Rambellisea gigliensis and Rambellisea halocynthiae, gen. et spp. nov. (Lulworthiaceae) from the Marine Tunicate Halocynthia papillosa
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Isolation
- (i)
- Direct plating: tunic was cut into pieces of about 1 cm3 and directly plated (5 pieces for each plate) onto Petri dishes (90 mm) containing Malt Extract Agar seawater (MEAsw; 50 g MEA—Sigma-Aldrich dissolved in 1 L of seawater) and Corn Meal Agar seawater (CMAsw; 17 g CMA–Fluka analytical, Buchs, Switzerland, dissolved in 1 L of seawater).
- (ii)
- Homogenization: 5 g of each district (T, I) was homogenized in 10 mL of sterile seawater using a sterile device (ULTRA-TURRAX, IKA, Staufen, Germany). A total of 500 μL of each suspension was plated onto Petri dishes (90 mm) containing MEAsw and CMAsw.
2.2. Morphology and Growth Studies on Different Media
2.3. DNA Extraction, PCR Amplification, and Data Assembling
2.4. Sequence Alignment and Phylogenetic Analyses
3. Results
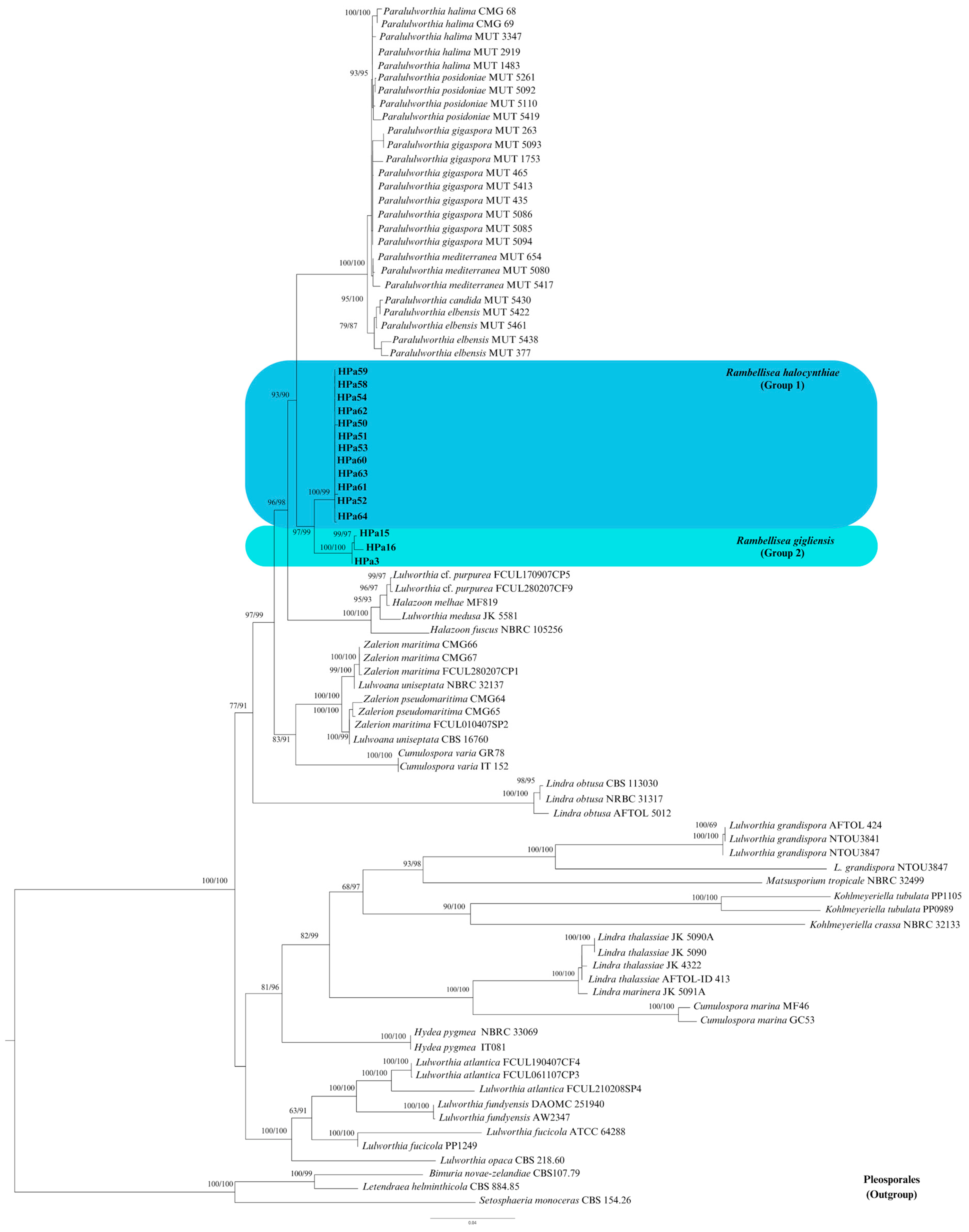
3.1. Phylogenetic Inference
3.2. Taxonomy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giovannini, V.; Barghini, P.; Gorrasi, S.; Massimiliano, F.; Pasqualetti, M. Marine fungi: A potential source of novel enzymes for environmental and biotechnological applications. J. Environ. Prot. Ecol. 2019, 20, 1214–1222. [Google Scholar]
- Pasqualetti, M.; Barghini, P.; Giovannini, V.; Fenice, M. High production of chitinolytic activity in halophilic conditions by a new marine strain of Clonostachys rosea. Molecules 2019, 24, 101880. [Google Scholar] [CrossRef] [PubMed]
- Botta, L.; Saladino, R.; Barghini, P.; Fenice, M.; Pasqualetti, M. Production and identification of two antifungal terpenoids from the Posidonia oceanica epiphytic Ascomycota Mariannaea humicola IG100. Microb. Cell Fact. 2020, 19, 184. [Google Scholar] [CrossRef] [PubMed]
- Pasqualetti, M.; Gorrasi, S.; Giovannini, V.; Braconcini, M.; Fenice, M. Polyextremophilic chitinolytic activity by a marine strain (IG119) of Clonostachys rosea. Molecules 2022, 27, 688. [Google Scholar] [CrossRef] [PubMed]
- Poli, A.; Bovio, E.; Ranieri, L.; Varese, G.C.; Prigione, V. News from the sea: A new genus and seven new species in the Pleosporalean families Roussoellaceae and Thyridariaceae. Diversity 2020, 12, 144. [Google Scholar] [CrossRef]
- Pasqualetti, M.; Giovannini, V.; Barghini, P.; Gorrasi, S.; Fenice, M. Diversity and ecology of culturable marine fungi associated with Posidonia oceanica leaves and their epiphytic algae Dictyota dichotoma and Sphaerococcus coronopifolius. Fungal Ecol. 2020, 44, 100906. [Google Scholar] [CrossRef]
- Poli, A.; Bovio, E.; Perugini, I.; Varese, G.C.; Prigione, V. Corollospora mediterranea: A novel species complex in the Mediterranean Sea. Appl. Sci. 2021, 11, 5452. [Google Scholar] [CrossRef]
