Evaluation of Cordyceps sinensis Quality in 15 Production Areas Using Metabolomics and the Membership Function Method
Abstract
1. Introduction
2. Materials and Methods
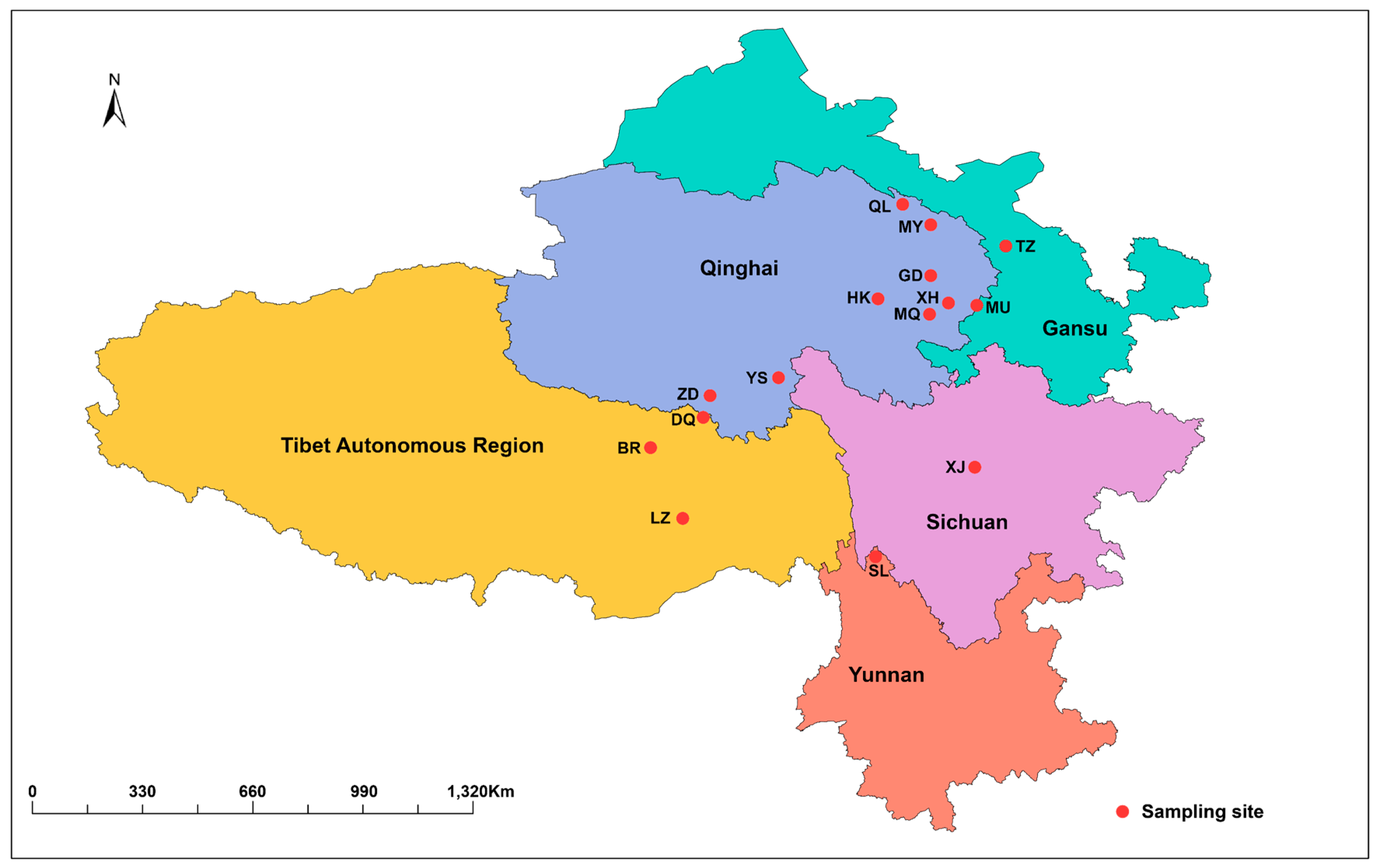
2.1. Sample and Data Collection
2.2. Sample Pretreatment and Metabolite Extraction
2.3. LC-MS/MS
2.4. Metabolite Identification and Quantitative Analysis
2.5. Acquisition of the Integrated Membership Function
3. Results
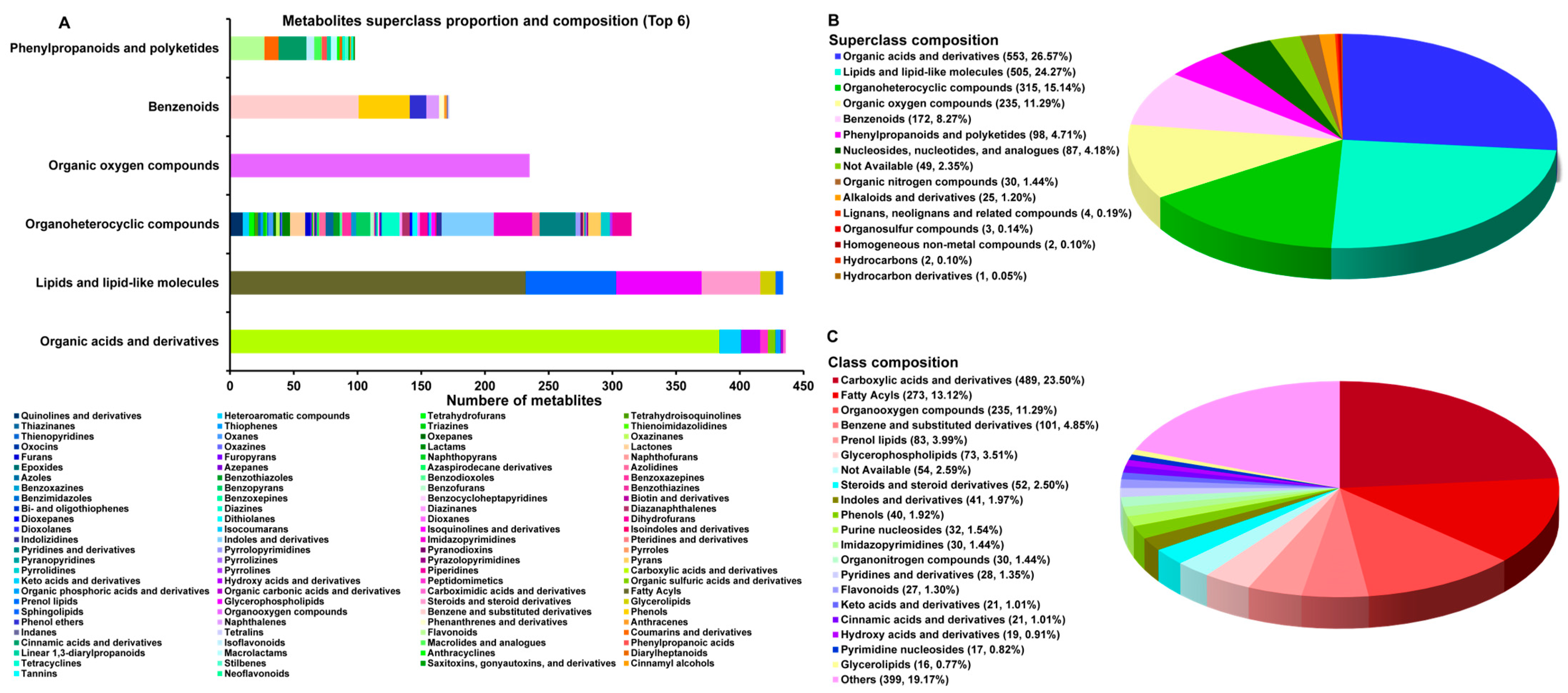
3.1. Overview of C. sinensis Metabolites
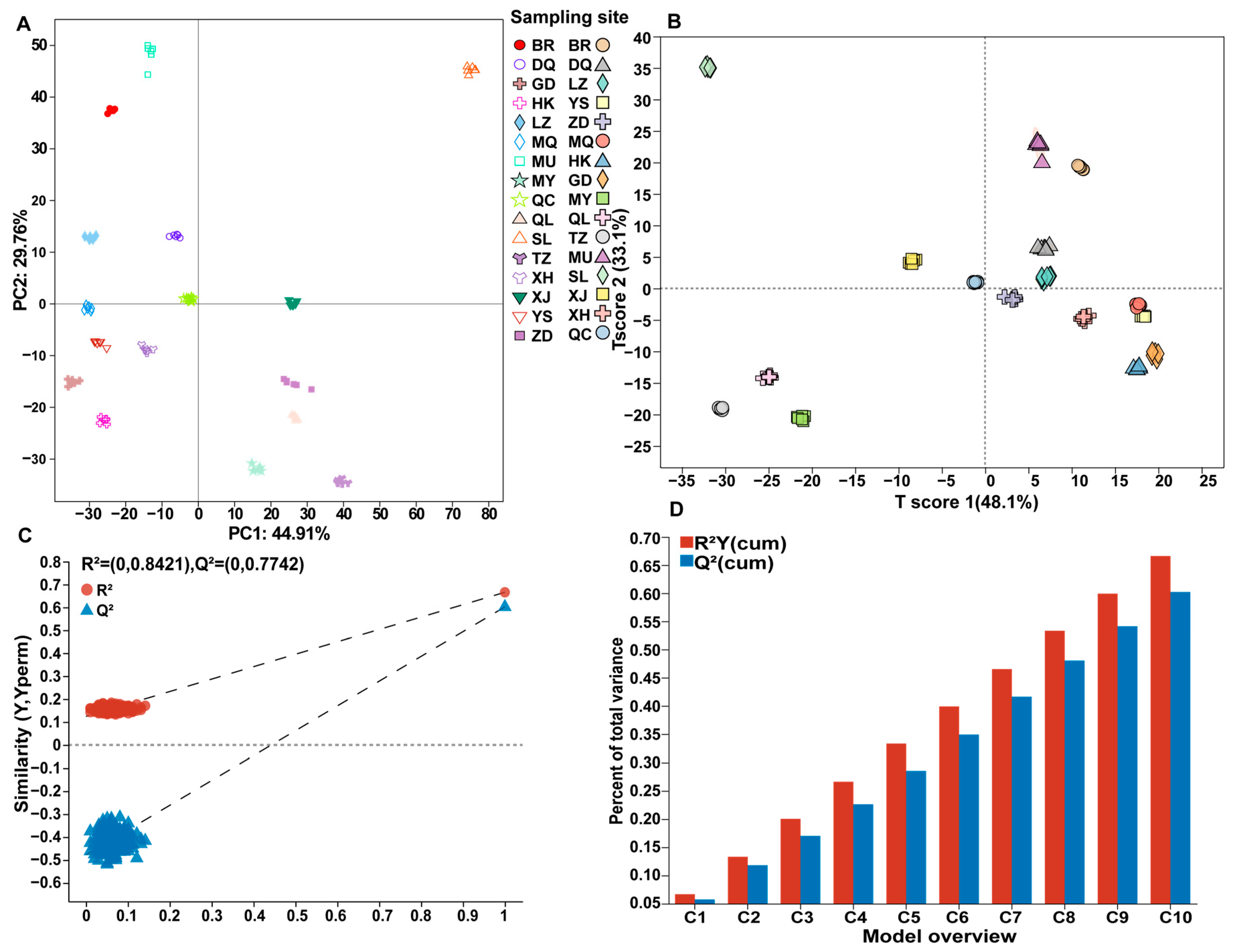
3.2. Multivariate Statistical Analysis of Metabolites
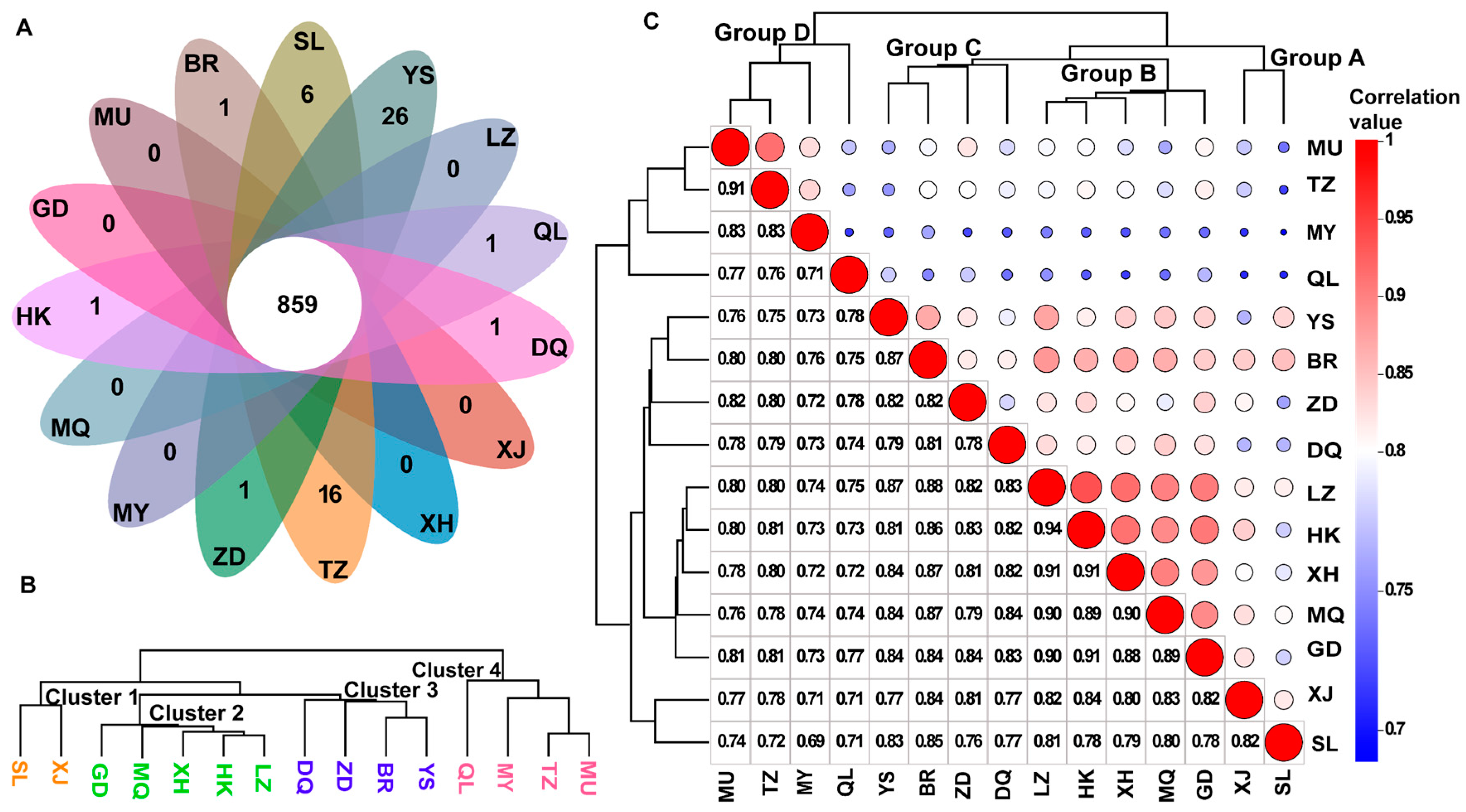
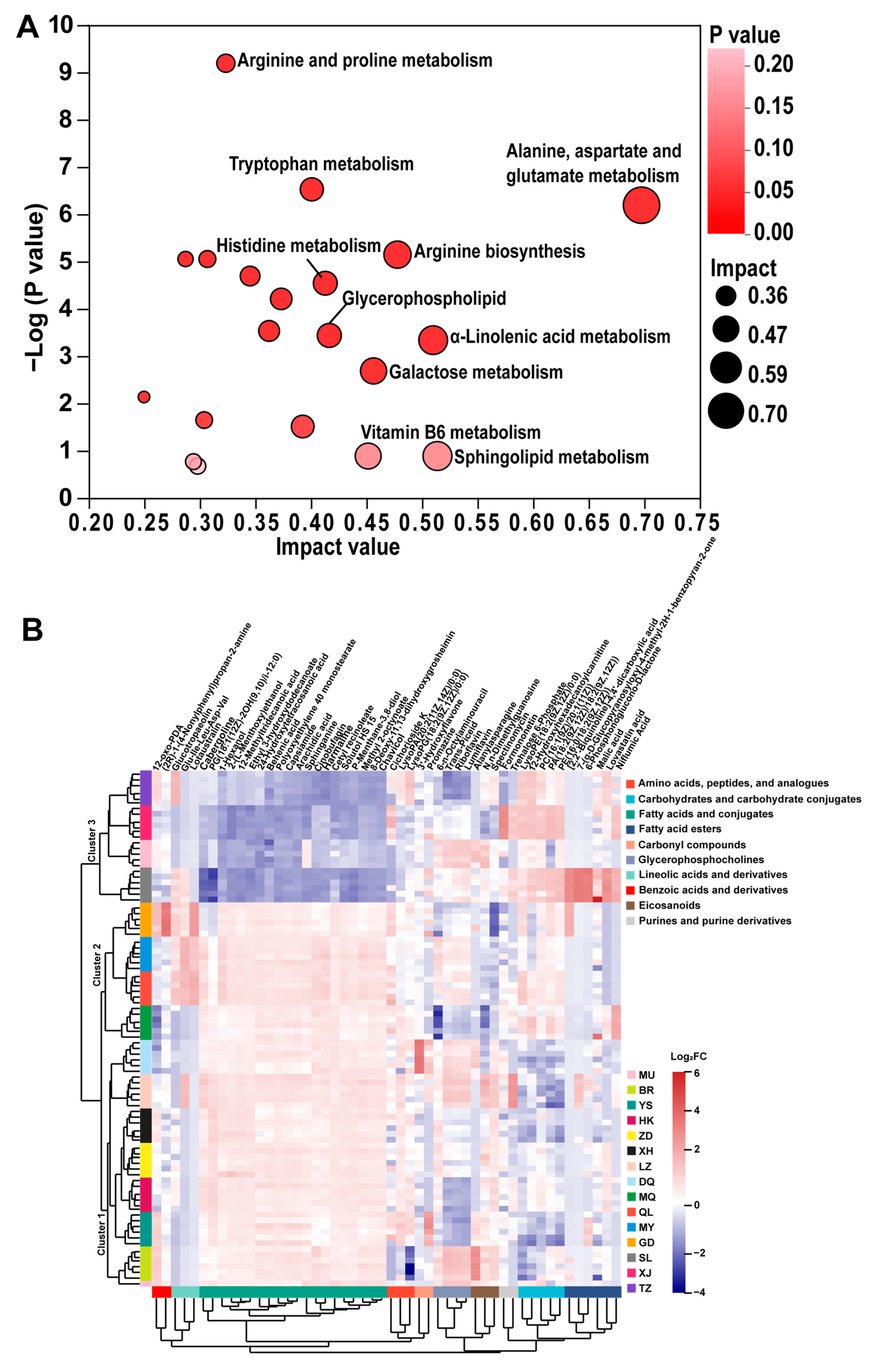
3.3. Identification of DAMs and Enrichment Pathway Analysis
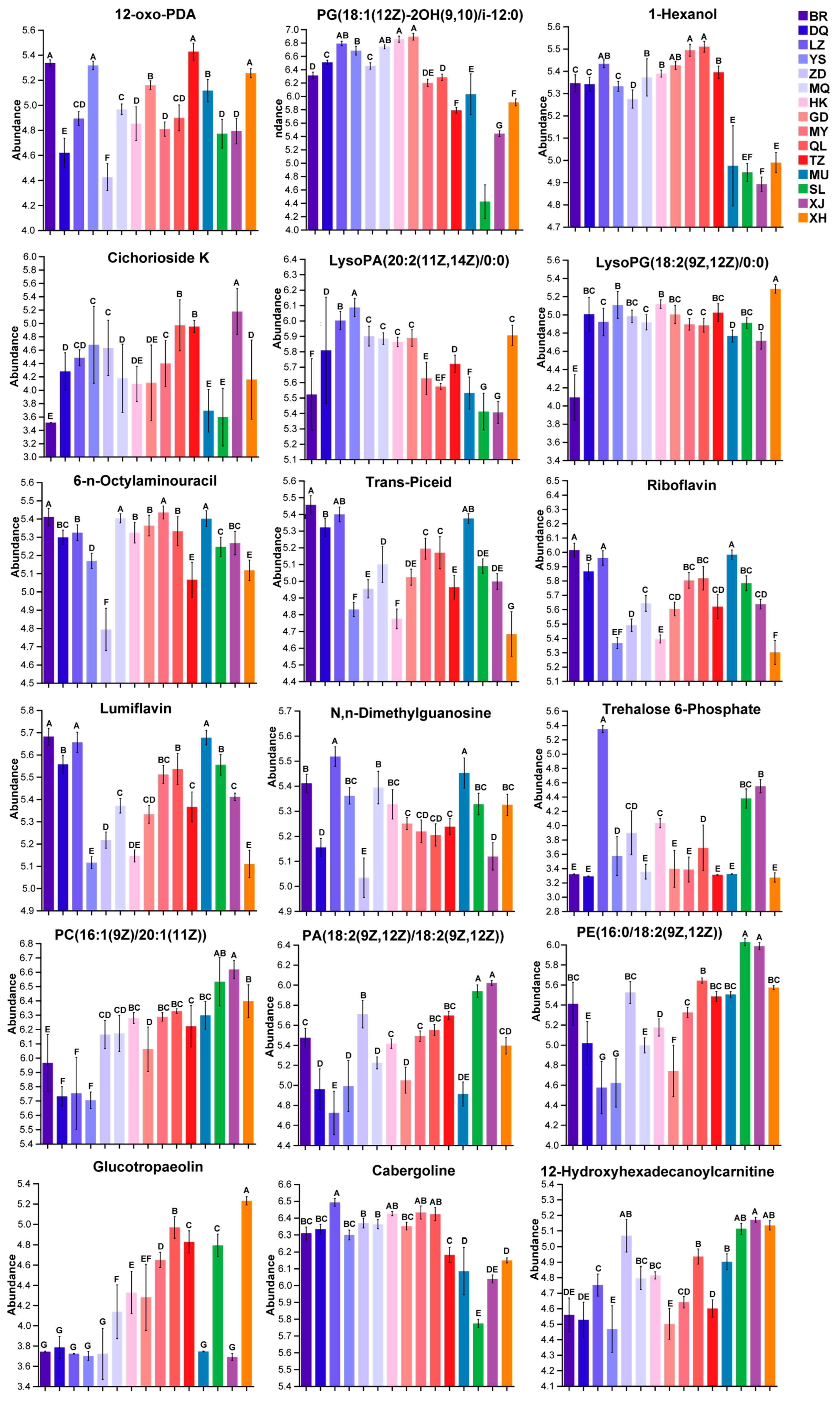
3.4. Validation of Biomarkers
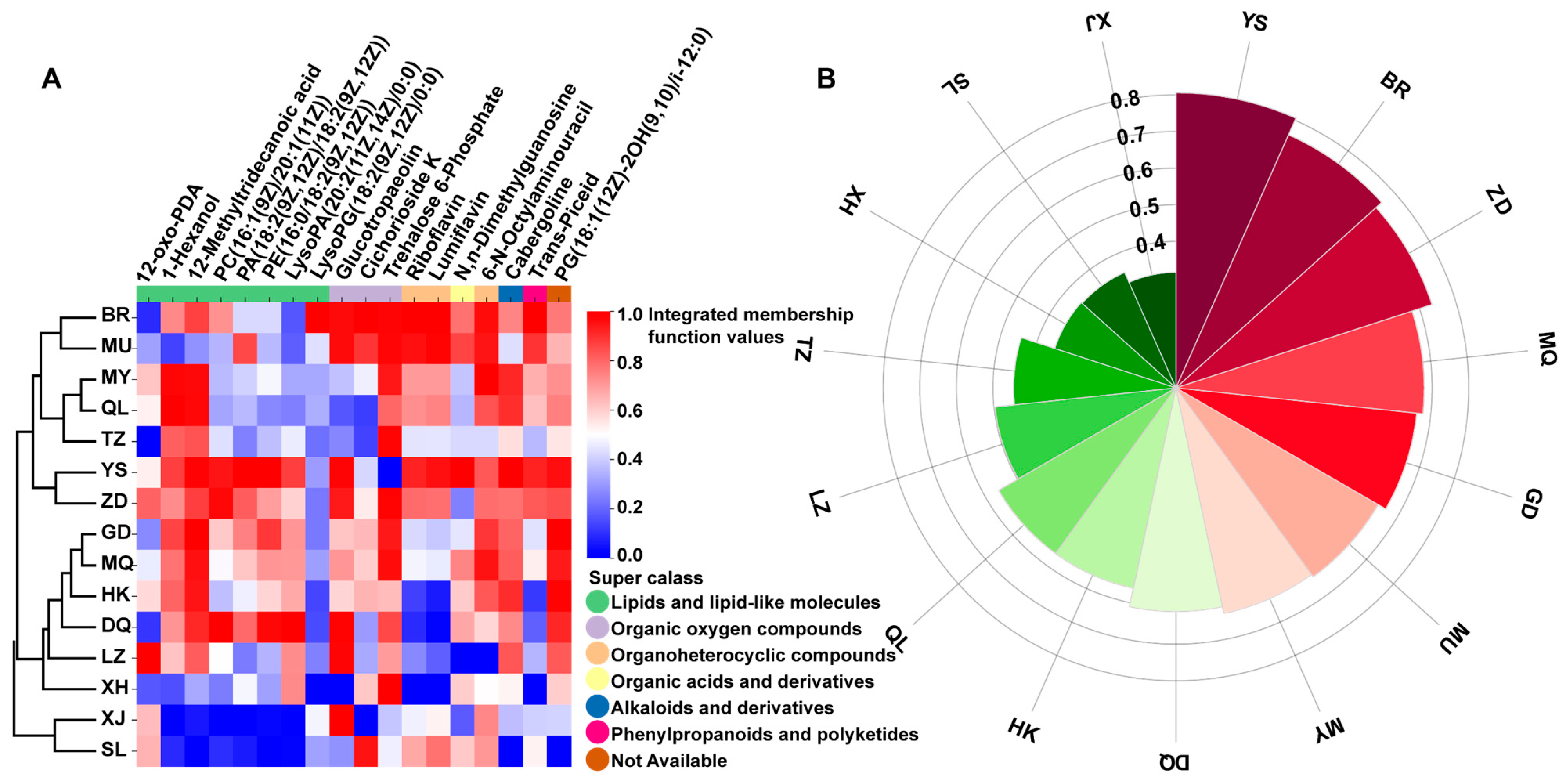
3.5. Comprehensive Quality Evaluation of C. sinensis from 15 Producing Areas Based on Biomarkers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, J.; Li, X.Z.; Chen, J.B.; Tang, C.Y.; Wang, T.; Li, Y.L. Suitability and regionalization of Chinese cordyceps in Qinghai Province Northwest China. Mycosystema 2022, 41, 1772–1785. [Google Scholar] [CrossRef]
- Li, S.P.; Yang, F.Q.; Tsim, K.W.K. Quality control of Cordyceps sinensis, a valued traditional Chinese medicine. J. Pharm. Biomed. 2006, 41, 1571–1584. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.W.; Gong, Z.H.; Su, Y.; Lin, J.; Tang, K.X. Cordyceps fungi: Natural products, pharmacological functions and developmental products. J. Pharm. Pharmacol. 