Influence of Salinity on the Extracellular Enzymatic Activities of Marine Pelagic Fungi
Abstract
1. Introduction
2. Materials and Methods
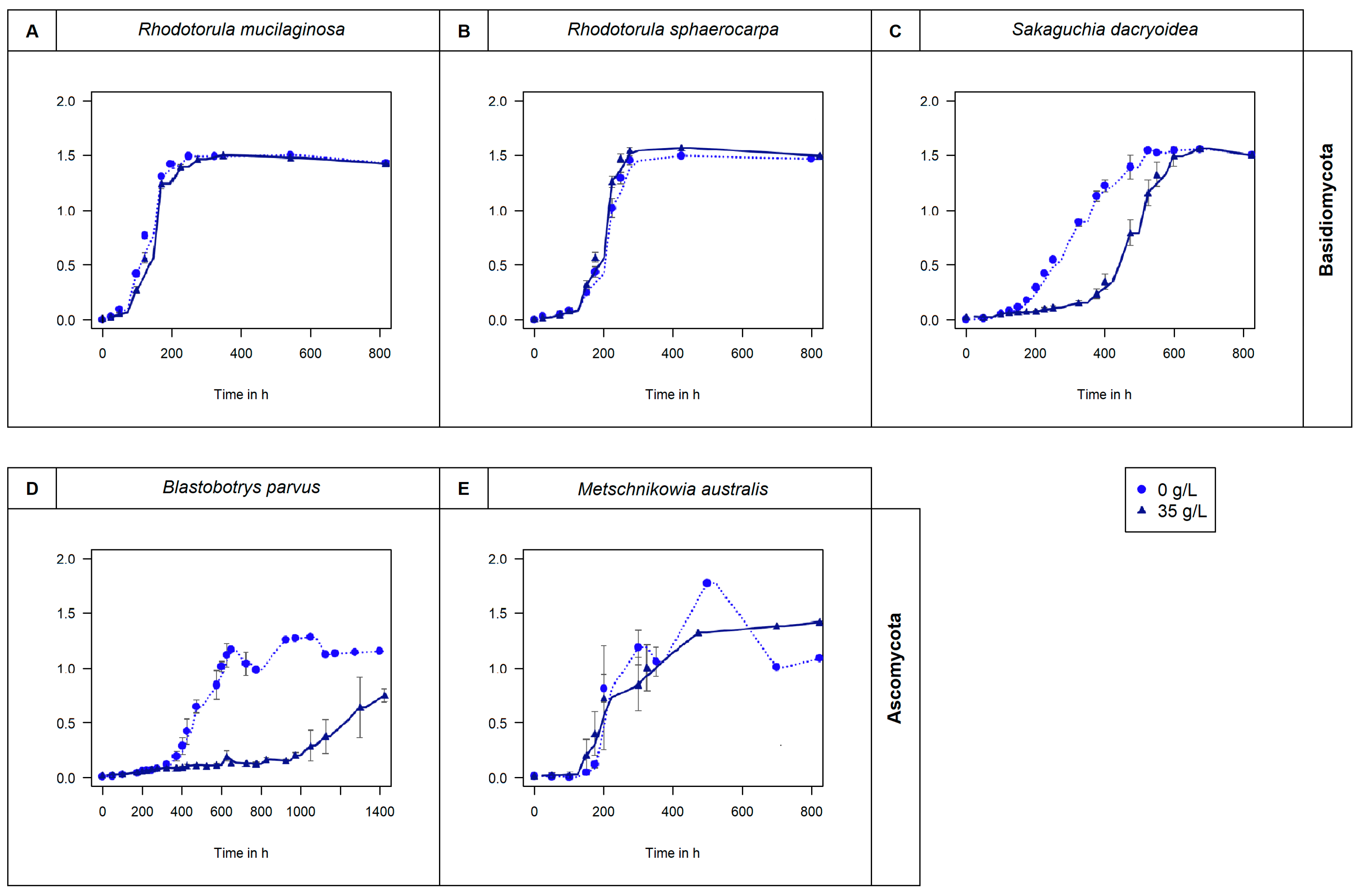
2.1. Culture of Fungal Species
2.2. Measuring Extracellular Enzymatic Activity and Fungal Biomass Determination
2.3. Determination of the Enzyme Kinetics
2.4. Statistical Analysis
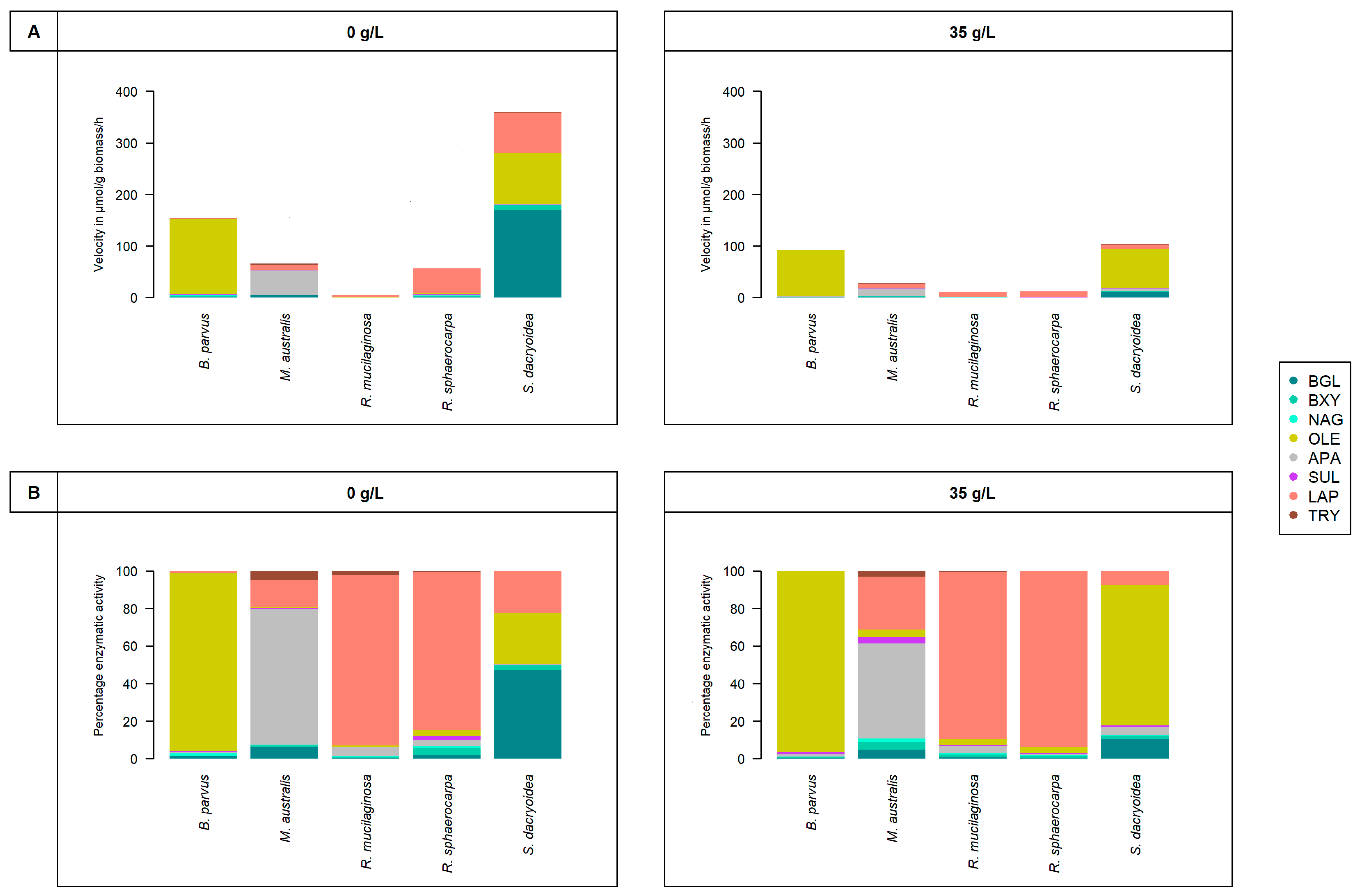
3. Results
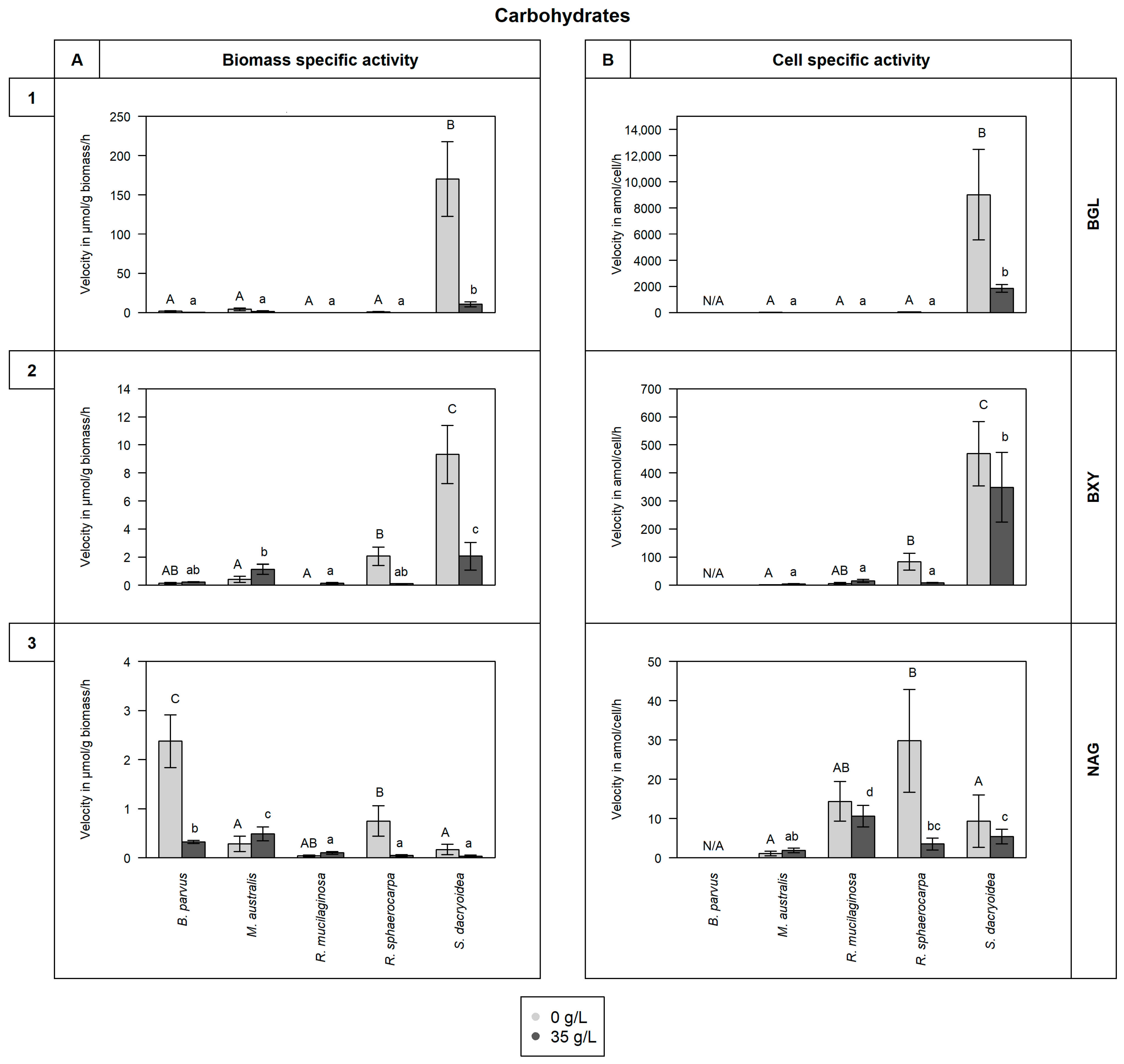
3.1. Carbohydrate Cleavage
3.1.1. β-Glucosidase (BGL)
3.1.2. β-Xylosidase (BXY)
3.1.3. N-Acetyl-β-D-glucosaminidase (NAG)
3.2. Hydrolytic Activity on Organic Compounds Containing Lipids, Phosphorus and Sulfur Moieties
3.2.1. Lipase (OLE)
3.2.2. Alkaline Phosphatase (APA)
3.2.3. Sulfatase (SUL)
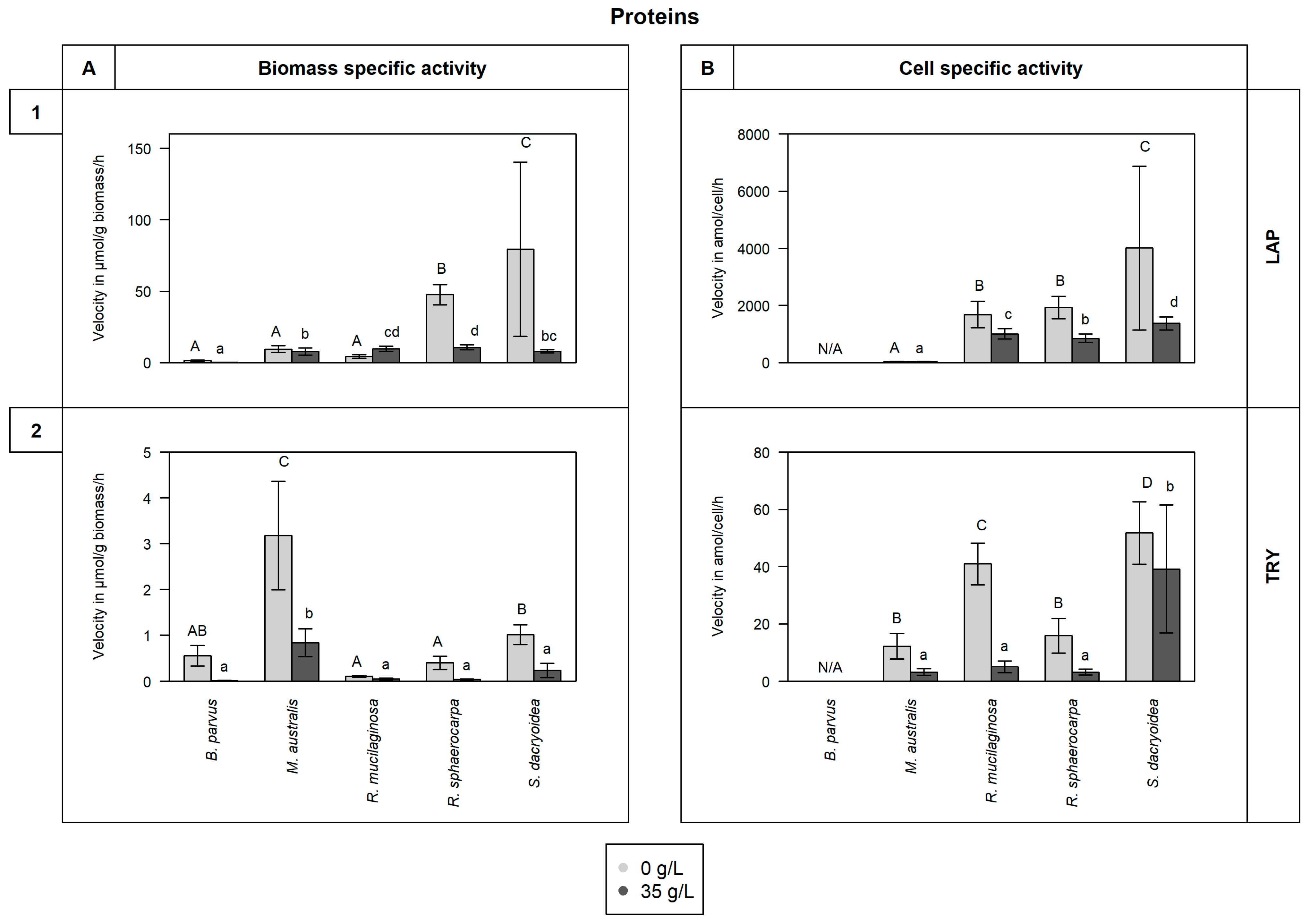
3.3. Proteins Cleavage
3.3.1. Leucine Aminopeptidase (LAP)
3.3.2. Trypsin (TRY)
4. Discussion
4.1. Extracellular Enzymatic Activities of Pelagic Fungal Strains
4.1.1. Release of Enzymes Hydrolytically Cleaving Carbohydrates
4.1.2. Release of Enzymes Cleaving Lipids, Phosphorus, and Sulfur Moieties
4.1.3. Release of Enzymes Hydrolytically Cleaving Proteins
4.2. Influence of Salinity on Extracellular Enzymatic Activity
4.3. Life Strategies
4.4. Potential Environmental Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jennings, D. Some aspects of the physiology and biochemistry of marine fungi. Biol. Rev. 1983, 58, 423–459. [Google Scholar] [CrossRef]
- Raghukumar, S. Fungi in Coastal and Oceanic Marine Ecosystems; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Gladfelter, A.S.; James, T.Y.; Amend, A.S. Marine fungi. Curr. Biol. 2019, 29, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Ruisi, S.; Barreca, D.; Selbmann, L.; Zucconi, L.; Onofri, S. Fungi in Antarctica. Rev. Environ. Sci. Bio/Technol. 2007, 6, 127–141. [Google Scholar] [CrossRef]
- Shivaji, S.; Prasad, G. Antarctic yeasts: Biodiversity and potential applications. In Yeast Biotechnology: Diversity and Applications; Satyanarayana, T., Kunze, G., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 3–18. [Google Scholar]
- Plemenitaš, A.; Lenassi, M.; Konte, T.; Kejžar, A.; Zajc, J.; Gostinčar, C.; Gunde-Cimerman, N. Adaptation to high salt concentrations in halotolerant/halophilic fungi: A molecular perspective. Front. Microbiol. 2014, 5, 199. [Google Scholar] [CrossRef] [PubMed]
- Śliżewska, W.; Struszczyk-Świta, K.; Marchut-Mikołajczyk, O. Metabolic potential of halophilic filamentous fungi-current perspective. Int. J. Mol. Sci. 2022, 23, 4189. [Google Scholar] [CrossRef] [PubMed]
- Norkrans, B.; Kylin, A. Regulation of the potassium to sodium ratio and of the osmotic potential in relation to salt tolerance in yeasts. J. Bacteriol. 1969, 100, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Oren, A. Bioenergetic aspects of halophilism. Microbiol. Mol. Biol. Rev. 1999, 63, 334–348. [Google Scholar] [CrossRef]
- Madern, D.; Ebel, C.; Zaccai, G. Halophilic adaptation of enzymes. Extremophiles 2000, 4, 91–98. [Google Scholar] [CrossRef]
- Gostinčar, C.; Lenassi, M.; Gunde-Cimerman, N.; Plemenitaš, A. Fungal adaptation to extremely high salt concentrations. In Advances in Applied Microbiology; Laskin, A., Sariaslani, S., Gadd, G., Eds.; Academic Press: Cambridge, MA, USA, 2011; Volume 77, pp. 71–96. [Google Scholar]
- Luard, E.J. Activity of isocitrate dehydrogenase from three filamentous fungi in relation to osmotic and solute effects. Arch. Microbiol. 1983, 134, 233–237. [Google Scholar] [CrossRef]
- Blomberg, A.; Adler, L. Physiology of osmotolerance in fungi. Adv. Microb. Physiol. 1992, 33, 145–212. [Google Scholar]
- Dihazi, H.; Kessler, R.; Eschrich, K. High osmolarity glycerol (HOG) pathway-induced phosphorylation and activation of 6-phosphofructo-2-kinase are essential for glycerol accumulation and yeast cell proliferation under hyperosmotic stress. J. Biol. Chem. 2004, 279, 23961–23968. [Google Scholar] [CrossRef]
- Velez, P.; Alejandri-Ramírez, N.D.; González, M.C.; Estrada, K.J.; Sanchez-Flores, A.; Dinkova, T.D. Comparative transcriptome analysis of the cosmopolitan marine fungus Corollospora maritima under two physiological conditions. G3 Genes Genomes Genet. 2015, 5, 1805–1814. [Google Scholar] [CrossRef]
- Leger, R.J.S.; Joshi, L.; Roberts, D.W. Adaptation of proteases and carbohydrates of saprophytic, phytopathogenic and entomopathogenic fungi to the requirements of their ecological niches. Microbiology 1997, 143, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Arnosti, C. Microbial extracellular enzymes and the marine carbon cycle. Annu. Rev. Mar. Sci. 2011, 3, 401–425. [Google Scholar] [CrossRef] [PubMed]
- Muszewska, A.; Stepniewska-Dziubinska, M.M.; Steczkiewicz, K.; Pawlowska, J.; Dziedzic, A.; Ginalski, K. Fungal lifestyle reflected in serine protease repertoire. Sci. Rep. 2017, 7, 9147. [Google Scholar] [CrossRef] [PubMed]
- Richards, T.A.; Jones, M.D.; Leonard, G.; Bass, D. Marine fungi: Their ecology and molecular diversity. Annu. Rev. Mar. Sci. 2012, 4, 495–522. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.M. The central role of enzymes as biological catalysts. In The Cell: A Molecular Approach; Cooper, G.M., Ed.; Sinauer Associates: Sunderland, MA, USA, 2000; Volume 1, pp. 1–5. [Google Scholar]
- Kohler, T.J.; Peter, H.; Fodelianakis, S.; Pramateftaki, P.; Styllas, M.; Tolosano, M.; De Staercke, V.; Schön, M.; Busi, S.B.; Wilmes, P. Patterns and drivers of extracellular enzyme activity in New Zealand glacier-fed streams. Front. Microbiol. 2020, 11, 591465. [Google Scholar] [CrossRef] [PubMed]