- Gorrasi, S.; Franzetti, A.; Ambrosini, R.; Pittino, F.; Pasqualetti, M.; Fenice, M. Spatio-temporal variation of the bacterial communities along a salinity gradient within a thalassohaline environment (Saline di Tarquinia salterns Italy). Molecules 2021, 26, 1338. [Google Scholar] [CrossRef]
- Gorrasi, S.; Pesciaroli, C.; Barghini, P.; Pasqualetti, M.; Fenice, M. Structure and diversity of the bacterial community of an Arctic estuarine system (Kandalaksha Bay) subject to intense tidal currents. J. Mar. Syst. 2019, 196, 77–85. [Google Scholar] [CrossRef]
- Gorrasi, S.; Franzetti, A.; Brandt, A.; Minzlaff, U.; Pasqualetti, M.; Fenice, M. Insights into the prokaryotic communities of the abyssal-hadal benthic-boundary layer of the Kuril Kamchatka Trench. Environ. Microbiome 2023, 18, 67. [Google Scholar] [CrossRef]
- Amend, A.; Burgaud, G.; Cunliffe, M.; Edgcomb, V.P.; Ettinger, C.L.; Gutiérrez, M.H.; Heitman, J.; Hom, E.F.Y.; Ianiri, G.; Jones, A.; et al. Fungi in the marine environment: Open questions and unsolved problems. mBio 2019, 10, e01189-18. [Google Scholar] [CrossRef]
- Gladfelter, A.S.; James, T.Y.; Amend, A.S. Marine fungi. Curr. Biol. 2019, 29, R191–R195. [Google Scholar] [CrossRef] [PubMed]
- Grossart, H.P.; Van den Wyngaert, S.; Kagami, M.; Wurzbacher, C.; Cunliffe, M.; Rojas-Jimenez, K. Fungi in aquatic ecosystems. Nat. Rev. Microbiol. 2019, 17, 339–354. [Google Scholar] [CrossRef]
- Gonçalves, M.F.; Esteves, A.C.; Alves, A. Marine Fungi: Opportunities and Challenges. Encyclopedia 2022, 2, 559–577. [Google Scholar] [CrossRef]
- Jones, E.B.G.; Pang, K.L.; Abdel-Wahab, M.A.; Scholz, B.; Hyde, K.D.; Boekhout, T.; Ebel, R.; Rateb, M.E.; Henderson, L.; Sakayaroj, J.; et al. An online resource for marine fungi. Fungal Divers. 2019, 96, 347–433. [Google Scholar] [CrossRef]
- Jensen, P.R. Secondary metabolites from marine fungi. In Fungi in Marine Environments; Hyde, K.D., Ed.; Fungal Diversity Research Series; Fungal Diversity Press: Hong Kong, China, 2002; Volume 7, pp. 293–315. [Google Scholar]
- Overy, D.P.; Rämä, T.; Oosterhuis, R.; Walker, A.K.; Pang, K.L. The neglected marine fungi sensu stricto and their isolation for natural products’ discovery. Mar. Drugs 2019, 17, 42. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.; Inderbitzin, P.; Kohlmeyer, J.; Volkmann-Kohlmeyer, B. Koralionastetales a new order of marine Ascomycota in the Sordariomycetes. Mycol. Res. 2009, 113, 373–380. [Google Scholar] [CrossRef]
- Sakayaroj, J.; Pang, K.L.; Jones, E.G. Multi-gene phylogeny of the Halosphaeriaceae: Its ordinal status relationships between genera and morphological character evolution. Fungal Divers. 2011, 46, 87–109. [Google Scholar] [CrossRef]
- Kohlmeyer, J.; Spatafora, J.W.; Volkmann-Kohlmeyer, B. Lulworthiales a new order of marine Ascomycota. Mycologia 2000, 92, 453–458. [Google Scholar] [CrossRef]
- Azevedo, E.; Barata, M.; Marques, M.I.; Caeiro, M.F. Lulworthia atlantica: A new species supported by molecular phylogeny and morphological analysis. Mycologia 2017, 109, 287–295. [Google Scholar] [CrossRef]