2009, 61, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Jie, Y.; Cao, G.C.; Rong, Z.L.; Li, H.F. Global prediction of potential distribution of Ophiocordyceps sinensis in Chain based on Maxent model. Ecol. Sci. 2023, 42, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.J.; Yi, L.; Wang, W.J.; He, J.S.; Yang, R.H.; Wu, H.J.; Wang, X.L.; Jiao, L.; Tang, Z.Y.; Yao, Y.J. Range shifts in response to climate change of Ophiocordyceps sinensis, a fungus endemic to the Tibetan Plateau. Biol. Conserv. 2017, 206, 143–150. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, Y.; Cui, Y.; Liu, H.; Dong, C.; Sun, Y. Structural characterization, antioxidant and immunomodulatory activities of a neutral polysaccharide from Cordyceps militaris cultivated on hull-less barley. Carbohydr. Polym 2020, 235, 115969. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Shan, G.; Di, X.; Li, S.; Hu, H.K. Lipidomic profiling of wild cordyceps and its substituents by liquid chromatography-electrospray ionization-tandem mass spectrometry. LWT 2022, 163, 113497. [Google Scholar] [CrossRef]
- Zhou, J.; Hou, D.; Zou, W.; Wang, J.; Luo, R.; Wang, M.; Yu, H. Comparison of Widely Targeted Metabolomics and Untargeted Metabolomics of Wild Ophiocordyceps sinensis. Molecules 2022, 27, 3645. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Q.; Kan, L.J.; Nie, S.P.; Chen, H.; Steve, W.C.; Aled, O.P.; Glyn, O.P.; Li, Y.J.; Xie, M.Y. A comparison of chemical composition, bioactive components and antioxidant activity of natural and cultured Cordyceps sinensis. LWT-Food Sci. Technol. 2015, 63, 2–7. [Google Scholar] [CrossRef]
- Yang, M.L.; Kuo, P.C.; Hwang, T.L.; Wu, T.S. Anti-inflammatory principles from Cordyceps sinensis. J. Nat. Prod 2011, 74, 1996–2000. [Google Scholar] [CrossRef]
