scholar] [CrossRef]
- Zhan, P.; Geng, C.; Li, Z.; Jin, Y.; Jiang, Q.; Tao, L.; Luo, Y.; Xiong, Z.; Wu, S.; Li, D.; et al. Evolution of tinea capitis in the Nanchang area, Southern China: A 50-year survey (1965–2014). Mycoses 2015, 58, 261–266. [Google Scholar] [CrossRef]
- Mapelli, E.T.; Cerri, A.; Bombonato, C.; Menni, S. Tinea capitis in the paediatric population in Milan, Italy: The emergence of Trichophyton violaceum. Mycopathologia 2013, 176, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Li, L.; Wang, J.; Zhang, C.; Kang, K.; Zhang, Q. Tinea capitis in Southeastern China: A 16-year survey. Mycopathologia 2010, 169, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Fulgence, K.K.; Marie, K.P.C.; Akoua, V.B.; Massafoma, K.E.G.; Etienne, A.K.; Abibatou, K.; Henriette, V.A.; Sebastien, M.A.J.; Vincent, D.; William, Y.; et al. Dermatophytosis and the associated risk factors among primary school children in southern and central Côte d’Ivoire. Mycoses 2023, 66, 869–875. [Google Scholar] [CrossRef]
- Grigoryan, K.V.; Tollefson, M.M.; Olson, M.A.; Newman, C.C. Pediatric tinea capitis caused by Trichophyton violaceum and Trichophyton soudanense in Rochester, Minnesota, United States. Int. J. Dermatol. 2019, 58, 912–915. [Google Scholar] [CrossRef] [PubMed]
- Anton, A.; Plinet, M.; Peyret, T.; Cazaudarré, T.; Pesant, S.; Rouquet, Y.; Tricoteaux, M.-A.; Bernier, M.; Bayette, J.; Fournier, R.; et al. Rapid and Accurate Diagnosis of Dermatophyte Infections Using the DendrisCHIP® Technology. Diagnostics 2023, 13, 3430. [Google Scholar] [CrossRef]
- Keisham, C.; Sarkar, R.; Khurana, N.; Ghosh, N.; Garg, V.K.; Manoj, R.K. Black dot tinea capitis caused by Trichophyton rubrum in an adult female presenting with cicatricial alopecia. Indian. J. Dermatol. Venereol. Leprol. 2015, 81, 224. [Google Scholar] [CrossRef]
- Silverberg, N.B.; Weinberg, J.M.; DeLeo, V.A. Tinea capitis: Focus on African American women. J. Am. Acad. Dermatol. 2002, 46 (Suppl. S2), S120–S124. [Google Scholar] [CrossRef]
- Cremer, G.; Bournerias, I.; Vandemeleubroucke, E.; Houin, R.; Revuz, J. Tinea capitis in adults: Misdiagnosis or reappearance? Dermatology 1997, 194, 8–11. [Google Scholar] [CrossRef]
- Ginter-Hanselmayer, G.; Weger, W.; Ilkit, M.; Smolle, J. Epidemiology of tinea capitis in Europe: Current state and changing patterns. Mycoses 2007, 50 (Suppl. S2), 6–13. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Cerdeira, C.; Martínez-Herrera, E.; Szepietowski, J.C.; Pinto-Almazán, R.; Frías-De-León, M.; Espinosa-Hernández, V.; Chávez-Gutiérrez, E.; García-Salazar, E.; Vega-Sánchez, D.; Arenas, R.; et al. A systematic review of worldwide data on tinea capitis: Analysis of the last 20 years. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 844–883. [Google Scholar] [CrossRef] [PubMed]
