Population Genetic Analyses and Trichothecene Genotype Profiling of Fusarium pseudograminearum Causing Wheat Crown Rot in Henan, China
Abstract
1. Introduction
2. Materials and Methods
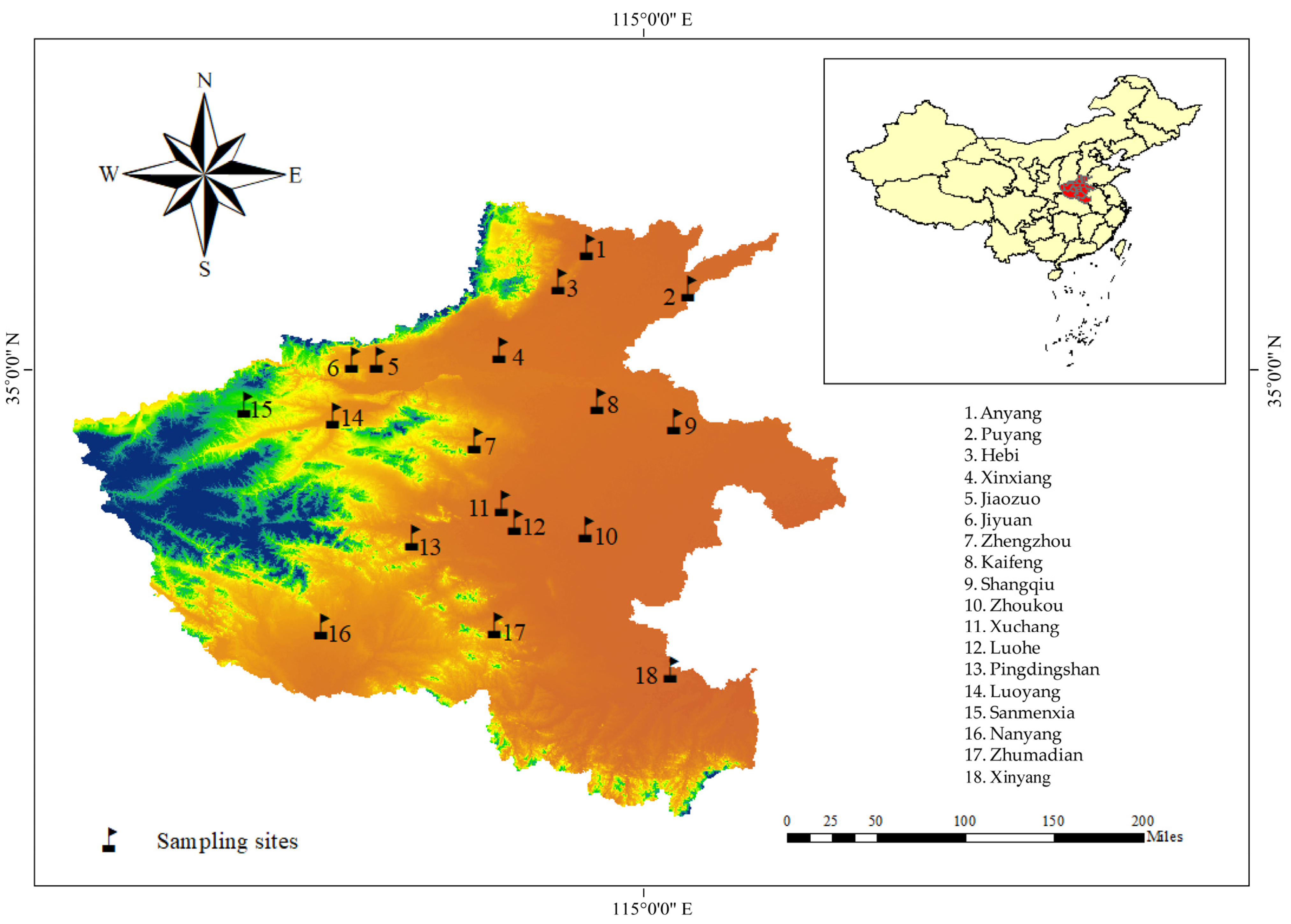
2.1. Sample Collection
2.2. Fungal Isolation and Culture
2.3. Genomic DNA Extraction
2.4. Fusarium pseudograminearum Confirmation by Specific Primers
2.5. Trichothecene Genotype Determination of Fusarium pseudograminearum
2.6. Mating Type Idiomorph Determination of Fusarium pseudograminearum
3. Results
3.1. Pathogen Identification
3.2. Trichothecene Genotype Determination
3.3. Mating Type Determination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Figueroa, M.; Hammond-Kosack, K.E.; Solomon, P. A review of wheat disease-a field perspective. Mol. Plant Pathol. 2018, 19, 1523–1536. [Google Scholar] [CrossRef]
- Serfling, A.; Kopahnke, D.; Habekuss, A.; Novakazi, F.; Ordon, F. Wheat diseases: An overview. In Achieving Sustainable Cultivation of Wheat; Langridge, P., Ed.; Burleigh Dodds Science Publishing: Cambridge, UK, 2017. [Google Scholar]
- Saad, A.; Christopher, J.; Martin, A.; McDonald, S.; Percy, C. Fusarium pseudograminearum and F. culmorum affect the root system architecture of bread wheat. Crop J. 2023, 11, 316–321. [Google Scholar] [CrossRef]
- Laraba, I.; Boureghda, H.; Abdallah, N.; Bouaicha, O.; Obanor, F.; Moretti, A.; Geiser, D.M.; Kim, H.; McCormick, S.P.; Proctor, R.H.; et al. Population genetic structure and mycotoxin potential of the wheat crown rot and head blight pathogen Fusarium culmorum in Algeria. Fungal Genet. Biol. 2017, 103, 34–41. [Google Scholar] [CrossRef]
- Kazan, K.; Gardiner, D.M. Fusarium crown rot caused by Fusarium pseudograminearum in cereal crops: Recent progress and future prospects. Mol. Plant Pathol. 2018, 19, 1547–1562. [Google Scholar] [CrossRef] [PubMed]
- Khudhair, M.; Thatcher, L.F.; Gardiner, D.M.; Kazan, K.; Roper, M.M.; Aitken, E.; Obanor, F. Comparative analysis of genetic structures and aggressiveness of Fusarium pseudograminearum populations from two surveys undertaken in 2008 and 2015 at two sites in the wheat belt of Western Australia. Plant Pathol. 2019, 68, 1337–1349. [Google Scholar] [CrossRef]
- Khudhair, M.; Obanor, F.; Kazan, K.; Gardiner, D.M.; Aitken, E.; McKay, A.; Giblot-Ducray, D.; Simpfendorfer, S.; Thatcher, L.F. Genetic diversity of Australian Fusarium pseudograminearum populations causing crown rot in wheat. Eur. J. Plant Pathol. 2021, 159, 741–753. [Google Scholar] [CrossRef]
- Bozoğlu, T.; Derviş, S.; Imren, M.; Amer, M.; Özdemir, F.; Paulitz, T.C.; Morgounov, A.; Dababat, A.A.; Özer, G. Fungal pathogens associated with crown and root rot of wheat in central, eastern, and southeastern Kazakhstan. J. Fungi 2022, 8, 417. [Google Scholar] [CrossRef] [PubMed]