- Mulder, C.; Hendriks, A.J. Half-saturation constants in functional responses. Glob. Ecol. Conserv. 2014, 2, 161–169. [Google Scholar] [CrossRef]
- Semenova, T.A.; Dunaevsky, Y.E.; Beljakova, G.A.; Borisov, B.A.; Shamraichuk, I.L.; Belozersky, M.A. Extracellular peptidases as possible markers of fungal ecology. Appl. Soil Ecol. 2017, 113, 591465. [Google Scholar] [CrossRef]
- Boddy, L. Fungi, ecosystems, and global change. In The Fungi; Watkinson, S.C., Boddy, L., Money, N.P., Eds.; Academic Press: Cambridge, MA, USA, 2016; Volume 3, pp. 361–400. [Google Scholar]
- Arnosti, C.; Bell, C.; Moorhead, D.L.; Sinsabaugh, R.L.; Steen, A.D.; Stromberger, M.; Wallenstein, M.; Weintraub, M.N. Extracellular enzymes in terrestrial, freshwater, and marine environments: Perspectives on system variability and common research needs. Biogeochemistry 2014, 117, 5–21. [Google Scholar] [CrossRef]
- Caruso, G. Leucine aminopeptidase, β-glucosidase and alkaline phosphatase activity rates and their significance in nutrient cycles in some coastal Mediterranean sites. Mar. Drugs 2010, 8, 916–940. [Google Scholar] [CrossRef] [PubMed]
- Morales, S.E.; Biswas, A.; Herndl, G.J.; Baltar, F. Global structuring of phylogenetic and functional diversity of pelagic fungi by depth and temperature. Front. Mar. Sci. 2019, 6, 131. [Google Scholar] [CrossRef]
- Baltar, F.; Zhao, Z.; Herndl, G.J. Potential and expression of carbohydrate utilization by marine fungi in the global ocean. Microbiome 2021, 9, 106. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, M.H.; Pantoja, S.; Tejos, E.; Quiñones, R.A. The role of fungi in processing marine organic matter in the upwelling ecosystem off Chile. Mar. Biol. 2011, 158, 205–219. [Google Scholar] [CrossRef]
- Caruso, G.; Zaccone, R. Estimates of leucine aminopeptidase activity in different marine and brackish environments. J. Appl. Microbiol. 2000, 89, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Urvoy, M.; Labry, C.; Delmas, D.; Creac’h, L.; l’Helguen, S. Microbial enzymatic assays in environmental water samples: Impact of Inner Filter Effect and substrate concentrations. Limnol. Oceanogr. Methods 2020, 18, 725–738. [Google Scholar] [CrossRef]
- Cullings, K.; Courty, P.-E. Saprotrophic capabilities as functional traits to study functional diversity and resilience of ectomycorrhizal community. Oecologia 2009, 161, 661–664. [Google Scholar] [CrossRef]
- Orsi, W.; Biddle, J.F.; Edgcomb, V. Deep sequencing of subseafloor eukaryotic rRNA reveals active fungi across marine subsurface provinces. PLoS ONE 2013, 8, e56335. [Google Scholar] [CrossRef]
- Bochdansky, A.B.; Clouse, M.A.; Herndl, G.J. Eukaryotic microbes, principally fungi and labyrinthulomycetes, dominate biomass on bathypelagic marine snow. ISME J. 2017, 11, 362–373. [Google Scholar] [CrossRef]
- Picard, K.T. Coastal marine habitats harbor novel early-diverging fungal diversity. Fungal Ecol. 2017, 25, 1–13. [Google Scholar] [CrossRef]
- Ueda, M.; Nomura, Y.; Doi, K.; Nakajima, M.; Honda, D. Seasonal dynamics of culturable thraustochytrids (Labyrinthulomycetes, Stramenopiles) in estuarine and coastal waters. Aquat. Microb. Ecol. 2015, 74, 187–204. [Google Scholar] [CrossRef]
- Amend, A.; Burgaud, G.; Cunliffe, M.; Edgcomb, V.P.; Ettinger, C.L.; Gutiérrez, M.H.; Heitman, J.; Hom, E.F.Y.; Ianiri, G.; Jones, A.C.; et al. Fungi in the marine environment: Open questions and unsolved problems. mBio 2019, 10, 1–18. [Google Scholar] [CrossRef]
- Taylor, J.D.; Cunliffe, M. Multi-year assessment of coastal planktonic fungi reveals environmental drivers of diversity and abundance. ISME J. 2016, 10, 2118–2128. [Google Scholar] [CrossRef]
- Martínez-García, S.; Bunse, C.; Pontiller, B.; Baltar, F.; Israelsson, S.; Fridolfsson, E.; Lindh, M.; Lundin, D.; Legrand, C.; Pinhassi, J. Seasonal dynamics in carbon cycling of marine bacterioplankton are lifestyle dependent. Front. Microbiol. 2022, 13, 834675. [Google Scholar] [CrossRef]
- Benner, R. Chemical composition and reactivity. Biogeochem. Mar. Dissolved Org. Matter 2002, 1, 59–90. [Google Scholar]
- Benner, R. Molecular indicators of the bioavailability of dissolved organic matter. In Aquatic Ecosystems; Findlay, S.E.G., Sinsabaugh, R.L., Eds.; Academic Press: Cambridge, MA, USA, 2003; Volume 1, pp. 121–137. [Google Scholar]
- Benner, R.; Pakulski, J.D.; McCarthy, M.; Hedges, J.I.; Hatcher, P.G. Bulk chemical characteristics of dissolved organic matter in the ocean. Science 1992, 255, 1561–1564. [Google Scholar] [CrossRef] [PubMed]
- Chróst, R.J. Microbial ectoenzymes in aquatic environments. In Aquatic Microbial Ecology; Overbeck, J., Chróst, R.J., Eds.; Springer: New York, NY, USA, 1990; Volume 1, pp. 47–78. [Google Scholar]
- Baltar, F.; Aristegui, J. Fronts at the surface ocean can shape distinct regions of microbial activity and community assemblages down to the bathypelagic zone: The Azores Front as a case study. Front. Mar. Sci. 2017, 4, 252. [Google Scholar] [CrossRef]
- Hoppe, H.-G. Significance of exoenzymatic activities in the ecology of brackish water: Measurements by means of methylumbelliferyl-substrates. Mar. Ecol. Prog. Ser. 1983, 11, 299–308. [Google Scholar] [CrossRef]
- Reid, P.C.; Fischer, A.C.; Lewis-Brown, E.; Meredith, M.P.; Sparrow, M.; Andersson, A.J.; Antia, A.; Bates, N.R.; Bathmann, U.; Beaugrand, G. Impacts of the oceans on climate change. Adv. Mar. Biol. 2009, 56, 1–150. [Google Scholar] [PubMed]
- Hutchins, D.A.; Fu, F. Microorganisms and ocean global change. Nat. Microbiol. 2017, 2, 17058. [Google Scholar] [CrossRef] [PubMed]