- Qiu, W.; Wu, J.; Choi, J.; Hirai, H.; Nishida, H.; Kawagishi, H. Cytotoxic compounds against cancer cells from Bombyx moriinoculated with Cordyceps militaris. Biosci. Biotechnol. Biochem. 2017, 81, 1224–1226. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.N.; Kim, J.; Lee, M.Y.; Park, D.K.; Hong, Y.S.; Lee, C.H. Metabolomics revealed novel isoflavones and optimal cultivation time of Cordyceps militaris fermentation. J. Agric. Food Chem. 2010, 58, 4258–4267. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Yin, Y.; Yu, G.; Jin, Y.; Ye, X.; Shrestha, A.; Liu, W.; Yu, W.; Sun, H. A novel protein with anti-metastasis activity on 4T1 carcinoma from medicinal fungus Cordyceps militaris. Int. J. Biol. Macromol. 2015, 80, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Chen, Y.; Abbasi, A.M.; Ma, H.; Mo, H.; Liu, B. Tannin fraction from Ampelopsis grossedentata leaves tea (Tengcha) as an antioxidant and α-glucosidase inhibitory nutraceutical. Int. J. Food Sci. Technol. 2016, 51, 2692–2700. [Google Scholar] [CrossRef]
- Liu, W.C.; Chuang, W.L.; Tsai, M.L.; Hong, J.H.; McBride, W.H.; Chiang, C.S. Cordyceps sinensis Health supplement enhances recovery from taxol-induced leukopenia. Exp. Biol. Med. 2008, 233, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.H.; Xu, X.R.; Wei, X.C.; Feng, W.W.; Huang, H.Z.; Liu, H.Y.; Xu, R.C.; Lin, J.Z.; Han, L.; Zhang, D.K. Natural medicines for the treatment of fatigue: Bioactive components, pharmacology, and mechanisms. Pharmacol. Res. 2019, 148, 104409. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Shi, T.; Zhu, W.; Chen, L.; Guan, Y.; Jin, C. Quality control method of sterols in fermented Cordyceps sinensis based on combined fingerprint and quantitative analysis of multicomponents by single marker. J. Food Sci. 2022, 85, 2994–3002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Sun, X.; Miao, Y.J.; Li, M.H.; Huang, L.F. Cordyceps cicadae and Cordyceps gunnii have closer species correlation with Cordyceps sinensis: From the perspective of metabonomic and MaxEnt models. Sci. Rep. 2022, 12, 20469. [Google Scholar] [CrossRef]