- Özer, G.; Erper, İ.; Yıldız, Ş.; Bozoğlu, T.; Zholdoshbekova, S.; Alkan, M.; Tekin, F.; Uulu, T.E.; İmren, M.; Dababat, A.A.; et al. Fungal pathogens associated with crown and root rot in wheat-growing areas of Northern Kyrgyzstan. J. Fungi 2023, 9, 124. [Google Scholar] [CrossRef] [PubMed]
- Özer, G.; Paulitz, T.C.; İmren, M.; Alkan, M.; Muminjanov, H.; Dababat, A.A. Identity and pathogenicity of fungi associated with crown and root rot of dryland winter wheat in Azerbaijan. Plant Dis. 2020, 104, 2149–2157. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Liu, C.J.; Mitter, V.; Scott, J.B.; Akinsanmi, O.A.; Ali, S.; Dill-Macky, R.; Nicol, J.; Backhouse, D.; Simpfendorfer, S. Pathogen population structure and epidemiology are keys to wheat crown rot and Fusarium head blight management. Australas. Plant Pathol. 2006, 35, 643–655. [Google Scholar] [CrossRef]
- Smiley, R.W.; Gourlie, J.A.; Easley, S.A.; Patterson, L.M. Pathogenicity of fungi associated with the wheat crown rot complex in Oregon and Washington. Plant Dis. 2005, 89, 949–957. [Google Scholar] [CrossRef]
- Davis, R.A.; Huggins, D.R.; Cook, J.R.; Paulitz, T.C. Nitrogen and crop rotation effects on fusarium crown rot in no-till spring wheat. Can. J. Plant Pathol. 2009, 31, 456–467. [Google Scholar] [CrossRef]
- Agustí-Brisach, C.; Raya-Ortega, M.C.; Trapero, C.; Roca, L.F.; Luque, F.; López-Moral, A.; Fuentes, M.; Trapero, A. First report of Fusarium pseudograminearum causing crown rot of wheat in Europe. Plant Dis. 2018, 102, 1670. [Google Scholar] [CrossRef]
- Murray, G.M.; Brennan, J.P. Estimating disease losses to the Australian wheat industry. Australas. Plant Pathol. 2009, 38, 558–570. [Google Scholar] [CrossRef]
- Scherm, B.; Balmas, V.; Spanu, F.; Pani, G.; Delogu, G.; Pasquali, M.; Migheli, Q. Fusarium culmorum: Causal agent of foot and root rot and head blight on wheat. Mol. Plant Pathol. 2013, 14, 323–341. [Google Scholar] [CrossRef] [PubMed]
- Obanor, F.; Neate, S.; Simpfendorfer, S.; Sabburg, R.; Wilson, P.; Chakraborty, S. Fusarium graminearum and Fusarium pseudograminearum caused the 2010 head blight epidemics in Australia. Plant Pathol. 2013, 62, 79–91. [Google Scholar] [CrossRef]
- Smiley, R.W.; Gourlie, J.A.; Easley, S.A.; Patterson, L.M.; Whittaker, R.G. Crop damage estimates for crown rot of wheat and barley in the Pacific Northwest. Plant Dis. 2005, 89, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Yuan, H.X.; Fu, B.; Xing, X.P.; Sun, B.J.; Tang, W.H. First report of Fusarium pseudograminearum causing crown rot of wheat in Henan, China. Plant Dis. 2012, 96, 1065. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Sun, H.Y.; Shen, C.M.; Li, W.; Yu, H.S.; Chen, H.G. Survey of Fusarium spp. causing wheat crown rot in major winter wheat growing regions of China. Plant Dis. 2015, 99, 1610–1615. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.J.; Kong, L.X.; Li, Q.S.; Wang, L.S.; Chen, D.; Ma, P. First report of Fusarium pseudograminearum causing Fusarium head blight of wheat in Hebei province, China. Plant Dis. 2015, 100, 220. [Google Scholar] [CrossRef]