- Hillary, T.; Suys, E. An outbreak of tinea capitis in elderly patients. Int. J. Dermatol. 2014, 53, e101–e103. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Zheng, X.; Song, G.; Zhang, M.; Liu, J.; Zang, X.; Fu, M.; Liu, W. Adult tinea capitis in China: A retrospective analysis from 2000 to 2019. Mycoses 2020, 63, 876–888. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Silverberg, N.B.; Howard, R.; Tran, C.T.; Laude, T.A.; Frieden, I.J. Do Hair Care Practices Affect the Acquisition of Tinea Capitis?: A Case-Control Study. Arch. Pediatr. Adolesc. Med. 2001, 155, 818–821. [Google Scholar] [CrossRef]
- Akhoundi, M.; Nasrallah, J.; Marteau, A.; Chebbah, D.; Izri, A.; Brun, S. Effect of Household Laundering, Heat Drying, and Freezing on the Survival of Dermatophyte Conidia. J. Fungi 2022, 8, 546. [Google Scholar] [CrossRef] [PubMed]
- Coulibaly, O.; Thera, M.A.; Piarroux, R.; Doumbo, O.K.; Ranque, S. High dermatophyte contamination levels in hairdressing salons of a West African suburban community. Mycoses 2015, 58, 65–68. [Google Scholar] [CrossRef]
- Müller, V.L.; Kappa-Markovi, K.; Hyun, J.; Georgas, D.; Silberfarb, G.; Paasch, U.; Uhrlaß, S.; Nenoff, P.; Schaller, J. Tinea capitis et barbae caused by Trichophyton tonsurans: A retrospective cohort study of an infection chain after shavings in barber shops. Mycoses 2021, 64, 428–436. [Google Scholar] [CrossRef]
- Lateur, N.; André, J.; De Maubeuge, J.; Poncin, M.; Song, M. Tinea capitis in two black african adults with HIV infection. Br. J. Dermatol. 1999, 140, 722–724. [Google Scholar] [CrossRef]
- Xie, W.; Chen, Y.; Liu, W.; Li, X.; Liang, G. Seborrheic dermatitis-like adult tinea capitis due to Trichophyton rubrum in an elderly man. Med. Mycol. Case Rep. 2023, 41, 16–19. [Google Scholar] [CrossRef]
- El-Khalawany, M.; Shaaban, D.; Hassan, H.; Abdalsalam, F.; Eassa, B.; Kader, A.A.; Shaheen, I. A multicenter clinicomycological study evaluating the spectrum of adult tinea capitis in Egypt. Acta Dermatovenerol. Alp. Pannonica Adriat. 2013, 22, 77–82. [Google Scholar]
- Vannini, P.; Guadagni, R.; Palleschi, G.M.; Difonzo, E.M.; Panconesi, E. Tinea capitis in the adult: Two case studies. Mycopathologia 1986, 96, 53–57. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lo, H.J.; Tu, M.G.; Ju, Y.-M.; Fan, Y.-C.; Lin, C.-C.; Chiang, Y.-T.; Yang, Y.-L.; Chen, K.-T.; Sun, P.-L. The survey of tinea capitis and scalp dermatophyte carriage in nursing home residents. Med. Mycol. 2018, 56, 180–185. [Google Scholar] [CrossRef]