- Guo, L.X.; Xu, X.M.; Liang, F.R.; Yuan, J.P.; Peng, J.; Wu, C.F.; Wang, J.H. Morphological Observations and Fatty Acid Composition of Indoor-Cultivated Cordyceps sinensis at a High-Altitude Laboratory on Sejila Mountain, Tibet. PLoS ONE 2015, 10, e0126095. [Google Scholar] [CrossRef]
- Zhang, J.S.; Wang, N.; Chen, W.X.; Zhang, W.P.; Zhang, H.S.; Yu, H.; Yi, Y. Integrated metabolomics and transcriptomics reveal metabolites difference between wild and cultivated Ophiocordyceps sinensis. Food Res. Int. 2023, 163, 112275. [Google Scholar] [CrossRef]
- Yao, C.L.; Qian, Z.M.; Tian, W.S.; Xu, X.Q.; Yan, Y.; Shen, Y.; Lu, S.M.; Li, W.J.; Guo, D.A. Profiling and identification of aqueous extract of Cordyceps sinensis by ultra-high performance liquid chromatography tandem quadrupole-orbitrap mass spectrometry. Chin. J. Nat. Med. 2019, 17, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.K.; Li, J.Y.; Yuan, C.; Xi, B.; Yang, B.H.; Meng, X.Y.; Guo, T.T.; Yue, Y.J.; Guo, Y.Q.; Liu, J.B.; et al. Evaluation of Mutton Quality Characteristics of Dongxiang Tribute Sheep Based on Membership Function and Gas Chromatography and Ion Mobility Spectrometry. Front. Nutr. 2022, 9, e852399. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.W.; Guo, B.J.; Guo, Y.; Ma, X.L.; Luo, S.S.; Feng, L.L.; Pan, Z.P.; Deng, L.J.; Pan, S.H.; Wei, J.B.; et al. A comprehensive “quality-quantity-activity” approach based on portable near-infrared spectrometer and membership function analysis to systematically evaluate spice quality: Cinnamomum cassia as an example. Food Chem. 2024, 439, 138142. [Google Scholar] [CrossRef] [PubMed]