- Xu, F.; Song, Y.L.; Yang, G.Q.; Wang, J.M.; Liu, L.L.; Li, Y.H. First report of Fusarium pseudograminearum from wheat heads with Fusarium head blight in North China Plain. Plant Dis. 2015, 99, 156. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Song, Y.L.; Wang, J.M.; Liu, L.L.; Zhao, K. First report of Fusarium pseudograminearum causing crown rot on Aegilops tauschii in the North China Plain. Plant Dis. 2018, 102, 1041. [Google Scholar] [CrossRef]
- Jiang, H.; Ma, L.G.; Qi, K.; Zhang, Y.L.; Zhang, B.; Ma, G.P.; Qi, J.S. First report of maize seedling blight caused by Fusarium pseudograminearum in China. Plant Dis. 2022, 106, 2519. [Google Scholar] [CrossRef] [PubMed]
- Gratz, S.W.; Richardson, A.J.; Duncan, G.; Holtrop, G. Annual variation of dietary deoxynivalenol exposure during years of different Fusarium prevalence: A pilot biomonitoring study. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2014, 31, 1579–1585. [Google Scholar] [CrossRef]
- Obanor, F.; Chakraborty, S. Aetiology and toxigenicity of Fusarium graminearum and Fusarium pseudograminearum causing crown rot and head blight in Australia under natural and artificial infection. Plant Pathol. 2014, 32, 1218–1229. [Google Scholar] [CrossRef]
- Monds, R.D.; Cromey, M.G.; Lauren, D.R.; di Menna, M.; Marshall, J. Fusarium graminearum, F. cortaderiae and F. pseudograminearum in New Zealand: Molecular phylogenetic analysis, mycotoxin chemotypes and co-existence of species. Mycol. Res. 2005, 109, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Proctor, R.H.; Hohn, T.M.; McCormick, S.P. Reduced virulence of Gibberella zeae caused by disruption of a trichothecene toxin biosynthetic gene. Mol. Plant Microbe Interact. 1995, 8, 593–601. [Google Scholar] [CrossRef]
- Jansen, C.; Von Wettstein, D.; Schafer, W.; Kogel, K.H.; Felk, A.; Maier, F.J. Infection patterns in barley and wheat spikes inoculated with wild-type and trichodiene synthase gene disrupted Fusarium graminearum. Proc. Natl. Acad. Sci. USA 2005, 102, 16892–16897. [Google Scholar] [CrossRef]
- Xu, F.; Yang, G.; Wang, J.; Song, Y.; Liu, L.; Zhao, K.; Li, Y.; Han, Z. Spatial distribution of root and crown rot fungi associated with winter wheat in the North China Plain and its relationship with climate variables. Front. Microbiol. 2018, 9, 1054. [Google Scholar] [CrossRef]
- Zhou, H.; He, X.; Wang, S.; Ma, Q.; Sun, B.; Ding, S.; Chen, L.; Zhang, M.; Li, H. Diversity of the Fusarium pathogens associated with crown rot in the Huanghuai wheat-growing region of China. Environ. Microbiol. 2019, 21, 2740–2754. [Google Scholar] [CrossRef]