- Poli, A.; Bovio, E.; Ranieri, L.; Varese, G.C.; Prigione, V. Fungal Diversity in the Neptune Forest: Comparison of the Mycobiota of Posidonia oceanica; Flabellia petiolata and Padina pavonica. Front. Microbiol. 2020, 11, 933. [Google Scholar] [CrossRef]
- Poli, A.; Prigione, V.; Bovio, E.; Perugini, I.; Varese, G.C. Insights on Lulworthiales inhabiting the Mediterranean Sea and description of three novel species of the genus Paralulworthia. J. Fungi 2021, 7, 940. [Google Scholar] [CrossRef]
- Goncalves, M.F.; Abreu, A.C.; Hilario, S.; Alves, A. Diversity of marine fungi associated with wood baits in the estuary Ria de Aveiro with descriptions of Paralulworthia halima comb. nov. Remispora submersa sp. nov. and Zalerion pseudomaritima sp. nov. Mycologia 2021, 113, 664–683. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.W.; Boers, J.; Holdom, D.; Steinrucken, T.V.; Tan, Y.P.; Vitelli, J.S.; Shivas, R.G.; Barret, M.; Boxshall, A.G.; Broadbridge, J.; et al. Fungal Planet description sheets: 1383–1435. Persoonia 2022, 48, 261–371. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.B.G.; Pang, K.L. Introduction marine fungi. In Marine Fungi and Fungal-Like Organisms; Walter de Gruyter: Berlin, Germany, 2012; pp. 1–13. [Google Scholar]
- Abdel-Wahab, M.A.; Hodhod, M.S.; Bahkali, A.H.; Jones, E.G. Marine fungi of Saudi Arabia. Bot. Mar. 2014, 57, 323–335. [Google Scholar] [CrossRef]
- Aime, M.C.; Miller, A.N.; Aoki, T.; Bensch, K.; Cai, L.; Crous, P.W.; Hawksworth, D.L.; Hyde, K.D.; Kirk, P.M. How to publish a new fungal species or name version 3.0. IMA Fungus 2021, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Knapp, D.G.; Kovács, G.M.; Zajta, E.; Groenewald, J.Z.; Crous, P.W. Dark septate endophytic pleosporalean genera from semiarid areas. Persoonia 2015, 35, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Koch, R.A.; Jean Lodge, D.; Sourell, S.; Nakasone, K.; McCoy, A.G.; Aime, M.C. Tying up loose threads: Revised taxonomy and phylogeny of an avian-dispersed Neotropical rhizomorph-forming fungus. Mycol. Prog. 2018, 17, 989–998. [Google Scholar] [CrossRef]
- Crous, P.W.; Carnegie, A.J.; Wingfield, M.J.; Sharma, R.; Mughini, G.; Noordeloos, M.E.; Santini, A.; Shouche, Y.S.; Bezerra, J.D.P.; Dima, B.; et al. Fungal Planet description sheets: 868–950. Persoonia 2019, 42, 291. [Google Scholar] [CrossRef] [PubMed]
- Noumeur, S.R.; Teponno, R.B.; Helaly, S.E.; Wang, X.W.; Harzallah, D.; Houbraken, J.; Crous, P.W.; Stadler, M. Diketopiperazines from Batnamyces globulariicola gen. sp. nov. (Chaetomiaceae) a fungus associated with roots of the medicinal plant Globularia alypum in Algeria. Mycol. Prog. 2020, 19, 589–603. [Google Scholar] [CrossRef]
- Hyde, K.D.; Norphanphoun, C.; Maharachchikumbura, S.S.N.; Bhat, D.J.; Jones, E.; Bundhun, D.; Chen, Y.J.; Bao, D.F.; Boonmee, S.; Calabon, M.S.; et al. Refined families of Sordariomyetes. Mycosphere 2020, 11, 305–1059. [Google Scholar] [CrossRef]