- Keller, N.P. Fungal secondary metabolism: Regulation, function and drug discovery. Nat. Rev. Microbiol. 2019, 17, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.H.; Wu, F.F.; Zou, Z.C.; Mao, L.Y.; Shan, L. Discovery of the chemical constituents, structural characteristics, and pharmacological functions of Chinese caterpillar fungus. Open Chem. 2023, 21, 20220337. [Google Scholar] [CrossRef]
- Wang, T.; Tang, C.Y.; Xiao, M.J.; Cao, Z.F.; He, M.; Qi, J.Z.; Li, Y.L.; Li, X.Z. Effect of Air Drying on the Metabolic Profile of Fresh Wild and Artificial Cordyceps sinensis. Foods 2024, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Ahn, B.; Kim, M.; Ji, H.J.; Shin, S.K.; Hong, I.P.; Kim, C.Y.; Hwang, B.Y.; Lee, M.K. Effect of Cordyceps militaris extract and active constituents on metabolic parameters of obesity induced by high-fat diet in C58BL/6J mice. J. Ethnopharmacol. 2014, 151, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Chen, C.; Zou, Y.; Lou, H.; Zheng, Q.; Guo, L.; Lin, J.; Ye, Z.; Yun, F. Purification and structural characterization of a novel natural pigment: Cordycepene from edible and medicinal mushroom Cordyceps militaris. Appl. Microbiol. Biotechnol. 2019, 103, 7943–7952. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Omega-3 fatty acids in inflammation and autoimmune diseases. J. Am. Coll. Nutr. 2002, 21, 495–505. [Google Scholar] [CrossRef]
- Young, G.; Conquer, J. The emerging role of omega-3 fatty acids and neuropsychiatric disorders. Reprod. Nutr. Dev. 2005, 45, 1–28. [Google Scholar] [CrossRef]
- Lee, K.H.; Seong, H.J.; Kim, G.; Jeong, G.H.; Kim, J.Y.; Park, H.; Jung, E.Y.; Kronbichler, A.; Eisenhut, M.; Stubbs, B.; et al. Consumption of fish and ω-3 fatty acids and cancer risk: An umbrella review of meta-analyses of observational studies. Adv. Nutr. 2020, 11, 1134–1149. [Google Scholar] [CrossRef] [PubMed]