- Zhang, J.B.; Wang, J.H.; Gong, A.D.; Chen, F.F.; Song, B.; Li, X.; Li, H.P.; Peng, C.H.; Liao, Y.C. Natural occurrence of Fusarium head blight, mycotoxins and mycotoxin-producing isolates of Fusarium in commercial fields of wheat in Hubei. Mycol. Res. 2013, 62, 92–102. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Dang, F.; Qu, B.; Xu, Y.; Zhao, C.; Liao, Y. Determination of the trichothecene mycotoxin chemotypes and associated geographical distribution and phylogenetic species of the Fusarium graminearum clade from China. Mycol. Res. 2007, 111, 967–975. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Qu, B.; Zhang, J.; Huang, T.; Chen, F.; Liao, Y. Development of a generic PCR detection of 3-acetyldeoxynivalenol-, 15-acetyldeoxynivalenol- and nivalenol-chemotypes of Fusarium graminearum clade. Int. J. Mol. Sci. 2008, 9, 2495–2504. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; O’Donnell, K. Morphological and molecular characterization of F. pseudograminearum sp. nov., formerly recognized as the Group 1 population of Fusarium graminearum. Mycologia 1999, 91, 597–609. [Google Scholar] [CrossRef]
- Nicholson, P.; Simpson, D.R.; Weston, G.; Rezanoor, H.N.; Lees, A.K.; Parry, D.W.; Joyce, D. Detection and quantification of Fusarium culmorum and Fusarium graminearum in cereals using PCR assays. Physiol. Mol. Plant Pathol. 1998, 53, 17–37. [Google Scholar] [CrossRef]
- Deng, Y.; Liu, Z.; Chen, C.; Sun, H.; Cao, S.; Li, W.; Chen, H. A rapid method for chemotype identification of Fusarium pseudograminearum and its application on the analysis of the isolates from Huanghuai wheat-growing region of China. Acta Phytopathol. Sin. 2023. (In Chinese) [Google Scholar] [CrossRef]
- Kerényi, Z.; Moretti, A.; Waalwijk, C.; Oláh, B.; Hornok, L. Mating type sequences in asexually reproducing Fusarium species. Appl. Environ. Microbiol. 2004, 70, 4419–4423. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, Z.; Yang, X.; Yang, J.; Gong, A.; Zhang, J.; Chen, L.; Zhou, C. Fusarium graminearum species complex and trichothecene genotype. In Mycotoxins and Food Safety; Sabuncuoglu, S., Ed.; IntechOpen: London, UK, 2019. [Google Scholar]
- Deng, Y.Y.; Li, W.; Zhang, P.; Sun, H.Y.; Zhang, X.X.; Zhang, A.X.; Chen, H.G. Fusarium pseudograminearum as an emerging pathogen of crown rot of wheat in eastern China. Plant Pathol. 2020, 69, 240–248. [Google Scholar] [CrossRef]
- Bentley, A.R.; Leslie, J.F.; Liew, E.C.; Burgess, L.W.; Summerell, B.A. Genetic structure of Fusarium pseudograminearum populations from the Australian grain belt. Phytopathology 2008, 98, 250–255. [Google Scholar] [CrossRef]