- Mebazaa, A.; Oumari, K.E.L.; Said, M.B.; Ghariani, N.; Denguezli, M.; Mili, A.F.; Kenani, N.; Belajouza, C.; Nouira, R. Tinea capitis in adults in Tunisia. Int. J. Dermatol. 2010, 49, 513–516. [Google Scholar] [CrossRef]
- Hällgren, J.; Petrini, B.; Wahlgren, C.F. Increasing tinea capitis prevalence in Stockholm reflects immigration. Med. Mycol. 2004, 42, 505–509. [Google Scholar] [CrossRef]
- Ergin, S.; Ergin, C.; Erdoğan, B.S.; Kaleli, I.; Evliyaoğlu, D. An experience from an outbreak of tinea capitis gladiatorum due to Trichophyton tonsurans. Clin. Exp. Dermatol. 2006, 31, 212–214. [Google Scholar] [CrossRef]
- Galili, E.; Goldsmith, T.; Khanimov, I.; Arbel, C.; Sharvit, S.; Lyakhovitsky, A.; Shemer, A.; Barzilai, A.; Astman, N. Tinea capitis caused by Trichophyton tonsurans among adults: Clinical characteristics and treatment response. Mycoses 2023, 66, 144–149. [Google Scholar] [CrossRef]
- Baumgardner, D.J. Fungal Infections from Human and Animal Contact. J. Patient Cent. Res. Rev. 2017, 4, 78–89. [Google Scholar] [CrossRef]
- Hammer, T.R.; Mucha, H.; Hoefer, D. Infection risk by dermatophytes during storage and after domestic laundry and their temperature-dependent inactivation. Mycopathologia 2011, 171, 43–49. [Google Scholar] [CrossRef]
- Blasco Melguizo, J.; Ruiz Villaverde, R.; Delgado Florencio, V.; Buendía Eisman, A. Tinea capitis by Trichophyton violaceum in immuno-suppressed elderly man. J. Eur. Acad. Dermatol. Venereol. 2004, 18, 100–102. [Google Scholar] [CrossRef]
- Ooka, S.; Kashima, M.; Kubota, Y.; Noguchi, A.; Kawai, S.; Nakamura, Y.; Kano, R.; Watanabe, S.; Hasegawa, A.; Mizoguchi, M. A case of black dot ringworm with a review of Japanese cases. J. Dermatol. 2000, 27, 658–663. [Google Scholar] [CrossRef]
- Finielz, P. Tinea capitis in adult renal transplant recipient. Nephron 1999, 82, 86. [Google Scholar] [CrossRef]
- Park, S.K.; Park, S.W.; Yun, S.K.; Kim, H.U.; Park, J. Tinea capitis in adults: A 18-year retrospective, single-centre study in Korea. Mycoses 2019, 62, 609–616. [Google Scholar] [CrossRef]
- Barbosa, V.; Hight, R.; Grullon, K. Scalp Infection, Inflammation, and Infestation. Dermatol. Clin. 2023, 41, 539–545. [Google Scholar] [CrossRef]
- Elsaie, M.L. Update on Tinea Capitis Diagnosis and Treatment. Cutis 2022, 110, 238–240. [Google Scholar] [CrossRef]