- Munk, W. Ocean freshening, sea level rising. Science 2003, 300, 2041–2043. [Google Scholar] [CrossRef]
- Myers, R.A.; Akenhead, S.A.; Drinkwater, K. The influence of Hudson Bay runoff and ice-melt on the salinity of the inner Newfoundland Shelf. Atmos.-Ocean 1990, 28, 241–256. [Google Scholar] [CrossRef][Green Version]
- Balaguru, K.; Foltz, G.R.; Leung, L.R.; Emanuel, K.A. Global warming-induced upper-ocean freshening and the intensification of super typhoons. Nat. Commun. 2016, 7, 13670. [Google Scholar] [CrossRef]
- Fell, J.W.; Statzell, A.C. Sympodiomyces gen. n., a yeast-like organism from southern marine waters. Antonie Van Leeuwenhoek 1971, 37, 359–367. [Google Scholar] [CrossRef]
- Newell, S.Y.; Fell, J.W. The perfect form of a marine-occurring yeast of the genus Rhodotorula. Mycologia 1970, 62, 272–281. [Google Scholar] [CrossRef]
- Fell, J.W.; Hunter, I.L. Isolation of heterothallic yeast strains of Metschnikowia Kamienski and their mating reactions with Chlamydozyma Wickerham spp. Antonie Van Leeuwenhoek 1968, 34, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Fell, J.W.; Hunter, I.L.; Tallman, A.S. Marine basidiomycetous yeasts (Rhodosporidium spp. n.) with tetrapolar and multiple allelic bipolar mating systems. Can. J. Microbiol. 1973, 19, 643–657. [Google Scholar] [CrossRef] [PubMed]
- Wickerham, L.J. A taxonomic study of Monilia albicans with special emphasis on morphology and morphological variation. J. Trop. Med. Hyg. 1939, 42, 174–179. [Google Scholar]
- Wickerham, L.J. Taxonomy of Yeasts; US Department of Agriculture: Washington, DC, USA, 1951.
- Salazar Alekseyeva, K.; Mähnert, B.; Berthiller, F.; Breyer, E.; Herndl, G.J.; Baltar, F. Adapting an ergosterol extraction method with marine yeasts for the quantification of oceanic fungal biomass. J. Fungi 2021, 7, 690. [Google Scholar] [CrossRef]
- Morris, S.; Nicholls, J. An evaluation of optical density to estimate fungal spore concentrations in water suspensions. Strain 1978, 1, 1240–1242. [Google Scholar] [CrossRef]
- Pointing, S.B.; Vrijmoed, L.L.P.; Jones, E.B.G. A qualitative assessment of lignocellulose degrading enzyme activity in marine fungi. Bot. Mar. 1998, 41, 293–298. [Google Scholar] [CrossRef]
- Sinsabaugh, R.; Moorhead, D. Resource allocation to extracellular enzyme production: A model for nitrogen and phosphorus control of litter decomposition. Soil Biol. Biochem. 1994, 26, 1305–1311. [Google Scholar] [CrossRef]
- Collins, T.; Gerday, C.; Feller, G. Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiol. Rev. 2005, 29, 3–23. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, J.A.; Vieira, J.M.F.; Videira, A.; Meirelles, L.A.; Rodrigues, A.; Taniwaki, M.H.; Sette, L.D. Marine-derived fungus Aspergillus cf. tubingensis LAMAI 31: A new genetic resource for xylanase production. AMB Express 2016, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Ravn, J.L.; Engqvist, M.K.; Larsbrink, J.; Geijer, C. CAZyme prediction in ascomycetous yeast genomes guides discovery of novel xylanolytic species with diverse capacities for hemicellulose hydrolysis. Biotechnol. Biofuels 2021, 14, 150. [Google Scholar] [CrossRef] [PubMed]
- Joo, Y.C.; Seo, E.S.; Kim, Y.S.; Kim, K.R.; Park, J.B.; Oh, D.K. Production of 10-hydroxystearic acid from oleic acid by whole cells of recombinant Escherichia coli containing oleate hydratase from Stenotrophomonas maltophilia. J. Biotechnol. 2012, 158, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Prem, S.; Helmer, C.P.; Dimos, N.; Himpich, S.; Brück, T.; Garbe, D.; Loll, B. Towards an understanding of oleate hydratases and their application in industrial processes. Microb. Cell Factories 2022, 21, 58. [Google Scholar] [CrossRef]
- Dubovenko, A.G.; Dunaevsky, Y.E.; Belozersky, M.A.; Oppert, B.; Lord, J.C.; Elpidina, E.N. Trypsin-like proteins of the fungi as possible markers of pathogenicity. Fungal Biol. 2010, 114, 151–159. [Google Scholar] [CrossRef]
- Salazar Alekseyeva, K.; Herndl, G.J.; Baltar, F. Extracellular enzymatic activities of oceanic pelagic fungal strains and the influence of temperature. J. Fungi 2022, 8, 571. [Google Scholar] [CrossRef]
- Huitema, C.; Horsman, G. Analyzing enzyme kinetic data using the powerful statistical capabilities of R. bioRxiv 2018. [Google Scholar] [CrossRef]
- Johnson, K.A.; Goody, R.S. The original Michaelis constant: Translation of the 1913 Michaelis–Menten paper. Biochemistry 2011, 50, 8264–8269. [Google Scholar] [CrossRef]
- Brown, H.E.; Esher, S.K.; Alspaugh, J.A. Chitin: A “hidden figure” in the fungal cell wall. Fungal Cell Wall 2019, 1, 83–111. [Google Scholar]
- Norkrans, B. Degradation of cellulose. Annu. Rev. Phytopathol. 1963, 1, 325–350. [Google Scholar] [CrossRef]
- Metreveli, E.; Khardziani, T.; Elisashvili, V. The carbon source controls the secretion and yield of polysaccharide-hydrolyzing enzymes of Basidiomycetes. Biomolecules 2021, 11, 1341. [Google Scholar] [CrossRef] [PubMed]
- Kuhad, R.C.; Gupta, R.; Singh, A. Microbial cellulases and their industrial applications. Enzym. Res. 2011, 2011, 280696. [Google Scholar] [CrossRef] [PubMed]
- Lindenmuth, B.E.; McDonald, K.A. Production and characterization of Acidothermus cellulolyticus endoglucanase in Pichia pastoris. Protein Expr. Purif. 2011, 77, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Pointing, S.; Buswell, J.; Jones, E.G.; Vrijmoed, L. Extracellular cellulolytic enzyme profiles of five lignicolous mangrove fungi. Mycol. Res. 1999, 103, 696–700. [Google Scholar] [CrossRef]