- Elagizi, A.; Lavie, C.J.; O’Keefe, E.; Marshall, K.; O’Keefe, J.H.; Milani, R.V. An update on omega-3 polyunsaturated fatty acids and cardiovascular health. Nutrients 2021, 13, 204. [Google Scholar] [CrossRef] [PubMed]
- Astore, C.; Gibson, G. Integrative polygenic analysis of the protective effects of fatty acid metabolism on disease as modified by obesity. Front. Nutr 2024, 10, 1308622. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, H.; Lu, H.; Tang, X.; Zhang, H.; Chen, Y.Q.; Chen, W. Carbohydrate analysis of Mortierella alpina by colorimetry and HPLC–ELSD to reveal accumulation differences of sugar and lipid. Biotechnol. Lett. 2021, 43, 1289–1301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, J.; Fei, Z.; Dai, H.; Fan, Q.; Yang, Q.; Chen, Y.; Wang, B.; Wang, C. 3D Printing Scaffold Vaccine for Antitumor Immunity. Adv. Mater. 2021, 33, 2106768. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, X.M.; Huang, J.X.; Lin, Q.; Qin, B.; Jiang, M.S.; Shan, X.Y.; Luo, Z.Y.; Zhang, J.L.; Shi, Y.Y.; et al. Recruiting T cells and sensitizing tumors to NKG2D immune surveillance for robust antitumor immune response. J. Control. Release 2023, 353, 943–955. [Google Scholar] [CrossRef]
- Yu, Y.; Wen, Q.; Song, A.; Liu, Y.; Wang, F.; Jiang, B. Isolation and immune activity of a new acidic Cordyceps militaris exopolysaccharide. Int. J. Biol. Macromol. 2022, 194, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Siu, K.C.; Wang, W.Q.; Liu, X.X.; Wu, J.Y. Structure and antioxidant activity of a novel poly-N-acetylhexosamine produced by a medicinal fungus. Carbohydr. Polym. 2013, 94, 332–338. [Google Scholar] [CrossRef]