- Hay, R.J. Tinea Capitis: Current Status. Mycopathologia 2017, 182, 87–93. [Google Scholar] [CrossRef]
- Ilkit, M. Favus of the scalp: An overview and update. Mycopathologia 2010, 170, 143–154. [Google Scholar] [CrossRef]
- Elewski, B.E. Tinea capitis: A current perspective. J. Am. Acad. Dermatol. 2000, 42, 1–20. [Google Scholar] [CrossRef]
- Mochizuki, T.; Kawasaki, M.; Anzawa, K.; Kojima, K.; Hatta, J.; Tababe, H.; Higaki, S.; Fujita, S. Extra-scalp black dot ringworm caused by Trichophyton tonsurans among contact sports players. Mycopathologia 2012, 173, 241–244. [Google Scholar] [CrossRef]
- Poppe, H.; Kolb-Mäurer, A.; Wobser, M.; Trautmann, A. Pitfall scarring alopecia: Favus closely mimicking lichen planus. Mycoses 2013, 56, 382–384. [Google Scholar] [CrossRef]
- Schwartz, R.A.; Janniger, C.K. Tinea capitis. Cutis 1995, 55, 29–33. [Google Scholar] [PubMed]
- Zaraa, I.; Hawilo, A.; Aounallah, A.; Trojjet, S.; El Euch, D.; Mokni, M.; Ben Osman, A. Inflammatory Tinea capitis: A 12-year study and a review of the literature. Mycoses 2013, 56, 110–116. [Google Scholar] [CrossRef] [PubMed]
- John, A.M.; Schwartz, R.A.; Janniger, C.K. The kerion: An angry tinea capitis. Int. J. Dermatol. 2018, 57, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Khunger, N. Diagnostic Trichoscopy of Tinea Capitis. J. Cutan. Med. Surg. 2023, 27, 181. [Google Scholar] [CrossRef] [PubMed]
- Waśkiel-Burnat, A.; Rakowska, A.; Sikora, M.; Ciechanowicz, P.; Olszewska, M.; Rudnicka, L. Trichoscopy of Tinea Capitis: A Systematic Review. Dermatol. Ther. 2020, 10, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Genedy, R.M.; Sorour, O.A.; Elokazy, M.A.W. Trichoscopic signs of tinea capitis: A guide for selection of appropriate antifungal. Int. J. Dermatol. 2021, 60, 471–481. [Google Scholar] [CrossRef]
- Kumar, P.; Pandhi, D.; Bhattacharya, S.N.; Das, S. Trichoscopy as a monitoring tool in assessing treatment response in 98 children with tinea capitis: A prospective clinical study. Dermatol. Ther. 2021, 34, e15010. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.C.; Caplan, A.S.; Elewski, B.; Gold, J.A.W.; Lockhart, S.R.; Smith, D.J.; Lipner, S.R. Expert Panel Review of Skin and Hair Dermatophytoses in an Era of Antifungal Resistance. Am. J. Clin. Dermatol. 2024, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Chanyachailert, P.; Leeyaphan, C.; Bunyaratavej, S. Cutaneous Fungal Infections Caused by Dermatophytes and Non-Dermatophytes: An Updated Comprehensive Review of Epidemiology, Clinical Presentations, and Diagnostic Testing. J. Fungi 2023, 9, 669. [Google Scholar] [CrossRef] [PubMed]