- van den Brink, J.; de Vries, R.P. Fungal enzyme sets for plant polysaccharide degradation. Appl. Microbiol. Biotechnol. 2011, 91, 1477–1492. [Google Scholar] [CrossRef]
- Ab Wahab, A.F.F.; Karim, N.A.A.; Ling, J.G.; Hasan, N.S.; Yong, H.Y.; Bharudin, I.; Kamaruddin, S.; Bakar, F.D.A.; Murad, A.M.A. Functional characterisation of cellobiohydrolase I (Cbh1) from Trichoderma virens UKM1 expressed in Aspergillus niger. Protein Expr. Purif. 2019, 154, 52–61. [Google Scholar] [CrossRef]
- Baraldo Junior, A.; Borges, D.G.; Tardioli, P.W.; Farinas, C.S. Characterization of β-glucosidase produced by Aspergillus niger under solid-state fermentation and partially purified using MANAE-Agarose. Biotechnol. Res. Int. 2014, 2014, 317092. [Google Scholar] [CrossRef]
- Elyas, K.; Mathew, A.; Sukumaran, R.K.; Ali, P.M.; Sapna, K.; Kumar, S.R.; Mol, K.R. Production optimization and properties of beta glucosidases from a marine fungus Aspergillus-SA 58. New Biotechnol. 2010, 27, 347–351. [Google Scholar] [CrossRef]
- Hong, J.-H.; Jang, S.; Heo, Y.M.; Min, M.; Lee, H.; Lee, Y.M.; Lee, H.; Kim, J.-J. Investigation of marine-derived fungal diversity and their exploitable biological activities. Mar. Drugs 2015, 13, 4137–4155. [Google Scholar] [CrossRef]
- Lee, S.; Park, M.S.; Lee, H.; Kim, J.-J.; Eimes, J.A.; Lim, Y.W. Fungal diversity and enzyme activity associated with the macroalgae, Agarum clathratum. Mycobiology 2019, 47, 50–58. [Google Scholar] [CrossRef]
- MacDonald, M.J.; Hartley, D.L.; Speedie, M.K. Location of cellulolytic enzyme activity in the marine fungus Trichocladium achrasporum. Can. J. Microbiol. 1985, 31, 145–148. [Google Scholar] [CrossRef]
- Meyers, S.; Scott, E. Cellulose degradation by Lulworthia floridana and other lignicolous marine fungi. Mar. Biol. 1968, 2, 41–46. [Google Scholar] [CrossRef]
- Pilgaard, B.; Wilkens, C.; Herbst, F.-A.; Vuillemin, M.; Rhein-Knudsen, N.; Meyer, A.S.; Lange, L. Proteomic enzyme analysis of the marine fungus Paradendryphiella salina reveals alginate lyase as a minimal adaptation strategy for brown algae degradation. Sci. Rep. 2019, 9, 12338. [Google Scholar] [CrossRef] [PubMed]
- Hudson, H.J. Fungal Saprophytism; Hodder Education: London, UK, 1980; Volume 1. [Google Scholar]
- Vaz, A.B.M.; Rosa, L.H.; Vieira, M.L.A.; de Garcia, V.; Brandão, L.R.; Teixeira, L.C.R.S.; Moliné, M.; Libkind, D.; van Broock, M.; Rosa, C.A. The diversity, extracellular enzymatic activities and photoprotective compounds of yeasts isolated in Antarctica. Braz. J. Microbiol. 2011, 42, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.F.; Paço, A.; Escada, L.F.; Albuquerque, M.S.; Pinto, C.A.; Saraiva, J.; Duarte, A.S.; Rocha-Santos, T.A.; Esteves, A.C.; Alves, A. Unveiling biological activities of marine fungi: The effect of sea salt. Appl. Sci. 2021, 11, 6008. [Google Scholar] [CrossRef]
- Singh, G.; Verma, A.; Kumar, V. Catalytic properties, functional attributes and industrial applications of β-glucosidases. 3 Biotech 2016, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Ishihara, M. Action of fungal β-glucosidase on the mono-O-methylated p-nitrophenyl β-d-glucopyranoside. Holzforschung 2007, 63, 47–51. [Google Scholar] [CrossRef]
- Behera, B.; Sethi, B.; Mishra, R.; Dutta, S.; Thatoi, H. Microbial cellulases–diversity & biotechnology with reference to mangrove environment: A review. J. Genet. Eng. Biotechnol. 2017, 15, 197–210. [Google Scholar] [PubMed]
- Bucher, V.; Hyde, K.; Pointing, S.; Reddy, C. Production of wood decay enzymes, mass loss and lignin solubilization in wood by marine ascomycetes and their anamorphs. Fungal Divers. 2004, 15, 14. [Google Scholar]
- Domozych, D.; Ciancia, M.; Fangel, J.; Mikkelsen, M.; Ulvskov, P.; Willats, W. The cell walls of green algae: A journey through evolution and diversity. Front. Plant Sci. 2012, 3, 82. [Google Scholar] [CrossRef] [PubMed]
- Hernes, P.J.; Benner, R. Terrigenous organic matter sources and reactivity in the North Atlantic Ocean and a comparison to the Arctic and Pacific oceans. Mar. Chem. 2006, 100, 66–79. [Google Scholar] [CrossRef]
- Opsahl, S.; Benner, R. Distribution and cycling of terrigenous dissolved organic matter in the ocean. Nature 1997, 386, 480–482. [Google Scholar] [CrossRef]
- Pakulski, J.D.; Benner, R. Abundance and distribution of carbohydrates in the ocean. Limnol. Oceanogr. 1994, 39, 930–940. [Google Scholar] [CrossRef]
- Arora, D.K. Handbook of Applied Mycology: Soil and Plants; CRC Press: Boca Ratón, FL, USA, 1991; Volume 1. [Google Scholar]
- Duo-Chuan, L. Review of fungal chitinases. Mycopathologia 2006, 161, 345–360. [Google Scholar] [CrossRef]
- Numata, K.; Kaplan, D.L. Biologically derived scaffolds. In Advanced Wound Repair Therapies; Farrar, D., Ed.; Woodhead Publishing: Cambridge, UK, 2011; Volume 1, pp. 524–551. [Google Scholar]
- Ruiz-Herrera, J. Fungal Cell Wall: Structure, Synthesis, and Assembly; CRC Press: Boca Ratón, FL, USA, 1991; Volume 1. [Google Scholar]
- Suresh, P.; Anil Kumar, P. Enhanced degradation of α-chitin materials prepared from shrimp processing byproduct and production of N-acetyl-D-glucosamine by thermoactive chitinases from soil mesophilic fungi. Biodegradation 2012, 23, 597–607. [Google Scholar] [CrossRef]