- Balmas, V.; Scherm, B.; Marcello, A.; Beyer, M.; Hoffmann, L.; Migheli, Q.; Pasquali, M. Fusarium species and chemotypes associated with Fusarium head blight and Fusarium root rot on wheat in Sardinia. Plant Pathol. 2015, 64, 972–979. [Google Scholar] [CrossRef]
- Ward, T.J.; Clear, R.M.; Rooney, A.P.; O’Donnell, K.; Gaba, D.; Patrick, S.; Starkey, D.E.; Gilbert, J.; Geiser, D.M.; Nowicki, T.W. An adaptive evolutionary shift in Fusarium head blight pathogen populations is driving the rapid spread of more toxigenic Fusarium graminearum in North America. Fungal Genet. Biol. 2008, 45, 473–484. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Z.; van der Lee, T.; Chen, W.Q.; Xu, J.; Xu, J.S.; Yang, L.; Yu, D.; Waalwijk, C.; Feng, J. Population genetic analyses of Fusarium asiaticum populations from barley suggest a recent shift favoring 3ADON producers in Southern China. Phytopathology 2010, 100, 328–336. [Google Scholar] [CrossRef]
- Chen, L.; Yang, J.; Wang, H.; Yang, X.; Zhang, C.; Zhao, Z.; Wang, J. NX toxins: New threat posed by Fusarium graminearum species complex. Trends Food Sci. Technol. 2022, 119, 179–191. [Google Scholar] [CrossRef]
- Ward, T.J.; Bielawski, J.P.; Kistler, H.C.; Sullivan, E.; O’Donnell, K. Ancestral polymorphism and adaptive evolution in the trichothecene mycotoxin gene cluster of phytopathogenic Fusarium. Proc. Natl. Acad. Sci. USA 2002, 99, 9278–9283. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Sun, H.Y.; Li, W.; Xia, Y.L.; Deng, Y.Y.; Zhang, A.X.; Chen, H.G. Fitness of three chemotypes of Fusarium graminearum species complex in major winter wheat-producing areas of China. PLoS ONE 2017, 12, e0174040. [Google Scholar] [CrossRef] [PubMed]
- Nicolli, C.P.; Machado, F.J.; Spolti, P.; Del Ponte, E.M. Fitness traits of deoxynivalenol and nivalenolproducing Fusarium graminearum species complex strains from wheat. Plant Dis. 2018, 102, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Sabburg, R.; Obanor, F.; Aitken, E.; Chakraborty, S. Changing fitness of a necrotrophic plant pathogen under increasing temperature. Glob. Chang. Biol. 2015, 21, 3126–3137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Van der Lee, T.; Waalwijk, C.; Chen, W.; Xu, J.; Xu, J.; Zhang, Y.; Feng, J. Population analysis of the Fusarium graminearum species complex from wheat in China show a shift to more aggressive isolates. PLoS ONE 2012, 7, e31722. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Obanor, F.; Westecott, R.; Abeywickrama, K. Wheat crown rot pathogen Fusarium graminearum and F. pseudograminearum lacks specialisation. Phytopathology 2010, 100, 1057–1065. [Google Scholar] [CrossRef]
- Akinsanmi, O.A.; Mitter, V.; Simpfendorfer, S.; Backhouse, D.; Chakraborty, S. Identity and pathogenicity of Fusarium spp. isolated from wheat fields in Queensland and northern New South Wales. Aust. J. Agric. Res. 2004, 55, 97–107. [Google Scholar] [CrossRef]
