Morphological Changes and Strong Cytotoxicity in Yarrowia lipolytica by Overexpressing Delta-12-Desaturase
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
2.2. Analytical Methods
2.3. Construction of the 1312-12 and 1292-12 Strains
2.4. Lipid Extraction and Gas Chromatography for Fatty Acid Analysis
2.5. Cell Viability Assay
2.6. Supplementation of Vitamin C
2.7. Detection of ROS and MDA
2.8. CAT and SOD Enzyme Activity Assays
2.9. Supplementation of Fish and Colza Oil
2.10. Statistical Analysis
3. Results
3.1. Influence of Delta-12 Desaturase Expression on the Fatty Acid Profile
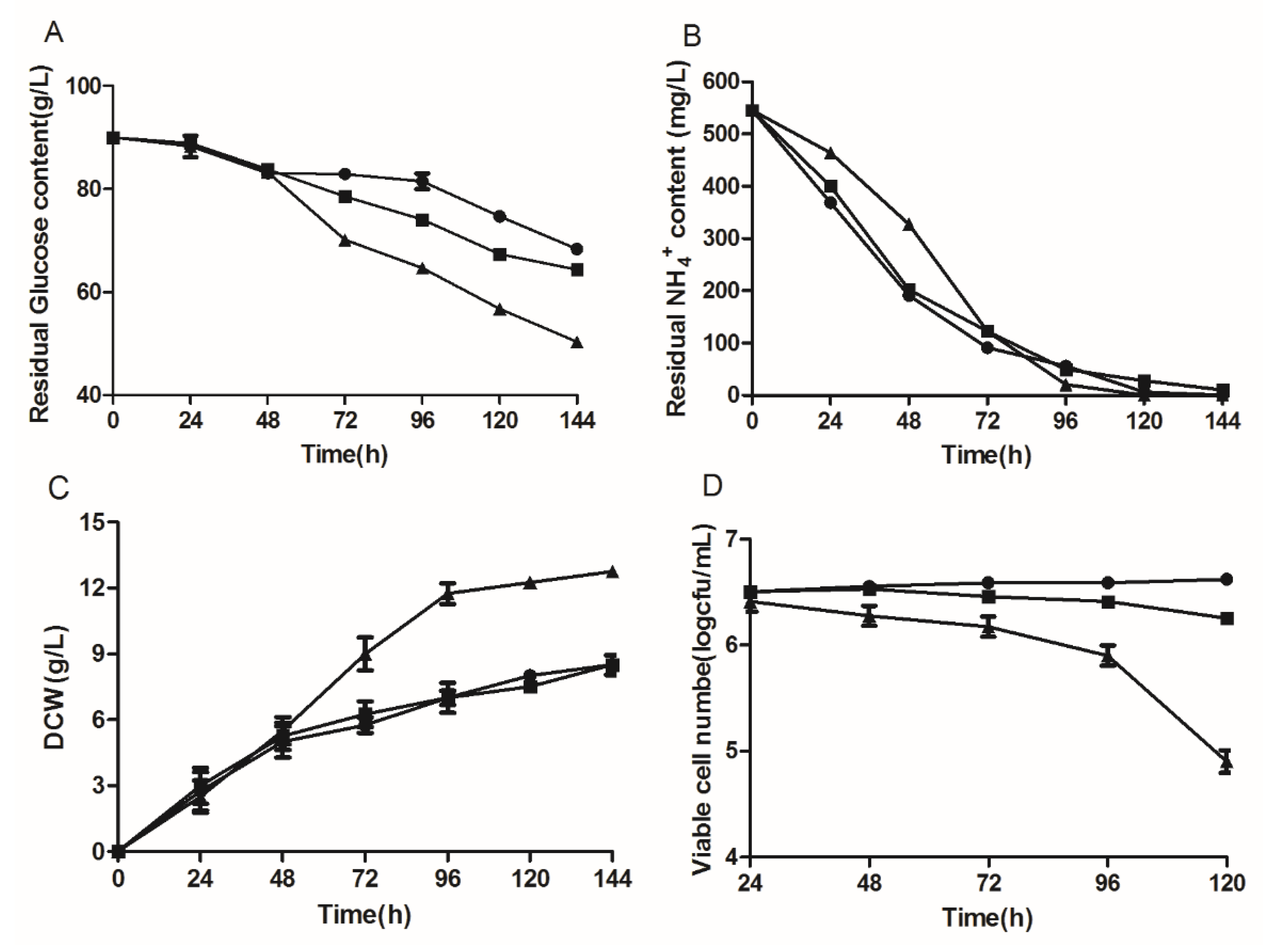
3.2. Influence of Delta-12 Desaturase Overexpression on the Growth of Y. lipolytica
3.3. Effects of Linoleic Acid Toxicity on the Cell Morphology of Y. lipolytica
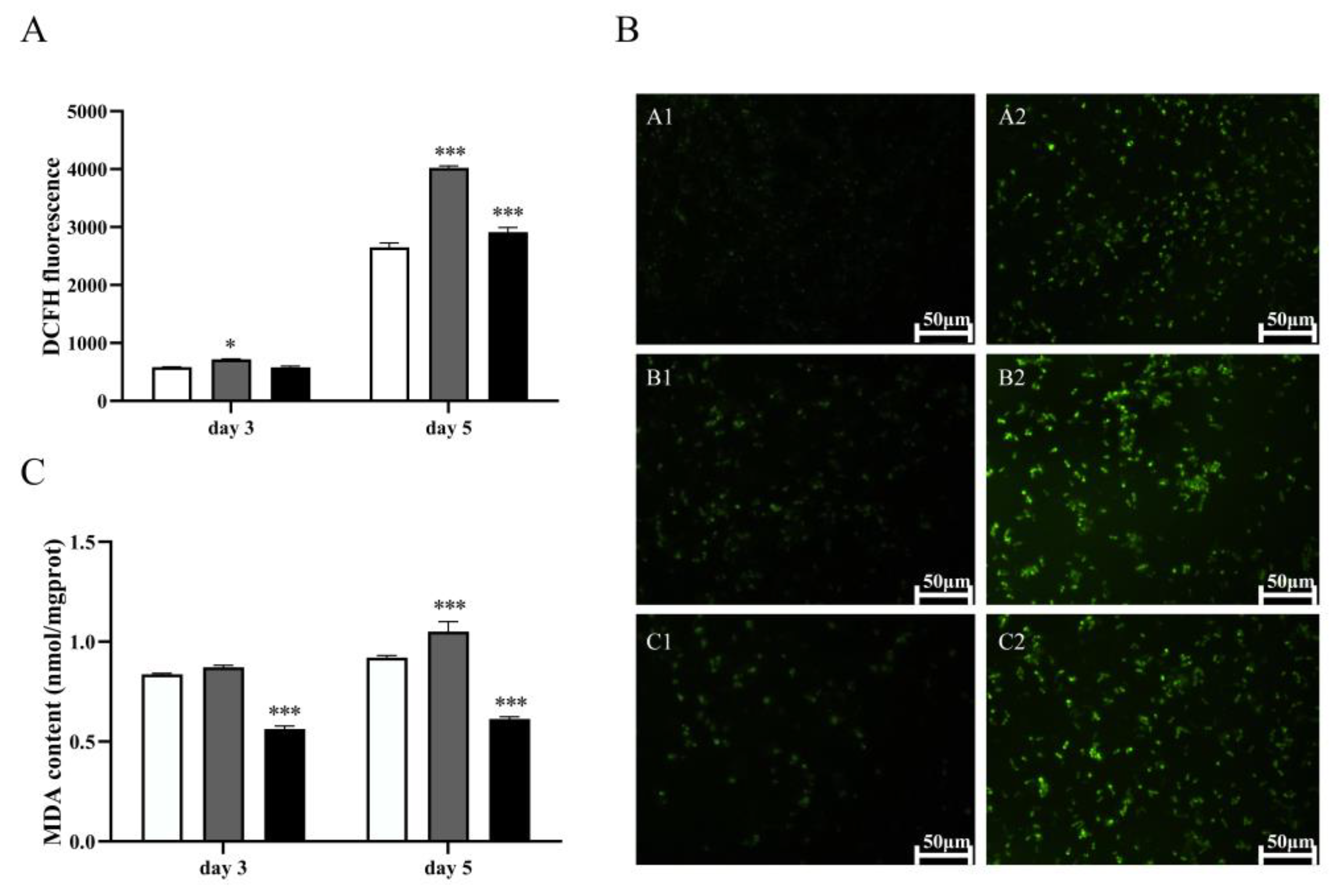
3.4. The Lipotoxicity of Excess LA in the Multicopy Strain 1291-12 Is Associated with Oxidative Stress
3.5. Effect of Fish or Colza Oil Supplementation on the Fatty Acid Profile of Y. lipolytica
3.6. Effects of Fish or Colza Oil Supplementation on Cell Growth
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carracedo, A.; Cantley, L.C.; Pandolfi, P.P. Cancer metabolism: Fatty acid oxidation in the limelight. Nat. Rev. Cancer 2013, 13, 227–232. [Google Scholar] [CrossRef]
- Arita, K.; Kobuchi, H.; Utsumi, T.; Takehara, Y.; Akiyama, J.; Horton, A.A.; Utsumi, K. Mechanism of apoptosis in HL-60 cells induced by n-3 and n-6 polyunsaturated fatty acids. Biochem. Pharmacol. 2001, 62, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Artwohl, M.; Lindenmair, A.; Roden, M.; Waldhausl, W.K.; Freudenthaler, A.; Klosner, G.; Klosner, C.; Ilhan, A.; Luger, A.; Baumgartner-Parzer, S.M. Fatty acids induce apoptosis in human smooth muscle cells depending on chain length, saturation, and duration of exposure. Atherosclerosis 2009, 202, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Phoon, M.C.; Desbordes, C.; Howe, J.; Chow, V.T. Linoleic and linolelaidic acids differentially influence proliferation and apoptosis of MOLT-4 leukaemia cells. Cell Biol. Int. 2001, 25, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Toborek, M.; Blanc, E.M.; Kaiser, S.; Mattson, M.P.; Hennig, B. Linoleic acid potentiates TNF-mediated oxidative stress, disruption of calcium homeostasis, and apoptosis of cultured vascular endothelial cells. J. Lipid Res. 1997, 38, 2155–2167. [Google Scholar] [CrossRef] [PubMed]