- Chen, J.-K.; Shen, C.-R.; Yeh, C.-H.; Fang, B.-S.; Huang, T.-L.; Liu, C.-L. N-acetyl glucosamine obtained from chitin by chitin degrading factors in Chitinbacter tainanesis. Int. J. Mol. Sci. 2011, 12, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Gaderer, R.; Seidl-Seiboth, V.; de Vries, R.P.; Seiboth, B.; Kappel, L. N-acetylglucosamine, the building block of chitin, inhibits growth of Neurospora crassa. Fungal Genet. Biol. 2017, 107, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Slámová, K.; Bojarová, P.; Petrásková, L.; Křen, V. β-N-acetylhexosaminidase: What’s in a name…? Biotechnol. Adv. 2010, 28, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yip, V.L.Y.; Withers, S.G. Mechanisms of enzymatic glycosyl transfer. In Comprehensive Natural Products II; Hung-Wen, L., Mander, L., Eds.; Elsevier: Oxford, UK, 2010; pp. 385–422. [Google Scholar]
- Fenice, M. The psychrotolerant Antarctic fungus Lecanicillium muscarium CCFEE 5003: A powerful producer of cold-tolerant chitinolytic enzymes. Molecules 2016, 21, 447. [Google Scholar] [CrossRef] [PubMed]
- Fenice, M.; Selbmann, L.; Di Giambattista, R.; Federici, F. Chitinolytic activity at low temperature of an Antarctic strain (A3) of Verticillium lecanii. Res. Microbiol. 1998, 149, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Edson, C.; Brody, S. Biochemical and genetic studies on galactosamine metabolism in Neurospora crassa. J. Bacteriol. 1976, 126, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Plíhal, O.; Sklenář, J.; Hofbauerová, K.; Novák, P.; Man, P.; Pompach, P.; Kavan, D.; Ryšlavá, H.; Weignerová, L.; Charvátová-Pišvejcová, A. Large propeptides of fungal β-N-acetylhexosaminidases are novel enzyme regulators that must be intracellularly processed to control activity, dimerization, and secretion into the extracellular environment. Biochemistry 2007, 46, 2719–2734. [Google Scholar] [CrossRef] [PubMed]
- Hartl, L.; Zach, S.; Seidl-Seiboth, V. Fungal chitinases: Diversity, mechanistic properties and biotechnological potential. Appl. Microbiol. Biotechnol. 2012, 93, 533–543. [Google Scholar] [CrossRef]
- Kubicek, C.P.; Herrera-Estrella, A.; Seidl-Seiboth, V.; Martinez, D.A.; Druzhinina, I.S.; Thon, M.; Zeilinger, S.; Casas-Flores, S.; Horwitz, B.A.; Mukherjee, P.K. Comparative genome sequence analysis underscores mycoparasitism as the ancestral life style of Trichoderma. Genome Biol. 2011, 12, R40. [Google Scholar] [CrossRef]
- Seidl, V. Chitinases of filamentous fungi: A large group of diverse proteins with multiple physiological functions. Fungal Biol. Rev. 2008, 22, 36–42. [Google Scholar] [CrossRef]
- Garcia-Rubio, R.; de Oliveira, H.C.; Rivera, J.; Trevijano-Contador, N. The fungal cell wall: Candida, Cryptococcus, and Aspergillus species. Front. Microbiol. 2020, 10, 2993. [Google Scholar] [CrossRef]
- Gkargkas, K.; Mamma, D.; Nedev, G.; Topakas, E.; Christakopoulos, P.; Kekos, D.; Macris, B.J. Studies on a N-acetyl-β-D-glucosaminidase produced by Fusarium oxysporum F3 grown in solid-state fermentation. Process Biochem. 2004, 39, 1599–1605. [Google Scholar] [CrossRef]
- Reyes, F.; Calatayud, J.; Vazquez, C.; Martínez, M.J. β-N-acetylglucosaminidase from Aspergillus nidulans which degrades chitin oligomers during autolysis. FEMS Microbiol. Lett. 1989, 65, 83–87. [Google Scholar] [CrossRef]
- Biely, P. Microbial xylanolytic systems. Trends Biotechnol. 1985, 3, 286–290. [Google Scholar] [CrossRef]
- Beg, Q.; Kapoor, M.; Mahajan, L.; Hoondal, G. Microbial xylanases and their industrial applications: A review. Appl. Microbiol. Biotechnol. 2001, 56, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Knob, A.; Terrasan, C.F.; Carmona, E. β-xylosidases from filamentous fungi: An overview. World J. Microbiol. Biotechnol. 2010, 26, 389–407. [Google Scholar] [CrossRef]
- Sunna, A.; Antranikian, G. Xylanolytic enzymes from fungi and bacteria. Crit. Rev. Biotechnol. 1997, 17, 39–67. [Google Scholar] [CrossRef]
- Bajpai, P. Microbial xylanolytic enzyme system: Properties and applications. In Advances in Applied Microbiology; Neidleman, S.L., Laskin, A.I., Eds.; Academic Press: Cambridge, MA, USA, 1997; Volume 43, pp. 141–194. [Google Scholar]
- Matsuo, M.; Yasui, T. β-xylosidases of several fungi. In Methods in Enzymology; Packer, L., Glazer, A.N., Eds.; Academic Press: Cambridge, MA, USA, 1988; Volume 160, pp. 684–695. [Google Scholar]
- Biely, P.; Vršanská, M.; Krátký, Z. Xylan-degrading enzymes of the yeast Cryptococcus albidus: Identification and cellular localization. Eur. J. Biochem. 1980, 108, 313–321. [Google Scholar] [CrossRef]
- Turkiewicz, M.; Kalinowska, H.; Zielińska, M.; Bielecki, S. Purification and characterization of two endo-1, 4-β-xylanases from Antarctic krill, Euphausia superba Dana. Comp. Biochem. Physiol. 2000, 127, 325–335. [Google Scholar] [CrossRef]
- Fell, J.W.; Pitt, J.I. Taxonomy of the yeast genus Metschnikowia: A correction and a new variety. J. Bacteriol. 1969, 98, 853–854. [Google Scholar] [CrossRef]
- Geoffry, K.; Achur, R.N. A novel halophilic extracellular lipase with both hydrolytic and synthetic activities. Biocatal. Agric. Biotechnol. 2017, 12, 125–130. [Google Scholar] [CrossRef]
- Basheer, S.M.; Chellappan, S.; Beena, P.; Sukumaran, R.K.; Elyas, K.; Chandrasekaran, M. Lipase from marine Aspergillus awamori BTMFW032: Production, partial purification and application in oil effluent treatment. New Biotechnol. 2011, 28, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Zalar, P.; Kocuvan, M.A.; Plemenitaš, A.; Gunde-Cimerman, N. Halophilic black yeasts colonize wood immersed in hypersaline water. Bot. Mar. 2005, 48, 323–326. [Google Scholar] [CrossRef]