- Smiley, R.W.; Patterson, L.M. Pathogenic fungi associated with Fusarium foot rot of winter wheat in the semiarid Pacific Northwest. Plant Dis. 1996, 80, 944–949. [Google Scholar] [CrossRef]
- Poole, G.J.; Smiley, R.W.; Walker, C.; Huggins, D.; Rupp, R.; Abatzoglou, J.; Garland-Campbell, K.; Paulitz, T.C. Effect of climate on the distribution of Fusarium spp. causing crown rot of wheat in the Pacific Northwest of the United States. Phytopathology 2013, 103, 1130–1140. [Google Scholar] [CrossRef]
- Backhouse, D. Forecasting the risk of crown rot between successive wheat crops. Aust. J. Exp. Agric. 2006, 46, 1499. [Google Scholar] [CrossRef]
- Knight, N.L.; Sutherland, M.W. Assessment of Fusarium pseudograminearum and F. culmorum biomass in seedlings of potential host cereal species. Plant Dis. 2017, 101, 2116–2122. [Google Scholar] [CrossRef]
- Župunski, V.; Jevtić, R.; Lalošević, M.; Mikić, S.; Orbović, B. The applicability of species- and trichothecene-specific primers in monitoring the Fusarium graminearum species complex and its impact on the surveillance of Fusarium head blight in winter wheat in Serbia. Agronomy 2021, 11, 778. [Google Scholar] [CrossRef]
- Boamah, S.; Zahang, S.; Xu, B.; Li, T.; Calderón-Urrea, A. Trichoderma longibrachiatum (TG1) enhances wheat seedlings tolerance to salt stress and resistance to Fusarium pseudograminearum in China. Front. Plant Sci. 2021, 12, 741231. [Google Scholar] [CrossRef]
- Li, Q.; Hao, X.; Guo, Z.; Qu, K.; Gao, M.; Song, G.; Yin, Z.; Yuan, Y.; Dong, C.; Niu, J.; et al. Screening and resistance locus identification of the mutant fcrZ22 resistant to crown rot caused by Fusarium pseudograminearum. Plant Dis. 2024, 108, 426–433. [Google Scholar] [CrossRef] [PubMed]
- McDonald, B.A.; Linde, C. The population genetics of plant pathogens and breeding strategies for durable resistance. Euphytica 2002, 124, 163–180. [Google Scholar] [CrossRef]
- Southwell, R.J.; Moore, K.J.; Manning, W.; Hayman, P.T. An outbreak of Fusarium head blight of durum wheat on the Liverpool Plains in northern New South Wales in 1999. Australas. Plant Pathol. 2003, 32, 465–471. [Google Scholar] [CrossRef]
| Sample Site | April | May | June | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean Temperature (°C) | Mean Relative Humidity (%) | Precipitation (mm) | Mean Temperature (°C) | Mean Relative Humidity (%) | Precipitation (mm) | Mean Temperature (°C) | Mean Relative Humidity (%) | Precipitation (mm) | |
| Anyang | 15.6 | 59.0 | 118.2 | 20.5 | 67.0 | 133.4 | 27.0 | 52.0 | 26.6 |
| Puyang | 15.5 | 65.0 | 194.0 | 20.5 | 66.0 | 24.2 | 27.5 | 62.0 | 35.7 |
| Hebi | 15.3 | 65.0 | 91.0 | 20.3 | 70.0 | 77.3 | 27.3 | 53.0 | 56.0 |
| Xinxiang | 15.8 | 65.0 | 76.2 | 20.8 | 70.0 | 95.4 | 27.4 | 57.0 | 11.3 |