- Akbaba, M.; Ilkit, M.; Sutoluk, Z.; Ates, A.; Zorba, H. Comparison of hairbrush, toothbrush and cotton swab methods for diagnosing asymptomatic dermatophyte scalp carriage. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 356–362. [Google Scholar] [CrossRef]
- Le, M.; Gabrielli, S.; Ghazawi, F.M.; Alkhodair, R.; Sheppard, D.C.; Jafarian, F. Efficacies and merits of the cotton swab technique for diagnosing tinea capitis in the pediatric population. J. Am. Acad. Dermatol. 2020, 83, 920–922. [Google Scholar] [CrossRef] [PubMed]
- Nasir, S.; Ralph, N.; O’Neill, C.; Cunney, R.; Lenane, P.; O’Donnell, B. Trends in Tinea Capitis in an Irish Pediatric Population and a Comparison of Scalp Brushings Versus Scalp Scrapings as Methods of Investigation. Pediatr. Dermatol. 2014, 31, 622–623. [Google Scholar] [CrossRef] [PubMed]
- Shemer, A.; Lyakhovitsky, A.; Kaplan, B.; Kassem, R.; Daniel, R.; Caspi, T.; Galili, E. Diagnostic approach to tinea capitis with kerion: A retrospective study. Pediatr. Dermatol. 2022, 39, 708–712. [Google Scholar] [CrossRef] [PubMed]
- Gautam, M.; Bhatia, S. Mount the Menace!—Potassium Hydroxide in Superficial Fungal Infections. Indian J. Paediatr. Dermatol. 2020, 21, 343. [Google Scholar]
- Mourad, B.; Ismail, M.; Hawwam, S.; Msseha, M.; Hassan, R. Evaluation of the Efficacy of Fluorescent Staining and Chicago Sky Blue Staining as Methods for Diagnosis of Dermatophytosis in Hair and Nails. Clin. Cosmet. Investig. Dermatol. 2019, 12, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Garg, J.; Tilak, R.; Garg, A.; Prakash, P.; Gulati, A.K.; Nath, G. Rapid detection of dermatophytes from skin and hair. BMC Res. Notes 2009, 2, 60. [Google Scholar] [CrossRef]
- Ndiaye, M.; Sacheli, R.; Diongue, K.; Adjetey, C.; Darfouf, R.; Seck, M.C.; Badiane, A.S.; Diallo, M.A.; Dieng, T.; Hayette, M.-P.; et al. Evaluation of the Multiplex Real-Time PCR DermaGenius® Assay for the Detection of Dermatophytes in Hair Samples from Senegal. J. Fungi 2021, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Cinotti, E.; Perrot, J.L.; Labeille, B.; Raberin, H.; Flori, P.; Cambazard, F. Hair dermatophytosis diagnosed by reflectance confocal microscopy: Six cases. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2257–2259. [Google Scholar] [CrossRef] [PubMed]
- Veasey, J.V.; Meneses, O.M.S.; da Silva, F.O. Reflectance confocal microscopy of tinea capitis: Comparing images with results of dermoscopy and mycological exams. Int. J. Dermatol. 2019, 58, 849–851. [Google Scholar] [CrossRef]