- Zuccaro, A.; Schoch, C.L.; Spatafora, J.W.; Kohlmeyer, J.; Draeger, S.; Mitchell, J.I. Detection and identification of fungi intimately associated with the brown seaweed Fucus serratus. Appl. Environ. Microbiol. 2008, 74, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Rämä, T.; Nordén, J.; Davey, M.L.; Mathiassen, G.H.; Spatafora, J.W.; Kauserud, H. Fungi ahoy! Diversity on marine wooden substrata in the high North. Fungal Ecol. 2014, 8, 46–58. [Google Scholar] [CrossRef]
- Garzoli, L.; Gnavi, G.; Varese, G.C.; Picco, A.M. Mycobiota associated with the rhodophyte alien species Asparagopsis taxiformis (Delile) Trevisan de Saint-Léon in the Mediterranean Sea. Mar. Ecol. 2015, 36, 959–968. [Google Scholar] [CrossRef]
- Calado, M.D.L.; Carvalho, L.; Barata, M.; Pang, K.L. Potential roles of marine fungi in the decomposition process of standing stems and leaves of Spartina maritima. Mycologia 2019, 111, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Hagestad, O.C.; Andersen, J.H.; Altermark, B.; Hansen, E.; Rämä, T. Cultivable marine fungi from the Arctic Archipelago of Svalbard and their antibacterial activity. Mycology 2019, 11, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Cha, H.J.; Chiang, M.W.; Guo, S.Y.; Lin, S.M.; Pang, K.L. Culturable fungal community of Pterocladiella capillacea in Keelung Taiwan: Effects of surface sterilization method and isolation medium. J. Fungi 2021, 7, 651. [Google Scholar] [CrossRef] [PubMed]
- Torta, L.; Burruano, S.; Giambra, S.; Conigliaro, G.; Piazza, G.; Mirabile, G.; Pirrotta, M.; Calvo, R.; Bellissimo, G.; Calvo, S.; et al. Cultivable fungal endophytes in roots rhizomes and leaves of Posidonia oceanica (L.) Delile along the coast of Sicily Italy. Plants 2022, 11, 1139. [Google Scholar] [CrossRef] [PubMed]
- Vohník, M. Are lulworthioid fungi dark septate endophytes of the dominant Mediterranean seagrass Posidonia oceanica? Plant Biol. 2022, 24, 127–133. [Google Scholar] [CrossRef]
- Bovio, E.; Gnavi, G.; Prigione, V.; Spina, F.; Denaro, R.; Yakimov, M.; Calogero, R.; Crisafi, F.; Varese, G.C. The culturable mycobiota of a Mediterranean marine site after an oil spill: Isolation identification and potential application in bioremediation. Sci. Total Environ. 2017, 576, 310–318. [Google Scholar] [CrossRef]
- Kohlmeyer, J.; Volkmann-Kohlmeyer, B. A new Lulworthia (Ascomycotina) from corals. Mycologia 1989, 81, 289–292. [Google Scholar] [CrossRef]
- Amend, A.S.; Barshis, D.J.; Oliver, T.A. Coral-associated marine fungi form novel lineages and heterogeneous assemblages. ISME J. 2012, 6, 1291–1301. [Google Scholar] [CrossRef]
- Bonthond, G.; Merselis, D.G.; Dougan, K.E.; Graff, T.; Todd, W.; Fourqurean, J.W.; Rodriguez-Lanetty, M. Inter-domain microbial diversity within the coral holobiont Siderastrea siderea from two depth habitats. PeerJ 2018, 6, e4323. [Google Scholar] [CrossRef] [PubMed]