| Jiaozuo | 15.6 | 62.0 | 115.9 | 21.5 | 67.0 | 45.9 | 27.4 | 57.0 | 98.5 |
| Jiyuan | 15.5 | 65.0 | 56.3 | 20.3 | 71.0 | 194.3 | 26.7 | 58.0 | 60.9 |
| Zhengzhou | 16.5 | 63.0 | 29.9 | 21.5 | 75.0 | 174.2 | 27.5 | 68.0 | 44.3 |
| Kaifeng | 16.5 | 61.0 | 65.8 | 21.3 | 67.0 | 119.0 | 27.3 | 60.0 | 31.7 |
| Shangqiu | 15.4 | 68.0 | 77.7 | 20.3 | 73.0 | 133.9 | 26.1 | 66.0 | 63.2 |
| Zhoukou | 16.3 | 67.0 | 63.2 | 20.9 | 73.0 | 140.9 | 26.6 | 67.0 | 81.2 |
| Xuchang | 15.5 | 69.0 | 54.0 | 20.5 | 73.0 | 131.0 | 26.3 | 66.0 | 57.2 |
| Luohe | 16.3 | 60.0 | 166.5 | 21.5 | 72.0 | 111.7 | 25.5 | 66.0 | 53.7 |
| Pingdingshan | 15.4 | 58.0 | 102.7 | 20.5 | 68.0 | 35.7 | 26.3 | 65.0 | 13.7 |
| Luoyang | 15.6 | 59.0 | 84.8 | 20.1 | 69.0 | 123.9 | 25.6 | 62.0 | 51.2 |
| Sanmenxia | 15.1 | 59.0 | 47.4 | 18.5 | 68.0 | 104.9 | 25.5 | 58.0 | 8.8 |
| Nanyang | 16.6 | 67.0 | 42.7 | 20.7 | 73.0 | 122.1 | 25.0 | 72.0 | 194.1 |
| Zhumadian | 16.4 | 68.0 | 102.2 | 20.6 | 76.0 | 177.1 | 25.7 | 70.0 | 213.0 |
| Xinyang | 16.5 | 67.0 | 39.5 | 20.5 | 76.0 | 143.2 | 27.5 | 71.0 | 85.5 |
| Primer | Nucleotide Sequence (5′ to 3′) | Reference |
|---|---|---|
| Tri13P1 | CTC(G/C)ACCGCATCGAAGA(G/C)TCTC | [34] |
| Tri13P2 | GAA(G/C)GTCGCA(A/G)GACCTTGTTTC | [34] |
| Fp1-1 | CGGGGTAGTTTCACATTTC(C/T)G | [35] |
| Fp1-2 | GAGAATGTGATGA(C/G)GACAATA | [35] |
| Fg16F | CTCCGGATATGTTGCGTCAA | [36] |
| Fg16R | GGTAGGTATCCGACATGGCAA | [36] |
| 3AT8-1 | CCTTATGACTCCCCCGATGTCG | [37] |
| 3AT8-2 | TGTTTACCACCAGACCGGAC | [37] |
| 15AT8-1 | AAGCGCGCTCATGTCAGTCCAAGTT | [37] |
| 15AT8-2 | GCCCACCGACAGTATTCCTT | [37] |
| NIVT8-1 | GTACACCGCGAGCGCTATTTCTTCT | [37] |
| NIVT8-2 | CGTGAGACCCAACAGCAT | [37] |
| fusALPHAfor | CGCCCTCT(G/T)AA(C/T)G(C/G)CTTCATG | [38] |
| fusALPHArev | GGA(A/G)TA(A/G)AC(C/T)TTAGCAAT(C/T)AGGGC | [38] |
| fusHMGfor | CGACCTCCCAA(C/T)GC(C/T)TACAT | [38] |
| fusHMGrev | TGGGCGGTACTGGTA(A/G)TC(A/G)GG | [38] |
| City | Sampling Site | Strain Number | Number of Strains | ||
|---|---|---|---|---|---|
| 3ADON | 15ADON | NIV | |||
| Anyang | 35°59′42.702″ N, 114°32′54.676″ E | 8 | 0 | 8 | 0 |
| Puyang | 35°39′27.836″ N, 115°22′20.830″ E | 8 | 1 | 7 | 0 |
| Hebi | 35°42′51.059″ N, 114°19′8.590″ E | 7 | 0 | 7 | 0 |
| Xinxiang | 35°9′28.497″ N, 113°50′0.940″ E | 5 | 1 | 4 | 0 |
| Jiaozuo | 35°5′5.527″ N, 112°49′58.265″ E | 6 | 1 | 5 | 0 |
| Jiyuan | 35°5′10.142″ N, 112°38′18.015″ E | 11 | 2 | 9 | 0 |
| Zhengzhou | 34°25′33.189″ N, 113°38′2.691″ E | 11 | 0 | 11 | 0 |
| Kaifeng | 34°44′36.787″ N, 114°37′56.559″ E | 10 | 1 | 9 | 0 |
| Shangqiu | 34°34′47.536″ N, 115°15′47.639″ E | 7 | 0 | 7 | 0 |
| Zhoukou | 33°42′38.148″ N, 114°32′32.892″ E | 5 | 0 | 5 | 0 |
| Xuchang | 33°54′55.083″ N, 113°51′4.283″ E | 14 | 1 | 13 | 0 |
| Luohe | 33°46′9.229″ N, 113°57′52.073″ E | 7 | 0 | 7 | 0 |