- Papaparaskevas, D.; Christakopoulos, P.; Kekos, D.; Macris, B.J. Optimizing production of extracellular lipase fromRhodotorula glutinis. Biotechnol. Lett. 1992, 14, 397–402. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Chevalot, I.; Galiotou-Panayotou, M.; Komaitis, M.; Marc, I.; Aggelis, G. Industrial derivative of tallow: A promising renewable substrate for microbial lipid, single-cell protein and lipase production by Yarrowia lipolytica. Electron. J. Biotechnol. 2007, 10, 425–435. [Google Scholar] [CrossRef]
- Louhasakul, Y.; Cheirsilp, B.; Prasertsan, P. Valorization of palm oil mill effluent into lipid and cell-bound lipase by marine yeast Yarrowia lipolytica and their application in biodiesel production. Waste Biomass Valoriz. 2016, 7, 417–426. [Google Scholar] [CrossRef]
- Duarte, A.W.F.; Bonugli-Santos, R.C.; Duarte, A.L.F.; Gomes, E.; Sette, L.D. Statistical experimental design applied to extracellular lipase production by the marine Antarctic yeast Leucosporidium scottii CRM 728. Biocatal. Agric. Biotechnol. 2021, 32, 101954. [Google Scholar] [CrossRef]
- Duarte, A.; Dayo-Owoyemi, I.; Nobre, F.; Pagnocca, F.C.; Chaud, L.C.S.; Pessoa, A.; Felipe, M.d.G.d.A.; Sette, L. Taxonomic assessment and enzymes production by yeasts isolated from marine and terrestrial Antarctic samples. Extremophiles 2013, 17, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chi, Z.; Wang, X.; Liu, Z.; Li, J. Diversity of lipase-producing yeasts from marine environments and oil hydrolysis by their crude enzymes. Ann. Microbiol. 2007, 57, 495–501. [Google Scholar] [CrossRef]
- Potumarthi, R.; Subhakar, C.; Vanajakshi, J.; Jetty, A. Effect of aeration and agitation regimes on lipase production by newly isolated Rhodotorula mucilaginosa–MTCC 8737 in stirred tank reactor using molasses as sole production medium. Appl. Biochem. Biotechnol. 2008, 151, 700–710. [Google Scholar] [CrossRef] [PubMed]
- Chrost, R.J.; Siuda, W.; HALEMEjko, G.Z. Longterm studies on alkaline phosphatase activity (APA) in a lake with fish-aquaculture in relation to lake eutrophication and phosphorus cycle. Arch. Für Hydrobiol. 1984, 70, 1–32. [Google Scholar]
- Karl, D.M. Phosphorus, the staff of life. Nature 2000, 406, 31–33. [Google Scholar] [CrossRef]
- Colman, A.S.; Blake, R.E.; Karl, D.M.; Fogel, M.L.; Turekian, K.K. Marine phosphate oxygen isotopes and organic matter remineralization in the oceans. Proc. Natl. Acad. Sci. USA 2005, 102, 13023–13028. [Google Scholar] [CrossRef] [PubMed]
- Paytan, A.; McLaughlin, K. The oceanic phosphorus cycle. Chem. Rev. 2007, 107, 563–576. [Google Scholar] [CrossRef]
- Brembu, T.; Mühlroth, A.; Alipanah, L.; Bones, A.M. The effects of phosphorus limitation on carbon metabolism in diatoms. Philos. Trans. R. Soc. London Ser. B Biol. Sci. 2017, 372, 20160406. [Google Scholar] [CrossRef]
- Hassan, H.; Pratt, D. Biochemical and physiological properties of alkaline phosphatases in five isolates of marine bacteria. J. Bacteriol. 1977, 129, 1607–1612. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Saavedra, D.E.M.; Thomson, B.; García, J.A.L.; Zhao, Z.; Patrick, W.M.; Herndl, G.J.; Baltar, F. Enzyme promiscuity in natural environments: Alkaline phosphatase in the ocean. ISME J. 2021, 15, 3375–3383. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.j.; Huang, X.f.; Hu, J.w.; Li, C.x.; Yi, Y.; Long, J. Distribution of several microorganisms and activity of alkaline phosphatase in sediments from Baihua Lake. Asia-Pac. J. Chem. Eng. 2009, 4, 711–716. [Google Scholar] [CrossRef]
- Baltar, F.; Lundin, D.; Palovaara, J.; Lekunberri, I.; Reinthaler, T.; Herndl, G.J.; Pinhassi, J. Prokaryotic responses to ammonium and organic carbon reveal alternative CO2 fixation pathways and importance of alkaline phosphatase in the mesopelagic North Atlantic. Front. Microbiol. 2016, 7, 1670. [Google Scholar] [CrossRef]
- Adler, L. Properties of alkaline phosphatase of the halotolerant yeast Debaryomyces hansenii. Biochim. Biophys. Acta (BBA)-Enzymol. 1978, 522, 113–121. [Google Scholar] [CrossRef]
- Wang, Y.; Barth, D.; Tamminen, A.; Wiebe, M.G. Growth of marine fungi on polymeric substrates. BMC Biotechnol. 2016, 16, 1–9. [Google Scholar] [CrossRef]
- Helbert, W. Marine polysaccharide sulfatases. Front. Mar. Sci. 2017, 4, 3. [Google Scholar] [CrossRef]
- Kloareg, B.; Quatrano, R. Structure of the cell walls of marine algae and ecophysiological functions of the matrix polysaccharides. Oceanogr. Mar. Biol. Annu. Rev. 1988, 26, 259–315. [Google Scholar]
- Aquino, R.S.; Grativol, C.; Mourão, P.A. Rising from the sea: Correlations between sulfated polysaccharides and salinity in plants. PLoS ONE 2011, 6, e18862. [Google Scholar] [CrossRef]
- Ciancia, M.; Fernández, P.V.; Leliaert, F. Diversity of sulfated polysaccharides from cell walls of coenocytic green algae and their structural relationships in view of green algal evolution. Front. Plant Sci. 2020, 11, 554585. [Google Scholar] [CrossRef] [PubMed]
- Aquino, R.S.; Landeira-Fernandez, A.M.; Valente, A.P.; Andrade, L.R.; Mourao, P.A. Occurrence of sulfated galactans in marine angiosperms: Evolutionary implications. Glycobiology 2005, 15, 11–20. [Google Scholar] [CrossRef]
- Hettle, A.G.; Vickers, C.J.; Boraston, A.B. Sulfatases: Critical enzymes for algal polysaccharide processing. Front. Plant Sci. 2022, 13, 837636. [Google Scholar] [CrossRef] [PubMed]
- Schultz-Johansen, M.; Bech, P.K.; Hennessy, R.C.; Glaring, M.A.; Barbeyron, T.; Czjzek, M.; Stougaard, P. A novel enzyme portfolio for red algal polysaccharide degradation in the marine bacterium Paraglaciecola hydrolytica S66T encoded in a sizeable polysaccharide utilization locus. Front. Microbiol. 2018, 9, 839. [Google Scholar] [CrossRef]