- Li, M.M.; Meng, Q.; Zhang, H.; Shu, R.H.; Zhao, Y.N.; Wu, P.P.; Zhang, J.H. Changes in transcriptomic and metabolomic profiles of morphotypes of Ophiocordyceps sinensis within the hemocoel of its host larvae, Thitarodes xiaojinensis. BMC Genom. 2020, 21, 789. [Google Scholar] [CrossRef]
- Tang, R.; Qiu, X.H.; Cao, L.; Long, H.L.; Han, R.C. Stage- and rearing dependent metabolomics profiling of Ophiocordyceps sinensis and its pipeline products. Insects 2021, 12, 666. [Google Scholar] [CrossRef]
- Carturan, L.; De, B.F.; Dinale, R.; Dragà, G.; Gabrielli, P.; Mair, V.; Seppi, R.; Tonidandel, D.; Zanoner, T.; Zendrini, T.L.; et al. Modern air, englacial and permafrost temperatures at high altitude on Mt Ortles (3905 m a.s.l.), in the eastern European Alps. Earth Syst. Sci. Data 2023, 15, 4661–4688. [Google Scholar] [CrossRef]
- Stanisław, B.; Mariusz, C.; Ulf, B.; Edyta, T.S. High oxidative stress despite low energy metabolism and vice versa: Insights through temperature acclimation in an ectotherm. J. Therm. Biol. 2018, 78, 36–41. [Google Scholar] [CrossRef] [PubMed]
- John, N.I.; Jonathan, C.E.; Mosope, F.A.; David, H.H.; Ann, L.G.; Wendy, M.S. Dynamics of mitochondrial adaptation and energy metabolism in rainbow trout (Oncorhynchus mykiss) in response to sustainable diet and temperature Get access Arrow. J. Anim. Sci. 2023, 101, e348. [Google Scholar] [CrossRef] [PubMed]
- Morgan-Kiss, R.M.; Priscu, J.C.; Pocock, T.; Gudynaite, S.L.; Huner, N.P. Adaptation and acclimation of photosynthetic microorganisms to permanently cold environments. Microbiol. Mol. Biol. Rev. 2006, 70, 222–252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yue, P.; Tong, X.; Gao, T.; Peng, T.; Guo, J. Comparative analysis of fatty acid metabolism based on transcriptome sequencing of wild and cultivated Ophiocordyceps sinensis. PeerJ 2021, 9, e11681. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Sun, C.; Xie, B.; Wang, T.; Liu, H.; Chen, X.; Huang, Q.; Zhang, C.; Li, T.; Deng, W. Cordyceps guangdongensis lipid-lowering formula alleviates fat and lipid accumulation by modulating gut microbiota and short-chain fatty acids in high-fat diet mice. Front. Nutr. 2022, 9, 1038740. [Google Scholar] [CrossRef]
- Li, R.F.; Zhou, X.B.; Zhou, H.X.; Yang, Z.F.; Jiang, H.M.; Wu, X.; Li, W.J.; Qiu, J.J.; Mi, J.N.; Chen, M.; et al. Novel Fatty Acid in Cordyceps Suppresses Influenza A (H1N1) Virus-Induced Proinflammatory Response Through Regulating Innate Signaling Pathways. ACS Omega 2021, 6, 1505–1515. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Tang, C.; He, H.; Cao, Z.; Xiao, M.; He, M.; Qi, J.; Li, Y.; Li, X. Evaluation of Cordyceps sinensis Quality in 15 Production Areas Using Metabolomics and the Membership Function Method. J. Fungi 2024, 10, 356. https://doi.org/10.3390/jof10050356
Wang T, Tang C, He H, Cao Z, Xiao M, He M, Qi J, Li Y, Li X. Evaluation of Cordyceps sinensis Quality in 15 Production Areas Using Metabolomics and the Membership Function Method. Journal of Fungi. 2024; 10(5):356. https://doi.org/10.3390/jof10050356
Chicago/Turabian StyleWang, Tao, Chuyu Tang, Hui He, Zhengfei Cao, Mengjun Xiao, Min He, Jianzhao Qi, Yuling Li, and Xiuzhang Li. 2024. "Evaluation of Cordyceps sinensis Quality in 15 Production Areas Using Metabolomics and the Membership Function Method" Journal of Fungi 10, no. 5: 356. https://doi.org/10.3390/jof10050356
APA StyleWang, T., Tang, C., He, H., Cao, Z., Xiao, M., He, M., Qi, J., Li, Y., & Li, X. (2024). Evaluation of Cordyceps sinensis Quality in 15 Production Areas Using Metabolomics and the Membership Function Method. Journal of Fungi, 10(5), 356. https://doi.org/10.3390/jof10050356