- Wei, L.W.; Qiao, J.J. Mini-Review: The Diagnostic Methods of Tinea Capitis. Mycopathologia 2023, 188, 563–569. [Google Scholar] [CrossRef]
- Ely, J.W.; Rosenfeld, S.; Seabury Stone, M. Diagnosis and management of tinea infections. Am. Fam. Physician 2014, 90, 702–710. [Google Scholar] [PubMed]
- Kimberlin, D.W.; Barnett, E.D.; Lynfield, R.; Sawyer, M.H. (Eds.) Red Book: 2021–2024 Report of the Committee on Infectious Diseases, 32nd ed.; American Academy of Pediatrics: Itasca, IL, USA, 2021. [Google Scholar]
- Vidimos, A.T.; Camisa, C.; Tomecki, K.J. Tinea capitis in three adults. Int. J. Dermatol. 1991, 30, 206–208. [Google Scholar] [CrossRef] [PubMed]
- Pomeranz, A.J.; Sabnis, S.S.; McGrath, G.J.; Esterly, N.B. Asymptomatic dermatophyte carriers in the households of children with tinea capitis. Arch. Pediatr. Adolesc. Med. 1999, 153, 483–486. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Elewski, B.E.; Hughey, L.C.; Sobera, J.O. Fungal diseases. In Dermatology, 3rd ed.; Bolognia, J.L., Jorizzo, J.L., Schaffer, J.V., Eds.; Elsevier Limited: Amsterdam, The Netherlands, 2012; Volume 2, p. 1251. [Google Scholar]
- Aste, N.; Pau, M. Tinea capitis caused by Microsporum canis treated with terbinafine. Mycoses 2004, 47, 428–430. [Google Scholar] [CrossRef] [PubMed]
- Aste, N.; Pau, M.; Biggio, P. Tinea capitis in adults. Mycoses 1996, 39, 299–301. [Google Scholar] [CrossRef] [PubMed]
- Tey, H.L.; Tan, A.S.; Chan, Y.C. Meta-analysis of randomized, controlled trials comparing griseofulvin and terbinafine in the treatment of tinea capitis. J. Am. Acad. Dermatol. 2011, 64, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Hofstader, S.L.; Summerbell, R.C.; Solomon, R.; Adam, P.; Alexis, M.; Raboobee, N.; De Doncker, P. Treatment of tinea capitis with itraconazole capsule pulse therapy. J. Am. Acad. Dermatol. 1998, 39, 216–219. [Google Scholar] [CrossRef]
- Foster, K.W.; Friedlander, S.F.; Panzer, H.; Ghannoum, M.A.; Elewski, B.E. A randomized controlled trial assessing the efficacy of fluconazole in the treatment of pediatric tinea capitis. J. Am. Acad. Dermatol. 2005, 53, 798–809. [Google Scholar] [CrossRef]
- Tangjaturonrusamee, C.; Piraccini, B.M.; Vincenzi, C.; Starace, M.; Tosti, A. Tinea capitis mimicking folliculitis decalvans. Mycoses 2011, 54, 87–88. [Google Scholar] [CrossRef]
- Lamb, S.R.; Rademaker, M. Tinea due to Trichophyton violaceum and Trichophyton soudanense in Hamilton, New Zealand. Australas. J. Dermatol. 2001, 42, 260–263. [Google Scholar] [CrossRef]