- Shvetsova, S.V.; Zhurishkina, E.V.; Bobrov, K.S.; Ronzhina, N.L.; Lapina, I.M.; Ivanen, D.R.; Gagkaeva, T.Y.; Kulminskaya, A.A. The novel strain Fusarium proliferatum LE1 (RCAM02409) produces α-l-fucosidase and arylsulfatase during the growth on fucoidan. J. Basic Microbiol. 2015, 55, 471–479. [Google Scholar] [CrossRef]
- Burley, S.K.; David, P.R.; Taylor, A.; Lipscomb, W.N. Molecular structure of leucine aminopeptidase at 2.7-A resolution. Proc. Natl. Acad. Sci. USA 1990, 87, 6878–6882. [Google Scholar] [CrossRef]
- Matsui, M.; Fowler, J.H.; Walling, L.L. Leucine aminopeptidases: Diversity in structure and function. Biol. Chem. 2006, 387, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Wahid, M.I.; Bitoon, S.R.; Fukunaga, T.; Yoshikawa, T.; Sakata, T. Comparative study of leucine aminopeptidases from marine labyrinthulid and thraustochytrid strains. Mem Fac Fish 2008, 1, 26–33. [Google Scholar]
- Huston, A.; Deming, J. Relationships between microbial extracellular enzymatic activity and suspended and sinking particulate organic matter: Seasonal transformations in the North Water. Deep Sea Res. Part II Top. Stud. Oceanogr. 2002, 49, 5211–5225. [Google Scholar] [CrossRef]
- Middelburg, J.J.; Vlug, T.; Jaco, F.; Van der Nat, W. Organic matter mineralization in marine systems. Glob. Planet. Chang. 1993, 8, 47–58. [Google Scholar] [CrossRef]
- Druffel, E.R.; Williams, P.M.; Bauer, J.E.; Ertel, J.R. Cycling of dissolved and particulate organic matter in the open ocean. J. Geophys. Res. Ocean. 1992, 97, 15639–15659. [Google Scholar] [CrossRef]
- Olsen, J.V.; Ong, S.-E.; Mann, M. Trypsin cleaves exclusively C-terminal to arginine and lysine residues. Mol. Cell. Proteom. 2004, 3, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A. Aspergillus nidulans: A potential resource of the production of the native and heterologous enzymes for industrial applications. Int. J. Microbiol. 2020, 2020, 8894215. [Google Scholar] [CrossRef] [PubMed]
- Souza, P.M.d.; Bittencourt, M.L.d.A.; Caprara, C.C.; Freitas, M.d.; Almeida, R.P.C.d.; Silveira, D.; Fonseca, Y.M.; Ferreira Filho, E.X.; Pessoa Junior, A.; Magalhães, P.O. A biotechnology perspective of fungal proteases. Braz. J. Microbiol. 2015, 46, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Bong, C.W.; Obayashi, Y.; Suzuki, S. Succession of protease activity in seawater and bacterial isolates during starvation in a mesocosm experiment. Aquat. Microb. Ecol. 2013, 69, 33–46. [Google Scholar] [CrossRef]
- Damare, S.; Raghukumar, C.; Muraleedharan, U.D.; Raghukumar, S. Deep-sea fungi as a source of alkaline and cold-tolerant proteases. Enzym. Microb. Technol. 2006, 39, 172–181. [Google Scholar] [CrossRef]
- You, Y.; Sun, X.; Ma, M.; He, J.; Li, L.; Porto, F.W.; Lin, S. Trypsin is a coordinate regulator of N and P nutrients in marine phytoplankton. Nat. Commun. 2022, 13, 4022. [Google Scholar] [CrossRef]
- Roussel, M.R. A Life Scientist’s Guide to Physical Chemistry; Cambridge University Press: Cambridge, UK, 2012; Volume 1. [Google Scholar]
- Jones, E.G.; Jennings, D. The effect of salinity on the growth of marine fungi in comparison with non-marine species. Trans. Br. Mycol. Soc. 1964, 47, 619–625. [Google Scholar] [CrossRef]
- Arfi, Y.; Chevret, D.; Henrissat, B.; Berrin, J.-G.; Levasseur, A.; Record, E. Characterization of salt-adapted secreted lignocellulolytic enzymes from the mangrove fungus Pestalotiopsis sp. Nat. Commun. 2013, 4, 1810. [Google Scholar] [CrossRef] [PubMed]
- Rietz, D.; Haynes, R. Effects of irrigation-induced salinity and sodicity on soil microbial activity. Soil Biol. Biochem. 2003, 35, 845–854. [Google Scholar] [CrossRef]
- Kornblatt, J.; Kornblatt, M. Water as it applies to the function of enzymes. Int. Rev. Cytol. 2002, 215, 49–73. [Google Scholar] [PubMed]
- Saenger, W. Structure and dynamics of water surrounding biomolecules. Annu. Rev. Biophys. Biophys. Chem. 1987, 16, 93–114. [Google Scholar] [CrossRef]
- Zaccai, G. The effect of water on protein dynamics. Philos. Trans. R. Soc. London Ser. B Biol. Sci. 2004, 359, 1269–1275. [Google Scholar] [CrossRef]
- Karan, R.; Capes, M.D.; DasSarma, S. Function and biotechnology of extremophilic enzymes in low water activity. Aquat. Biosyst. 2012, 8, 4. [Google Scholar] [CrossRef]
- Kuntz, I.D., Jr. Hydration of macromolecules. IV. Polypeptide conformation in frozen solutions. J. Am. Chem. Soc. 1971, 93, 516–518. [Google Scholar] [CrossRef] [PubMed]
- Danson, M.J.; Hough, D.W. The structural basis of protein halophilicity. Comp. Biochem. Physiol. 1997, 117, 307–312. [Google Scholar] [CrossRef]
- Mountain, R.D.; Thirumalai, D. Alterations in water structure induced by guanidinium and sodium ions. J. Phys. Chem. B 2004, 108, 19711–19716. [Google Scholar] [CrossRef]
- Sinha, R.; Khare, S.K. Protective role of salt in catalysis and maintaining structure of halophilic proteins against denaturation. Front. Microbiol. 2014, 5, 165. [Google Scholar] [CrossRef]
- Feller, G.; Gerday, C. Psychrophilic enzymes: Molecular basis of cold adaptation. Cell. Mol. Life Sci. 1997, 53, 830–841. [Google Scholar] [CrossRef]
- Larsen, H. Biochemical aspects of extreme halophilism. In Advances in Microbial Physiology; Rose, A.H., Wilkinson, J.F., Eds.; Academic Press: Cambridge, MA, USA, 1967; Volume 1, pp. 97–132. [Google Scholar]
- Lanyi, J.K. Salt-dependent properties of proteins from extremely halophilic bacteria. Bacteriol. Rev. 1974, 38, 272–290. [Google Scholar] [CrossRef]