- Edwards, I.J.; O’ Flaherty, J.T. Omega-3 Fatty Acids and PPARγ in Cancer. PPAR Res. 2008, 2008, 358052. [Google Scholar] [CrossRef] [PubMed]
- Lima, T.M.; Kanunfre, C.C.; Pompéia, C.; Verlengia, R.; Curi, R. Ranking the toxicity of fatty acids on Jurkat and Raji cells by flow cytometric analysis. Toxicol. Vitr. 2002, 16, 741–747. [Google Scholar] [CrossRef]
- Diggle, C.P. In vitro studies on the relationship between polyunsaturated fatty acids and cancer: Tumour or tissue specific effects? Prog. Lipid Res. 2001, 41, 240–253. [Google Scholar] [CrossRef]
- Sooksai, S.; Chewchanlertfa, P.; Kaneko, Y.; Harashima, S.; Laoteng, K. Alterations in growth and fatty acid profiles under stress conditions of Hansenula polymorpha defective in polyunsaturated fatty acid synthesis. Mol. Biol. Rep. 2013, 40, 4935–4945. [Google Scholar] [CrossRef]
- Cipak, A.; Hasslacher, M.; Tehlivets, O.; Collinson, E.J.; Zivkovic, M.; Matijevic, T.; Wonisch, W.; Waeg, G.; Dawes, W.I.; Zarkovic, N.; et al. Saccharomyces cerevisiae strain expressing a plant fatty acid desaturase produces polyunsaturated fatty acids and is susceptible to oxidative stress induced by lipid peroxidation. Free Radic. Biol. Med. 2006, 40, 897–906. [Google Scholar] [CrossRef]
- Zhang, H.; Kang, X.; Xiao, N.; Gao, M.; Zhao, Y.; Zhang, B.; Song, Y. Intracellular expression of Vitreoscilla haemoglobin improves lipid production in Yarrowia lipolytica. Lett. Appl. Microbiol. 2019, 68, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Hu, C.; Jin, G.; Zhao, X.; Zhao, Z.K. Phosphate-limitation mediated lipid production by Rhodosporidium toruloides. Bioresour. Technol. 2010, 101, 6124–6129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, L.; Chen, H.; Chen, Y.Q.; Chen, W.; Song, Y.; Ratledge, C. Enhanced lipid accumulation in the yeast Yarrowia lipolytica by over-expression of ATP:citrate lyase from Mus musculus. J. Biotechnol. 