- Góes-Neto, A.; Marcelino, V.R.; Verbruggen, H.; da Silva, F.F.; Badotti, F. Biodiversity of endolithic fungi in coral skeletons and other reef substrates revealed with 18S rDNA metabarcoding. Coral Reefs 2020, 39, 229–238. [Google Scholar] [CrossRef]
- Luna-Pérez, B.; Valle, C.; Fernández, T.V.; Sanchez-Lizaso, J.L.; Ramos-Espla, A.A. Halocynthia papillosa (Linnaeus 1767) as an indicator of SCUBA diving impact. Ecol. Ind. 2010, 10, 1017–1024. [Google Scholar] [CrossRef]
- Coppari, M.; Gori, A.; Rossi, S. Size spatial and bathymetrical distribution of the ascidian Halocynthia papillosa in Mediterranean coastal bottoms: Benthic–pelagic coupling implications. Mar. Biol. 2014, 161, 2079–2095. [Google Scholar] [CrossRef]
- Lübbering-Sommer, B.; Compère, P.; Goffinet, G. Cytochemical investigations on tunic morphogenesis in the sea peach Halocynthia papillosa (Tunicata Ascidiacea) 1: Demonstration of polysaccharides. Tissue Cell 1996, 28, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Delroisse, J.; Schoenaers, D.; Kim, H.; Nguyen, T.C.; Horbelt, N.; Leclère, P.; Hwang, D.S.; Harrington, M.J.; Flammang, P. Structure and composition of the tunic in the sea pineapple Halocynthia roretzi: A complex cellulosic composite biomaterial. Acta Biomater. 2020, 111, 290–301. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols; Elsevier: Amsterdam, The Netherlands; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Sym. 1990, 41, 95–98. [Google Scholar]
- Trifinopoulos, J.; Nguyen, L.T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 32: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Posada, D. jModelTest: Phylogenetic model averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
- Panebianco, C.; Tam, W.Y.; Jones, E.B.G. The effect of pre-inoculation of balsa wood by selected marine fungi and their effect on subsequent colonisation in the sea. Fungal Divers. 2002, 10, 77–88. [Google Scholar]
- Su, Y.Y.; Qi, Y.L.; Cai, L. Induction of sporulation in plant pathogenic fungi. Mycology 2012, 3, 195–200. [Google Scholar] [CrossRef]
- Senanayake, I.C.; Rathnayaka, A.R.; Marasinghe, D.S.; Calabon, M.S.; Gentekaki, E.; Lee, H.B.; Hurdeal, V.G.; Pem, D.; Dissanayake, L.S.; Wijesinghe, S.N.; et al. Morphological approaches in studying fungi: Collection examination isolation sporulation and preservation. Mycosphere 2020, 11, 2678–2754. [Google Scholar] [CrossRef]
- Raghukumar, S. The Role of Fungi in Marine Detrital Processes. In Marine Microbiology: Facets and Opportunities; Ramaiah, N., Ed.; National Institute of Oceanography: Goa, India, 2004; pp. 91–101. [Google Scholar]
- Morrison-Gardiner, S. Dominant fungi from Australian coral reefs. Fungal Divers. 2002, 9, 105–121. [Google Scholar]
- Damare, S.; Raghukumar, C.; Raghukumar, S. Fungi in deep-sea sediments of the Central Indian Basin. Deep Sea Res. Part I Oceanogr. Res. Pap. 2006, 53, 14–27. [Google Scholar] [CrossRef]