- Chen, C.; Koch, L.H.; Dice, J.E.; Dempsey, K.K.; Moskowitz, A.B.; Barnes-Eley, M.L.; Hubbard, T.W.; Williams, J.V. A randomized, double-blind study comparing the efficacy of selenium sulfide shampoo 1% and ciclopirox shampoo 1% as adjunctive treatments for tinea capitis in children. Pediatr. Dermatol. 2010, 27, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Ilkit, M.; Demirhindi, H. Asymptomatic dermatophyte scalp carriage: Laboratory diagnosis, epidemiology and management. Mycopathologia 2008, 165, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Buckley, D.A.; Fuller, L.C.; Higgins, E.M.; du Vivier, A.W. Lesson of the week. Tinea capitis in adults. BMJ 2000, 320, 1389–1390. [Google Scholar] [CrossRef] [PubMed]
- Mohta, A.; Singh, A.; Nyati, A.; Nyati, A.; Agrawal, A.; Nahar, D.; Lal, M.; Gupta, D.; Jain, S.K. Evaluation of Impact of Tinea Capitis on Quality of Life in Pediatric Patients Using Children’s Dermatology Life Quality Index and Its Correlation with Disease Duration. Int. J. Trichol. 2020, 12, 213–219. [Google Scholar] [CrossRef]
- Hay, R.J. The Spread of Resistant Tinea and the Ingredients of a Perfect Storm. Dermatology 2022, 238, 80–81. [Google Scholar] [CrossRef]
- Uhrlaß, S.; Verma, S.B.; Gräser, Y.; Rezaei-Matehkolaei, A.; Hatami, M.; Schaller, M.; Nenoff, P. Trichophyton indotineae—An Emerging Pathogen Causing Recalcitrant Dermatophytoses in India and Worldwide—A Multidimensional Perspective. J. Fungi 2022, 8, 757. [Google Scholar] [CrossRef]
- Gupta, A.K.; Venkataraman, M.; Hall, D.C.; Cooper, E.A.; Summerbell, R.C. The emergence of Trichophyton indotineae: Implications for clinical practice. Int. J. Dermatol. 2023, 62, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.S.; Chaturvedi, S.; Zhu, Y.; Todd, G.C.; Yin, L.; Lopez, A.; Travis, L.; Smith, D.J.; Chiller, T.; Lockhart, S.R.; et al. Notes from the Field: First Reported, U.S. Cases of Tinea Caused by Trichophyton indotineae—New York City, December 2021-March 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 536–537. [Google Scholar] [CrossRef] [PubMed]
- Elewski, B. A call for antifungal stewardship. Br. J. Dermatol. 2020, 183, 798–799. [Google Scholar] [CrossRef]
- Zeeshan, F.; Sabir, M.; Uddin, F. The rising menace of antifungal resistance in dermatophytes among the patients of tinea capitis. J. Pak. Med. Assoc. 2023, 73, 43–48. [Google Scholar] [CrossRef]
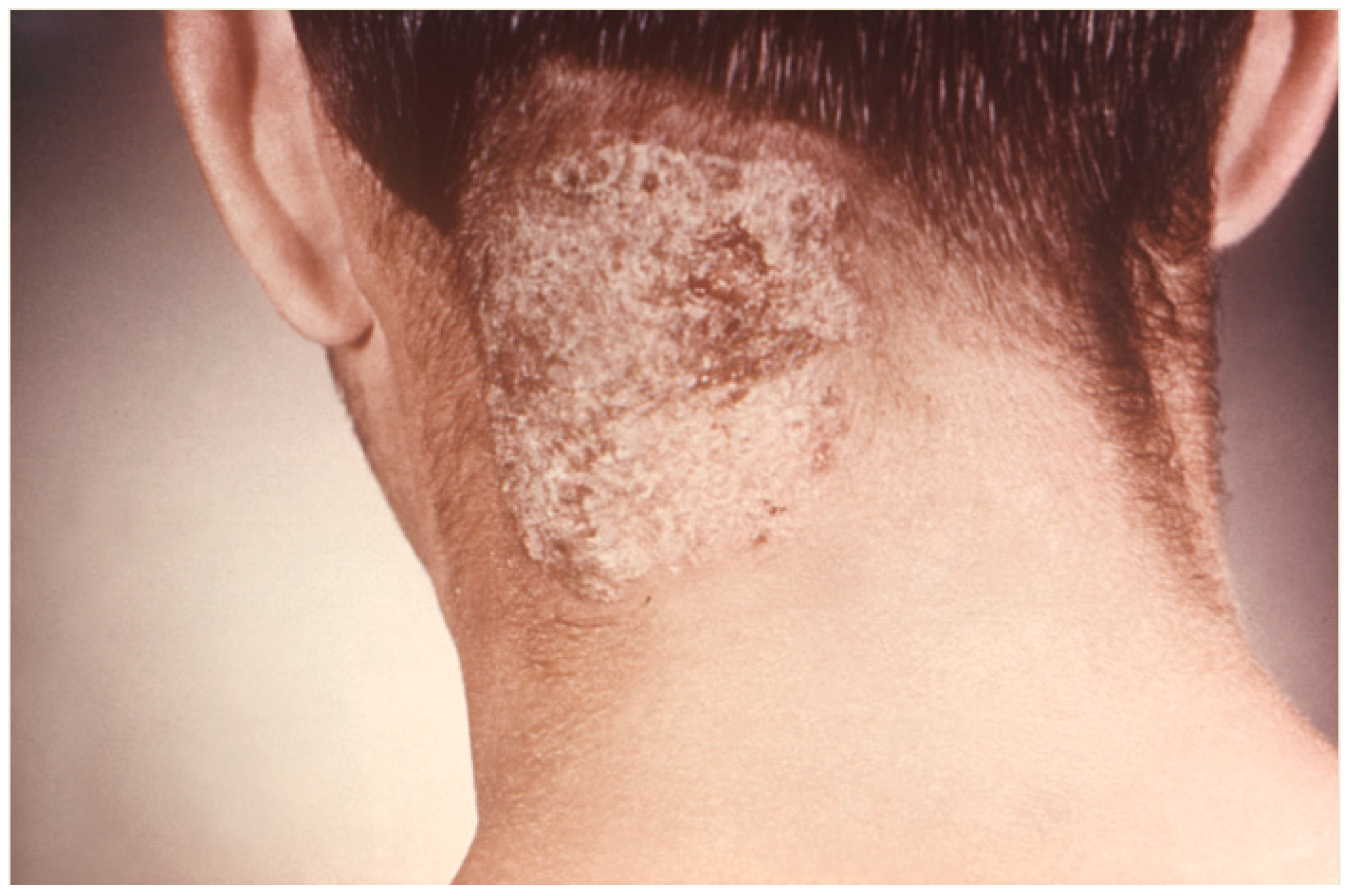
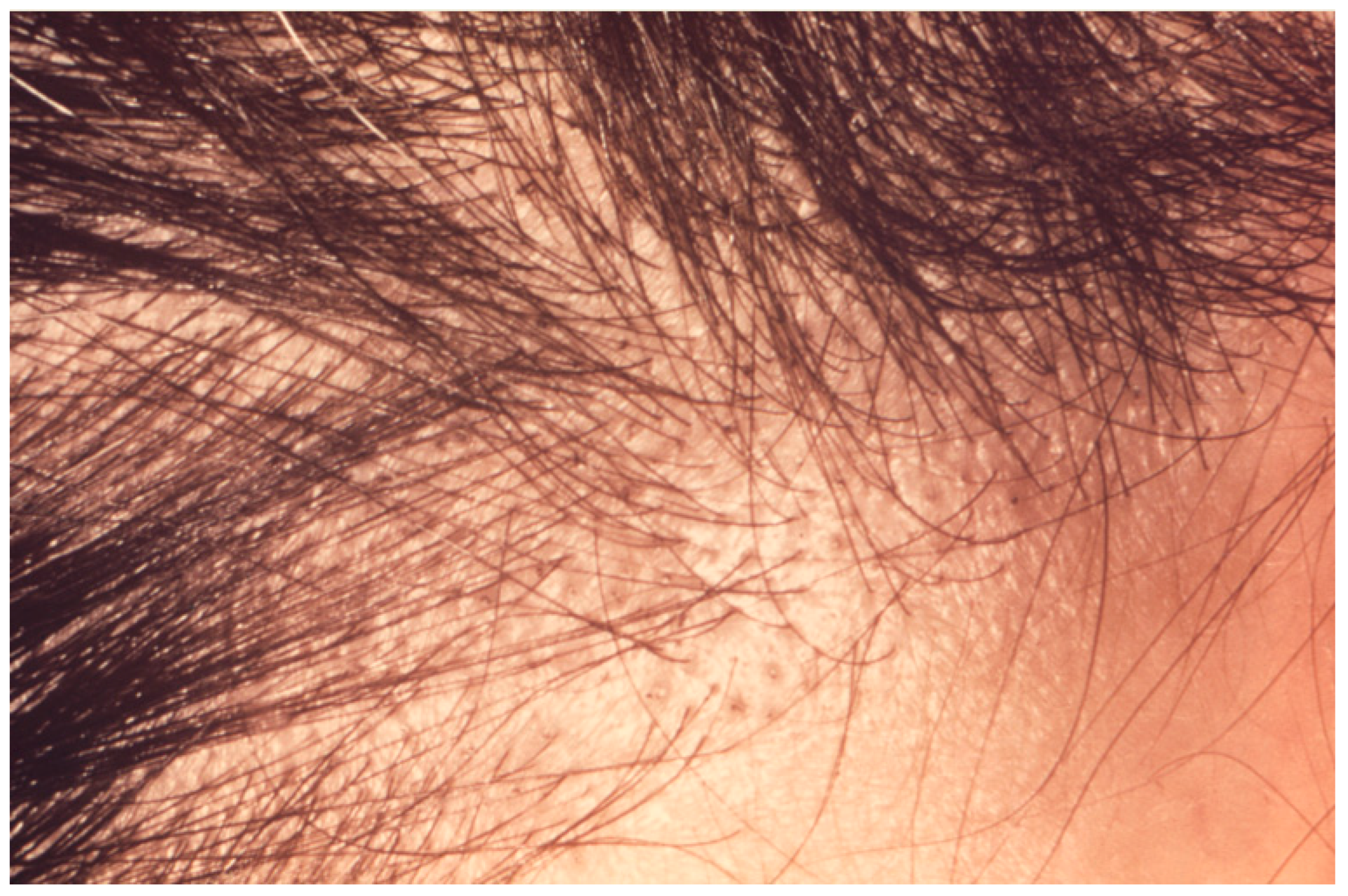
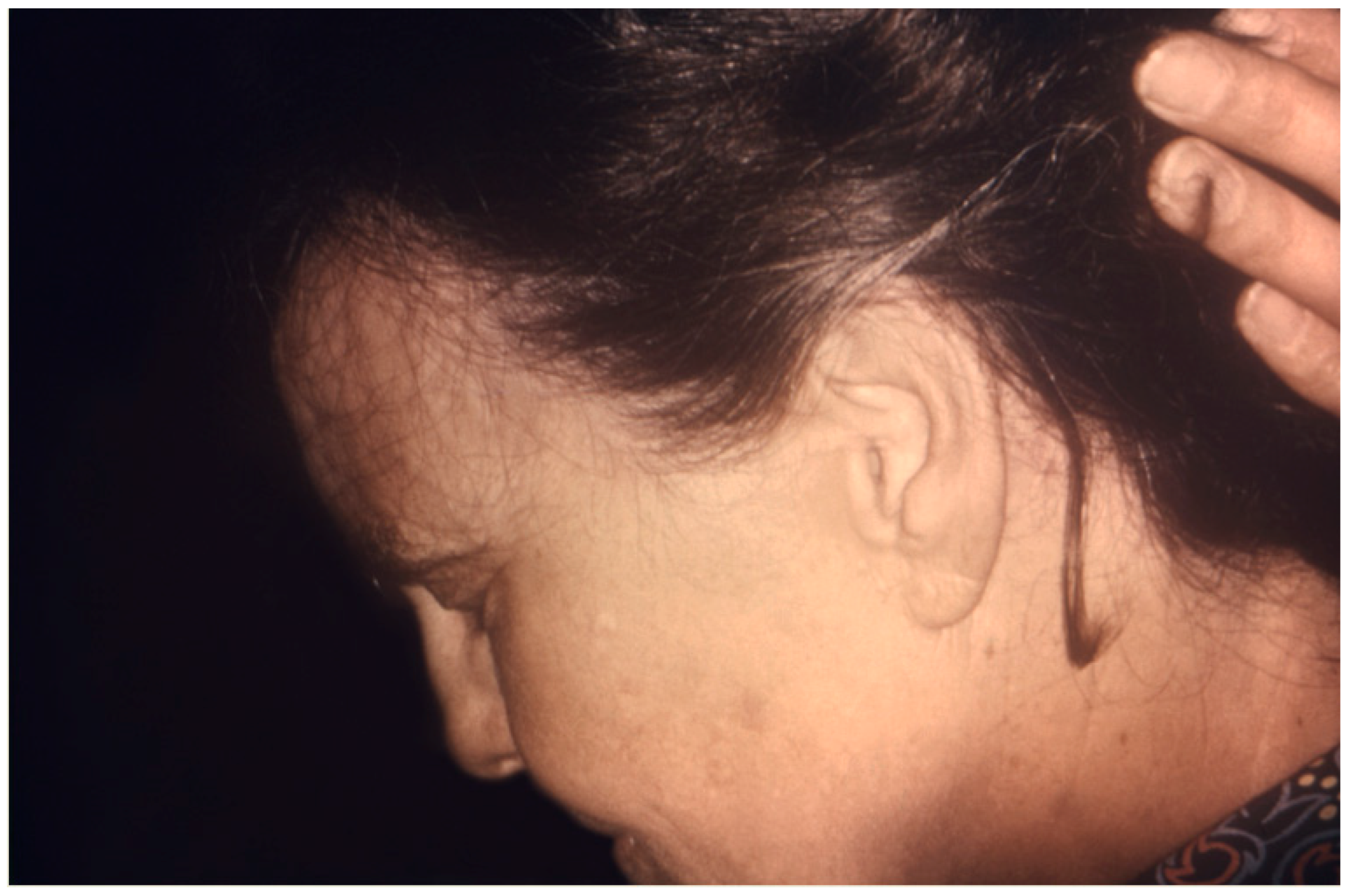
| Drug Name | Dose | Frequency | Laboratory Monitoring |