2014, 192, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.; Chen, X.; Milanova, S.; Santos, C.; Petranovic, D. Pufa-induced cell death is mediated by Yca1p-dependent and -independent pathways, and is reduced by vitamin C in yeast. FEMS Yeast Res. 2016, 16, fow007. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, Z.; Yu, Q.; Mao, J.; Zhang, B.; Xing, L.; Li, M. Deletion of genes encoding fatty acid desaturases leads to alterations in stress sensitivity in Pichia pastoris. FEMS Yeast Res. 2015, 15, fov020. [Google Scholar] [CrossRef] [PubMed]
- Elstner, E.F.; Heupel, A. Inhibition of nitrite formation from hydroxylammoniumchloride: A simple assay for superoxide dismutase. Anal. Biochem. 1976, 70, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Uemura, H. Synthesis and production of unsaturated and polyunsaturated fatty acids in yeast: Current state and perspectives. Appl. Microbiol. Biotechnol. 2012, 95, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cury-Boaventura, M.F.; Pompeia, C.; Curi, R. Comparative toxicity of oleic acid and linoleic acid on Jurkat cells. Clin. Nutr. 2004, 23, 721–732. [Google Scholar] [CrossRef]
- Rudolph, I.L.; Kelley, D.S.; Klasing, K.C.; Erickson, K.L. Regulation of cellular differentiation and apoptosis by fatty acids and their metabolites. Nutr. Res. 2001, 21, 381–393. [Google Scholar] [CrossRef]
- Ruenwai, R.; Neiss, A.; Laoteng, K.; Vongsangnak, W.; Dalfard, A.B.; Cheevadhanarak, S. Heterologous production of polyunsaturated fatty acids in Saccharomyces cerevisiae causes a global transcriptional response resulting in reduced proteasomal activity and increased oxidative stress. Biotechnol. J. 2011, 6, 343–356. [Google Scholar] [CrossRef]
- Priault, M.; Bessoule, J.J.; Grelaud-Coq, A.; Camougrand, N.; Manon, S. Bax-induced cell death in yeast depends on mitochondrial lipid oxidation. Eur. J. Biochem. 2002, 269, 5440–5450. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, H.; Halliwell, B. Damage to DNA by reactive oxygen and nitrogen species: Role in inflammatory disease and progression to cancer. Biochem. J. 1996, 313, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Munoz, M.F.; Arguelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, G.; Balzan, R. Oxidative stress and programmed cell death in yeast. Front. Oncol. 2012, 2, 64. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.L. Carbonyl modified proteins in cellular regulation, aging, and disease. Free. Radic. Biol. Med. 2002, 32, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Aydin, B.; Guler Sahin, C.; Sekeroglu, V.; Atli Sekeroglu, Z. Conjugated linoleic acid protects brain mitochondrial function in acrolein induced male rats. Toxicol. Mech. Methods 2021, 31, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kang, D.; Ok, S.-H.; Kwon, S.-C.; Kim, H.-J.; Kim, E.-J.; Hong, J.-M.; Kim, J.-Y.; Bae, S.I.; An, S.; et al. Linoleic acid attenuates the toxic dose of bupivacaine-mediated reduction of vasodilation evoked by the activation of adenosine triphosphate-sensitive potassium channels. Int. J. Mol. Sci. 2018, 19, 1876. [Google Scholar] [CrossRef]



| Time (h) | Strains | Fatty Acid Profile (%) | TFA (%) | ||||
|---|---|---|---|---|---|---|---|
| C16:0 | C16:1 | C18:0 | C18:1 | C18:2 | |||
| 72 | Control | 18.1 ± 0.1 a | 4.0 ± 0.2 a | 11.1 ± 0.4 b | 58.1 ± 0.1 a | 8.7 ± 0.4 d | 6.2 ± 0.2 a |
| 1312-12 | 18.6 ± 0.3 a | 3.7 ± 0.1 a | 12.9 ± 0.3 b | 50.2 ± 0.2 b | 14.6 ± 0.1 c | 6.1 ± 0.1 a | |
| 1292-12 | 19.4 ± 0. 5 a | 1.5 ± 0.2 c | 2.7 ± 0.2 c | 52.1 ± 0.1 b | 24.3 ± 0.5 b | 6.0 ± 0.5 a | |
| 120 | Control | 17.4 ± 0.1 b | 3.3 ± 0.2 a | 15.7 ± 0.3 a | 56.7 ± 0.4 a | 7.0 ± 0.3 d | 5.8 ± 0.4 a |
| 1312-12 | 19.5 ± 0.5 a | 2.7 ± 0.3 b | 10.1 ± 0.4 b | 51.0 ± 0.3 b | 18.7 ± 0.4 c | 6.3 ± 0.3 a | |
| 1292-12 | 21.8 ± 0.2 a | 1.3 ± 0.2 c | 3.4 ± 0.4 c | 42.7 ± 0.4 c | 30.8 ± 0.5 a | 6.0 ± 0.4 a | |
| Medium | Time | Strains | Fatty Acid Profile (%) | TFA (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C16:0 | C16:1 | C18:0 | C18:1 | C18:2 | C18:3 | C20:5 | C22:1 | C22:6 | ||||
| Added fish oil | 72 h | Control | 16.6 ± 0.3 b | 5.3 ± 0.1 a | 9.3 ± 0.2 a | 45.0 ± 0.3 b | 16.7 ± 0.1 b | - | 1.4 ± 0.1 b | - | 6.9 ± 0.1 a | 6.4 ± 0.2 a |