- Menezes, C.B.; Bonugli-Santos, R.C.; Miqueletto, P.B.; Passarini, M.R.; Silva, C.H.; Justo, M.R.; Leal, R.R.; Fantinatti-Garboggini, F.; Oliveira, V.M.; Berlinck, R.G.S.; et al. Microbial diversity associated with algae ascidians and sponges from the north coast of São Paulo state Brazil. Microbiol. Res. 2010, 165, 466–482. [Google Scholar] [CrossRef]
- López-Legentil, S.; Erwin, P.M.; Turon, M.; Yarden, O. Diversity of fungi isolated from three temperate ascidians. Symbiosis 2015, 66, 99–106. [Google Scholar] [CrossRef]
- Vacondio, B.; Birolli, W.G.; Ferreira, I.M.; Seleghim, M.H.; Gonçalves, S.; Vasconcellos, S.P.; Porto, A.L. Biodegradation of pentachlorophenol by marine-derived fungus Trichoderma harzianum CBMAI 1677 isolated from ascidian Didemnun ligulum. Biocatal. Agric. Biotechnol. 2015, 4, 266–275. [Google Scholar] [CrossRef]
- Godinho, V.M.; de Paula, M.T.R.; Silva, D.A.S.; Paresque, K.; Martins, A.P.; Colepicolo, P.; Rosa, C.A.; Rosa, L.H. Diversity and distribution of hidden cultivable fungi associated with marine animals of Antarctica. Fungal Biol. 2019, 123, 507–516. [Google Scholar] [CrossRef] [PubMed]


| PCR Steps | nrITS | nrLSU | nrSSU | |
|---|---|---|---|---|
| ITS5/ITS4 | LR0R/LR7 | NS1/NS4 | ||
| Initial denaturation | 94 °C for 2′ | 95 °C for 10′ | 95 °C for 10′ | |
| PCR cycle | denaturation | 94 °C for 20″ | 95 °C for 1′ | 95 °C for 1′ |
| annealing | 56 °C for 30″ | 50 °C for 50″ | 50 °C for 50″ | |
| elongation | 72 °C for 45″ | 72 °C for 1.5′ | 72 °C for 1.5′ | |
| Final elongation | 72 °C for 10′ | 72 °C for 10′ | 72 °C for 10′ | |
| Number of cycles | 35 | 40 | 40 |
| Species | Strain | Substrates | nrITS | nrSSU | nrLSU |
|---|---|---|---|---|---|
| Lulworthiales | |||||
| Cumulospora marina | MF46 | Submerged wood | - | GU252136 | GU252135 |
| GC53 | Submerged wood | - | GU256625 | GU256626 | |
| Cumulospora varia | GR78 | Submerged wood | - | EU848593 | EU848578 |
| IT 152 | Wood | - | EU848579 | - | |
| Halazoon melhae | MF819 | Submerged wood | - | GU252144 | GU252143 |
| Halazoon fuscus | NBRC 105256 | Driftwood | - | GU252148 | GU252147 |
| Hydea pygmea | NBRC 33069 | Driftwood | - | GU252134 | GU252133 |
| IT081 | Driftwood | - | GU256632 | GU256633 | |
| Kohlmeyeriella crassa | NBRC 32133 | Driftwood | LC146741 | - | LC146742 |
| Kohlmeyeriella tubulata | PP1105 | Sea foam | - | AY878998 | AF491265 |
| PP0989 | Marine environment | - | AY878997 | AF491264 | |
| Lindra marinera | JK 5091A | Marine environment | - | AY879000 | AY878958 |
| Lindra obtuse | NRBC 31317 | Sea foam | LC146744 | AY879002 | AY878960 |
| AFTOL 5012 | Marine environment | - | FJ176847 | FJ176902 | |
| CBS 113030 | - | - | AY879001 | AY878959 | |
| Lindra thalassiae | JK 5090A | Marine environment | - | U46874 | U46891 |
| AFTOL-ID 413 | Marine environment | DQ491508 | DQ470994 | DQ470947 | |