- DasSarma, S.; DasSarma, P. Halophiles and their enzymes: Negativity put to good use. Curr. Opin. Microbiol. 2015, 25, 120–126. [Google Scholar] [CrossRef]
- Baxter, R. An interpretation of the effects of salts on the lactic dehydrogenase of Halobacterium salinarium. Can. J. Microbiol. 1959, 5, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Hutcheon, G.W.; Vasisht, N.; Bolhuis, A. Characterisation of a highly stable α-amylase from the halophilic archaeon Haloarcula hispanica. Extremophiles 2005, 9, 487–495. [Google Scholar] [CrossRef]
- Karan, R.; Mathew, S.; Muhammad, R.; Bautista, D.B.; Vogler, M.; Eppinger, J.; Oliva, R.; Cavallo, L.; Arold, S.T.; Rueping, M. Understanding high-salt and cold adaptation of a polyextremophilic enzyme. Microorganisms 2020, 8, 1594. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.; Dolan, T.; Rees, D. Evidence for a common structural pattern in the polysaccharide sulphates of the Rhodophyceae. Nature 1965, 205, 1060–1062. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.F.; Hilário, S.; Van de Peer, Y.; Esteves, A.C.; Alves, A. Genomic and metabolomic analyses of the marine fungus Emericellopsis cladophorae: Insights into saltwater adaptability mechanisms and its biosynthetic potential. J. Fungi 2021, 8, 31. [Google Scholar] [CrossRef]
- Li, X.; Kondo, R.; Sakai, K. Studies on hypersaline-tolerant white-rot fungi L: Screening of lignin-degrading fungi in hypersaline conditions. J. Wood Sci. 2002, 48, 147–152. [Google Scholar] [CrossRef]
- Solis, M.J.L.; Draeger, S.; Cruz, T.E.E.d. Marine-derived fungi from Kappaphycus alvarezii and K. striatum as potential causative agents of ice-ice disease in farmed seaweeds. Bot. Mar. 2010, 53, 587–594. [Google Scholar] [CrossRef]
- Razzaq, A.; Shamsi, S.; Ali, A.; Ali, Q.; Sajjad, M.; Malik, A.; Ashraf, M. Microbial proteases applications. Front. Bioeng. Biotechnol. 2019, 7, 110. [Google Scholar] [CrossRef]
- Gzogyan, L.; Proskuryakov, M.; Ievleva, E.; Valueva, T. Trypsin-like proteinases and trypsin inhibitors in fruiting bodies of higher fungi. Appl. Biochem. Microbiol. 2005, 41, 538–541. [Google Scholar] [CrossRef]
- Allen, T.W.; Quayyum, H.A.; Burpee, L.L.; Buck, J.W. Effect of foliar disease on the epiphytic yeast communities of creeping bentgrass and tall fescue. Can. J. Microbiol. 2004, 50, 853–860. [Google Scholar] [CrossRef]
- Mauch, F.; Mauch-Mani, B.; Boller, T. Antifungal hydrolases in pea tissue: II. Inhibition of fungal growth by combinations of chitinase and β-1,3-glucanase. Plant Physiol. 1988, 88, 936–942. [Google Scholar] [CrossRef]
- Gooday, G.W.; Zhu, W.-Y.; O’Donnell, R.W. What are the roles of chitinases in the growing fungus? FEMS Microbiol. Lett. 1992, 100, 387–391. [Google Scholar] [CrossRef]
- Park, J.K.; Morita, K.; Fukumoto, I.; Yamasaki, Y.; Nakagawa, T.; Kawamukai, M.; Matsuda, H. Purification and characterization of the chitinase (ChiA) from Enterobacter sp. Gl. Biosci. Biotechnol. Biochem. 1997, 61, 684–689. [Google Scholar] [CrossRef]
- Gadanho, M.; Almeida, J.M.; Sampaio, J.P. Assessment of yeast diversity in a marine environment in the south of Portugal by microsatellite-primed PCR. Antonie Van Leeuwenhoek 2003, 84, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Francis, M.; Webb, V.; Zuccarello, G. Marine yeast biodiversity on seaweeds in New Zealand waters. N. Z. J. Bot. 2016, 54, 30–47. [Google Scholar] [CrossRef]
- Cook, A.; Fox, A.; Vaughan, D.; Ferrigno, J. Retreating glacier fronts on the Antarctic Peninsula over the past half-century. Science 2005, 308, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Meredith, M.P.; Venables, H.J.; Clarke, A.; Ducklow, H.W.; Erickson, M.; Leng, M.J.; Lenaerts, J.T.; van den Broeke, M.R. The freshwater system west of the Antarctic Peninsula: Spatial and temporal changes. J. Clim. 2013, 26, 1669–1684. [Google Scholar] [CrossRef]
- Dickson, R.R.; Meincke, J.; Malmberg, S.-A.; Lee, A.J. The “great salinity anomaly” in the northern North Atlantic 1968–1982. Prog. Oceanogr. 1988, 20, 103–151. [Google Scholar] [CrossRef]
- Rojas-Jimenez, K.; Rieck, A.; Wurzbacher, C.; Jürgens, K.; Labrenz, M.; Grossart, H.-P. A salinity threshold separating fungal communities in the Baltic Sea. Front. Microbiol. 2019, 10, 680. [Google Scholar] [CrossRef] [PubMed]


| Type | Code | Name | Standard |
|---|---|---|---|
| Carbohydrates | BGL | β-glucosidase | MUF |
| BXY | β-xylosidase | MUF | |
| NAG | N-acetyl-β-D-glucosaminidase | MUF | |
| Lipids, phosphorus, and sulfur moieties | OLE | Lipase | MUF |
| APA | Alkaline phosphatase | MUF | |
| SUL | Sulfatase | MUF | |
| Proteins | LAP | Leucine aminopeptidase | MCA |
| TRY | Trypsin | MCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salazar-Alekseyeva, K.; Herndl, G.J.; Baltar, F. Influence of Salinity on the Extracellular Enzymatic Activities of Marine Pelagic Fungi. J. Fungi 2024, 10, 152. https://doi.org/10.3390/jof10020152
Salazar-Alekseyeva K, Herndl GJ, Baltar F. Influence of Salinity on the Extracellular Enzymatic Activities of Marine Pelagic Fungi. Journal of Fungi. 2024; 10(2):152. https://doi.org/10.3390/jof10020152
Chicago/Turabian StyleSalazar-Alekseyeva, Katherine, Gerhard J. Herndl, and Federico Baltar. 2024. "Influence of Salinity on the Extracellular Enzymatic Activities of Marine Pelagic Fungi" Journal of Fungi 10, no. 2: 152. https://doi.org/10.3390/jof10020152
APA StyleSalazar-Alekseyeva, K., Herndl, G. J., & Baltar, F. (2024). Influence of Salinity on the Extracellular Enzymatic Activities of Marine Pelagic Fungi. Journal of Fungi, 10(2), 152. https://doi.org/10.3390/jof10020152