| 1292-12 | 23.5 ± 0.3 a | 1.3 ± 0.1 d | 2.1 ± 0.1 b | 42.4 ± 0.3 b | 23.9 ± 0.1 a | - | 1.6 ± 0.1 b | - | 3.5 ± 0.1 b | 6.7 ± 0.6 a | ||
| 120 h | Control | 20.4 ± 0.2 a | 6.5 ± 0.1 a | 10.3 ± 0.1 a | 40.6 ± 0.3 b | 12.3 ± 0.1 b | - | 2.6 ± 0.1 a | - | 7.5 ± 0.1 a | 6.0 ± 0.1 a | |
| 1292-12 | 20.7 ± 0.1 a | 3.7 ± 0.1 b | 2.1 ± 0.1 b | 45.9 ± 2.3 b | 20.4 ± 0.2 a | - | 2.1 ± 0.1 a | - | 5.2 ± 0.1 b | 6.1 ± 0.1 a | ||
| Added colza oil | 72 h | Control | 14.8 ± 0.1 c | 2.0 ± 0.1 c | 9.5 ± 0.1 a | 54.5 ± 0.4 a | 14.0 ± 0.1 b | 2.5 ± 0.1 a | - | 2.8 ± 0.1 b | - | 6.8 ± 0.3 a |
| 1292-12 | 16.7 ± 0.1 b | 2.4 ± 0.1 c | 1.9 ± 0.1 b | 52.1 ± 0.5 a | 22.0 ± 0.3 a | 1.0 ± 0.1 b | - | 4.1 ± 0.1 a | - | 6.0 ± 0.1 a | ||
| 120 h | Control | 12.6 ± 0.2 c | 2.1 ± 0.1 c | 8.4 ± 0.1 a | 56.7 ± 0.4 a | 15.3 ± 0.2 b | 2.3 ± 0.1 a | - | 2.7 ± 0.1 b | - | 6.3 ± 0.1 a | |
| 1292-12 | 16.3 ± 0.3 b | 1.8 ± 0.1 c | 2.1 ± 0.1 b | 51.6 ± 0.6 a | 23.5 ± 0.3 a | 2.4 ± 0.1 a | - | 2.3 ± 0.1 b | - | 6.8 ± 0.1 a | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.; Wang, Z.; Li, H.; Dang, W.; Song, Y.; Kang, X.; Zhang, H. Morphological Changes and Strong Cytotoxicity in Yarrowia lipolytica by Overexpressing Delta-12-Desaturase. J. Fungi 2024, 10, 126. https://doi.org/10.3390/jof10020126
Chang Y, Wang Z, Li H, Dang W, Song Y, Kang X, Zhang H. Morphological Changes and Strong Cytotoxicity in Yarrowia lipolytica by Overexpressing Delta-12-Desaturase. Journal of Fungi. 2024; 10(2):126. https://doi.org/10.3390/jof10020126
Chicago/Turabian StyleChang, Yufei, Zhen Wang, Hequn Li, Wenrui Dang, Yuanda Song, Xinxin Kang, and Huaiyuan Zhang. 2024. "Morphological Changes and Strong Cytotoxicity in Yarrowia lipolytica by Overexpressing Delta-12-Desaturase" Journal of Fungi 10, no. 2: 126. https://doi.org/10.3390/jof10020126
APA StyleChang, Y., Wang, Z., Li, H., Dang, W., Song, Y., Kang, X., & Zhang, H. (2024). Morphological Changes and Strong Cytotoxicity in Yarrowia lipolytica by Overexpressing Delta-12-Desaturase. Journal of Fungi, 10(2), 126. https://doi.org/10.3390/jof10020126