| JK 5090 | Marine environment | - | AF195634 | AF195635 | |
| JK 4322 | Thalassia testudinum | - | AF195632 | AF195633 | |
| Lulwoana uniseptate | NBRC 32137 | Submerged wood | LC146746 | LC146746 | LC146746 |
| CBS 16760 | Driftwood | - | AY879034 | AY878991 | |
| Lulworthia atlantica | FCUL210208SP4 | Sea water | KT347205 | KT347193 | JN886843 |
| FCUL190407CF4 | Sea water | KT347207 | KT347198 | JN886809 | |
| FCUL061107CP3 | Sea water | KT347208 | KT347196 | JN886825 | |
| Lulworthia fucicola | ATCC 64288 | Intertidal wood | - | AY879007 | AY878965 |
| PP1249 | Marine environment | - | AY879008 | AY878966 | |
| Lulworthia fundyensis | DAOMC 251940 | Marine wood | NR_178138 | - | - |
| AW2347 | Marine wood | MH465123 | MH465136 | MH458750 | |
| Lulworthia grandispora | AFTOL 424 | Dead Rhizophora sp. | - | DQ522855 | DQ522856 |
| NTOU3841 | Driftwood | - | KY026044 | KY026048 | |
| NTOU3847 | Mangrove wood | - | KY026046 | KY026049 | |
| NTOU3849 | Mangrove wood | - | KY026047 | KY026050 | |
| Lulworthia medusa | JK 5581 | Spartina sp. | - | AF195636 | AF195637 |
| Lulworthia opaca | CBS 218.60 | Driftwood | - | AY879003 | AY878961 |
| Lulworthia cf. purpurea | FCUL170907CP5 | Seawater | KT347219 | KT347201 | JN886824 |
| FCUL280207CF9 | Seawater | KT347218 | KT347202 | JN886808 | |
| Matsusporium tropicale | NBRC 32499 | Submerged wood | - | GU252142 | GU252141 |
| Paralulworthia candida | MUT 5430 | P. oceanica | MZ357724 | MZ357767 | MZ357746 |
| Paralulworthia elbensis | MUT 377 | P. oceanica | MZ357710 | MZ357753 | MZ357732 |
| MUT 5422 | P. oceanica | MZ357723 | MZ357766 | MZ357745 | |
| MUT 5438 | P. oceanica | MZ357712 | MZ357755 | MZ357734 | |
| MUT 5461 | P. oceanica | MZ357725 | MZ357768 | MZ357747 | |
| Paralulworthia gigaspora | MUT 435 | P. oceanica | MN649242 | MN649246 | MN649250 |
| MUT 5413 | P. oceanica | MN649243 | MN649247 | MN649251 | |
| MUT 263 | Seawater | MZ357729 | MZ357772 | MZ357751 | |
| MUT 465 | P. oceanica | MZ357726 | MZ357769 | MZ357748 | |
| MUT 1753 | Seawater | MZ357730 | MZ357773 | MZ357752 | |
| MUT 5085 | P. oceanica | MZ357715 | MZ357758 | MZ357737 | |
| MUT 5086 | P. oceanica | MZ357716 | MZ357759 | MZ357738 | |
| MUT 5093 | P. oceanica | MZ357718 | MZ357761 | MZ357740 | |
| MUT 5094 | P. oceanica | MZ357719 | MZ357762 | MZ357741 | |
| Paralulworthia halima | CMG 68 | Submerged wood | MT235736 | MT235712 | MT235753 |
| CMG 69 | Submerged wood | MT235737 | MT235713 | MT235754 | |
| MUT 1483 | Submerged wood | MZ357727 | MZ357770 | MZ357749 | |
| MUT 2919 | Submerged wood | MZ357713 | MZ357756 | MZ357735 | |
| MUT 3347 | Submerged wood | MZ357728 | MZ357771 | MZ357750 | |
| Paralulworthia mediterranea | MUT 654 | P. oceanica | MZ357711 | MZ357754 | MZ357733 |
| MUT 5080 | P. oceanica | MZ357714 | MZ357757 | MZ357736 | |
| MUT 5417 | P. oceanica | MZ357721 | MZ357764 | MZ357743 | |
| Paralulworthia posidoniae | MUT 5261 | P. oceanica | MN649245 | MN649249 | MN649253 |