|---|---|---|---|
| Griseofulvin ultramicrosize | 10–15 mg/kg per day (maximum 750 mg per day) | 6–12 weeks | Liver function panel and complete blood count if therapy extends ≥ 8 weeks |
| Terbinafine | 250 mg per day | 4–12 weeks depending on species 4–6 weeks for Trichophyton species 8–12 weeks for Microsporum species | Liver function panel before therapy begins and again if therapy extends ≥ 6 weeks CBC if therapy extends ≥ 6 weeks |
| Itraconazole | 5 mg/kg per day (maximum 400 mg per day) | 2–3 weeks, or consider pulse therapy | Liver function panel before therapy begins and again at 4 weeks |
| Fluconazole | 6 mg/kg per day (maximum 400 mg per day) | 3–6 weeks | No laboratory monitoring required |
| Group | Interventions | Frequency and Duration |
|---|---|---|
| Individual diagnosed with TC | Antifungal shampoo (ketoconazole 2% shampoo, selenium sulfide shampoo 1% or 2.5%, or ciclopirox 1% shampoo) | Twice weekly until complete cure |
| Disinfection of fomites (manual cleaning and chemical disinfection) | As needed | |
| Asymptomatic Carriers | Antifungal shampoo (ketoconazole 2% shampoo, selenium sulfide shampoo 1% or 2.5%, or ciclopirox 1% shampoo) if spore count low on culture | Twice weekly until complete cure |
| Addition of oral antifungals if spore count high on culture | Follow oral antifungal instructions | |
| Household contacts of children with TC | Antifungal shampoo (ketoconazole 2% shampoo, selenium sulfide shampoo 1% or 2.5%, or ciclopirox 1% shampoo) | Twice weekly for 4–6 weeks |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hill, R.C.; Gold, J.A.W.; Lipner, S.R. Comprehensive Review of Tinea Capitis in Adults: Epidemiology, Risk Factors, Clinical Presentations, and Management. J. Fungi 2024, 10, 357. https://doi.org/10.3390/jof10050357
Hill RC, Gold JAW, Lipner SR. Comprehensive Review of Tinea Capitis in Adults: Epidemiology, Risk Factors, Clinical Presentations, and Management. Journal of Fungi. 2024; 10(5):357. https://doi.org/10.3390/jof10050357
Chicago/Turabian StyleHill, Rachel C., Jeremy A. W. Gold, and Shari R. Lipner. 2024. "Comprehensive Review of Tinea Capitis in Adults: Epidemiology, Risk Factors, Clinical Presentations, and Management" Journal of Fungi 10, no. 5: 357. https://doi.org/10.3390/jof10050357
APA StyleHill, R. C., Gold, J. A. W., & Lipner, S. R. (2024). Comprehensive Review of Tinea Capitis in Adults: Epidemiology, Risk Factors, Clinical Presentations, and Management. Journal of Fungi, 10(5), 357. https://doi.org/10.3390/jof10050357