| Pingdingshan | 33°38′31.425″ N, 113°7′24.507″ E | 9 | 0 | 8 | 1 |
| Luoyang | 34°38′4.856″ N, 112°29′5.618″ E | 5 | 0 | 5 | 0 |
| Sanmenxia | 34°43′16.480″ N, 111°45′25.842″ E | 5 | 0 | 5 | 0 |
| Nanyang | 32°55′18.865″ N, 112°23′3.635″ E | 10 | 0 | 10 | 0 |
| Zhumadian | 32°55′33.584″ N, 113°48′8.004″ E | 8 | 2 | 6 | 0 |
| Xinyang | 32°32′12.907″ N, 115°13′54.549″ E | 7 | 0 | 7 | 0 |
| Total | … | 143 | 9 | 133 | 1 |
| City | Strain Number | Number and Frequency (%) | χ2 | p-Value | |
|---|---|---|---|---|---|
| MAT-1 | MAT-2 | ||||
| Anyang | 8 | 1 (12.5) | 7 (87.5) | 4.500 | 0.034 * |
| Puyang | 8 | 8 (100.0) | 0 (0) | 8.000 | 0.005 * |
| Hebi | 7 | 5 (71.4) | 2 (28.6) | 1.286 | 0.257 |
| Xinxiang | 5 | 2 (40.0) | 3 (60.0) | 0.200 | 0.655 |
| Jiaozuo | 6 | 2 (33.3) | 4 (66.7) | 0.667 | 0.414 |
| Jiyuan | 11 | 4 (36.4) | 7 (63.6) | 0.818 | 0.366 |
| Zhengzhou | 11 | 8 (72.7) | 3 (27.3) | 2.273 | 0.132 |
| Kaifeng | 10 | 3 (30.0) | 7 (70.0) | 1.600 | 0.206 |
| Shangqiu | 7 | 2 (28.6) | 5 (71.4) | 1.286 | 0.257 |
| Zhoukou | 5 | 2 (40.0) | 3 (60.0) | 0.200 | 0.655 |
| Xuchang | 14 | 11 (78.6) | 3 (21.4) | 4.571 | 0.033 * |
| Luohe | 7 | 2 (28.6) | 5 (71.4) | 1.286 | 0.257 |
| Pingdingshan | 9 | 5 (55.6) | 4 (44.4) | 0.111 | 0.739 |
| Luoyang | 5 | 3 (60.0) | 2 (40.0) | 0.200 | 0.655 |
| Sanmenxia | 5 | 4 (80.0) | 1 (20.0) | 1.800 | 0.180 |
| Nanyang | 10 | 7 (70.0) | 3 (30.0) | 1.600 | 0.206 |
| Zhumadian | 8 | 7 (87.5) | 1 (12.5) | 4.500 | 0.034 * |
| Xinyang | 7 | 4 (57.1) | 3 (42.9) | 0.143 | 0.705 |
| Total | 143 | 80 (55.9) | 63 (44.1) | 2.021 | 0.155 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhang, J.; Wang, J.; Zhang, M.; Li, C.; Wang, W.; Suo, Y.; Song, F. Population Genetic Analyses and Trichothecene Genotype Profiling of Fusarium pseudograminearum Causing Wheat Crown Rot in Henan, China. J. Fungi 2024, 10, 240. https://doi.org/10.3390/jof10040240
Zhang J, Zhang J, Wang J, Zhang M, Li C, Wang W, Suo Y, Song F. Population Genetic Analyses and Trichothecene Genotype Profiling of Fusarium pseudograminearum Causing Wheat Crown Rot in Henan, China. Journal of Fungi. 2024; 10(4):240. https://doi.org/10.3390/jof10040240
Chicago/Turabian StyleZhang, Jianzhou, Jiahui Zhang, Jianhua Wang, Mengyuan Zhang, Chunying Li, Wenyu Wang, Yujuan Suo, and Fengping Song. 2024. "Population Genetic Analyses and Trichothecene Genotype Profiling of Fusarium pseudograminearum Causing Wheat Crown Rot in Henan, China" Journal of Fungi 10, no. 4: 240. https://doi.org/10.3390/jof10040240
APA StyleZhang, J., Zhang, J., Wang, J., Zhang, M., Li, C., Wang, W., Suo, Y., & Song, F. (2024). Population Genetic Analyses and Trichothecene Genotype Profiling of Fusarium pseudograminearum Causing Wheat Crown Rot in Henan, China. Journal of Fungi, 10(4), 240. https://doi.org/10.3390/jof10040240