| MUT 5092 | P. oceanica | MZ357717 | MZ357760 | MZ357739 | |
| MUT 5110 | P. oceanica | MZ357720 | MZ357763 | MZ357742 | |
| MUT 5419 | P. oceanica | MZ357722 | MZ357765 | MZ357744 | |
| Rambellisea halocynthiae | HPa50 | H. papillosa | OR367481 | OR371485 | OR371457 |
| HPa51 | H. papillosa | OR367548 | OR371484 | OR371461 | |
| HPa52 | H. papillosa | OR367549 | - | OR371460 | |
| HPa53 | H. papillosa | OR367614 | - | - | |
| HPa54 | H. papillosa | OR378535 | - | - | |
| HPa58 | H. papillosa | OR367660 | - | - | |
| HPa59 | H. papillosa | OR367678 | - | - | |
| HPa60 | H. papillosa | OR367679 | - | - | |
| HPa61 | H. papillosa | OR367717 | - | - | |
| HPa62 | H. papillosa | OR378536 | - | - | |
| HPa63 | H. papillosa | OR378537 | - | - | |
| HPa64 | H. papillosa | OR378538 | - | - | |
| Rambellisea gigliensis | HPa3 | H. papillosa | OR367423 | OR371466 | OR369726 |
| HPa15 | H. papillosa | OR367447 | OR371482 | OR369725 | |
| HPa16 | H. papillosa | OR367450 | OR371483 | OR371456 | |
| Zalerion maritima | FCUL280207CP1 | Seawater | KT347216 | KT347203 | JN886806 |
| FCUL010407SP2 | Seawater | KT347217 | KT347204 | JN886805 | |
| CM66 | Submerged wood | MT235734 | MT235710 | MT235751 | |
| CM67 | Submerged wood | MT235735 | MT235711 | MT235752 | |
| Zalerion pseudomaritima | CMG64 | Submerged wood | MT235732 | MT235708 | MT235749 |
| CMG65 | Submerged wood | MT235733 | MT235709 | MT235750 | |
| Pleosporales | |||||
| Bimuria novae-zelandiae | CBS107.79 | soil | MH861181 | AY016338 | MH872950 |
| Setosphaeria monoceras | CBS 154.26 | - | DQ337380 | DQ238603 | AY016368 |
| Letendraea helminthicola | CBS 884.85 | Yerba mate | MK404145 | AY016345 | AY016362 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braconcini, M.; Gorrasi, S.; Fenice, M.; Barghini, P.; Pasqualetti, M. Rambellisea gigliensis and Rambellisea halocynthiae, gen. et spp. nov. (Lulworthiaceae) from the Marine Tunicate Halocynthia papillosa. J. Fungi 2024, 10, 127. https://doi.org/10.3390/jof10020127
Braconcini M, Gorrasi S, Fenice M, Barghini P, Pasqualetti M. Rambellisea gigliensis and Rambellisea halocynthiae, gen. et spp. nov. (Lulworthiaceae) from the Marine Tunicate Halocynthia papillosa. Journal of Fungi. 2024; 10(2):127. https://doi.org/10.3390/jof10020127
Chicago/Turabian StyleBraconcini, Martina, Susanna Gorrasi, Massimiliano Fenice, Paolo Barghini, and Marcella Pasqualetti. 2024. "Rambellisea gigliensis and Rambellisea halocynthiae, gen. et spp. nov. (Lulworthiaceae) from the Marine Tunicate Halocynthia papillosa" Journal of Fungi 10, no. 2: 127. https://doi.org/10.3390/jof10020127
APA StyleBraconcini, M., Gorrasi, S., Fenice, M., Barghini, P., & Pasqualetti, M. (2024). Rambellisea gigliensis and Rambellisea halocynthiae, gen. et spp. nov. (Lulworthiaceae) from the Marine Tunicate Halocynthia papillosa. Journal of Fungi, 10(2), 127. https://doi.org/10.3390/jof10020127










