Diversity of Colletotrichum Species Causing Anthracnose in Chayote in Brazil, with a Description of Two New Species in the C. magnum Complex
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Fungal Isolation
2.2. Molecular Characterization
2.3. Phylogenetic Reconstruction and Species Recognition
2.4. Phenotypical Characterization
2.5. Pathogenicity and Aggressiveness Testing in Fruits
3. Results
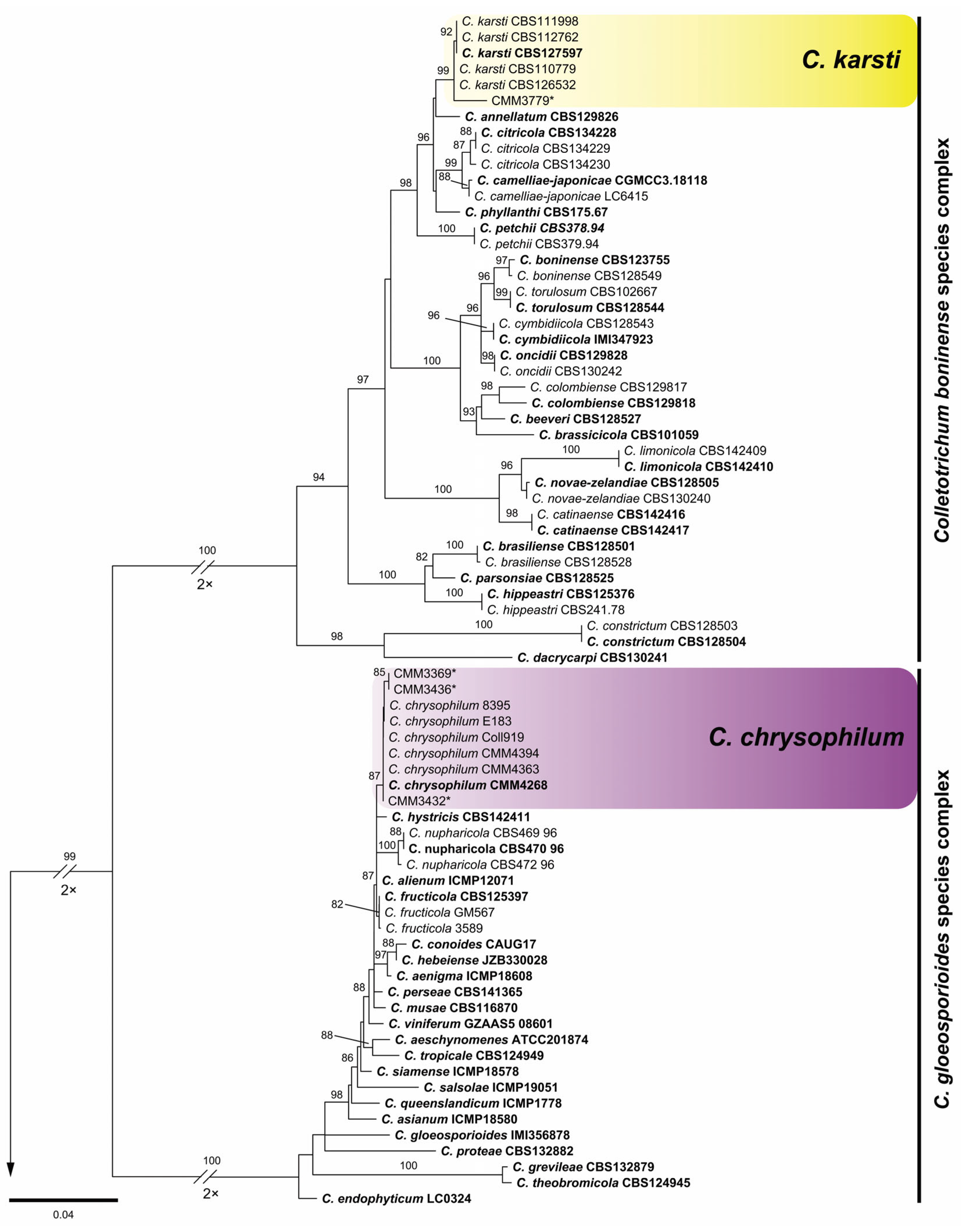
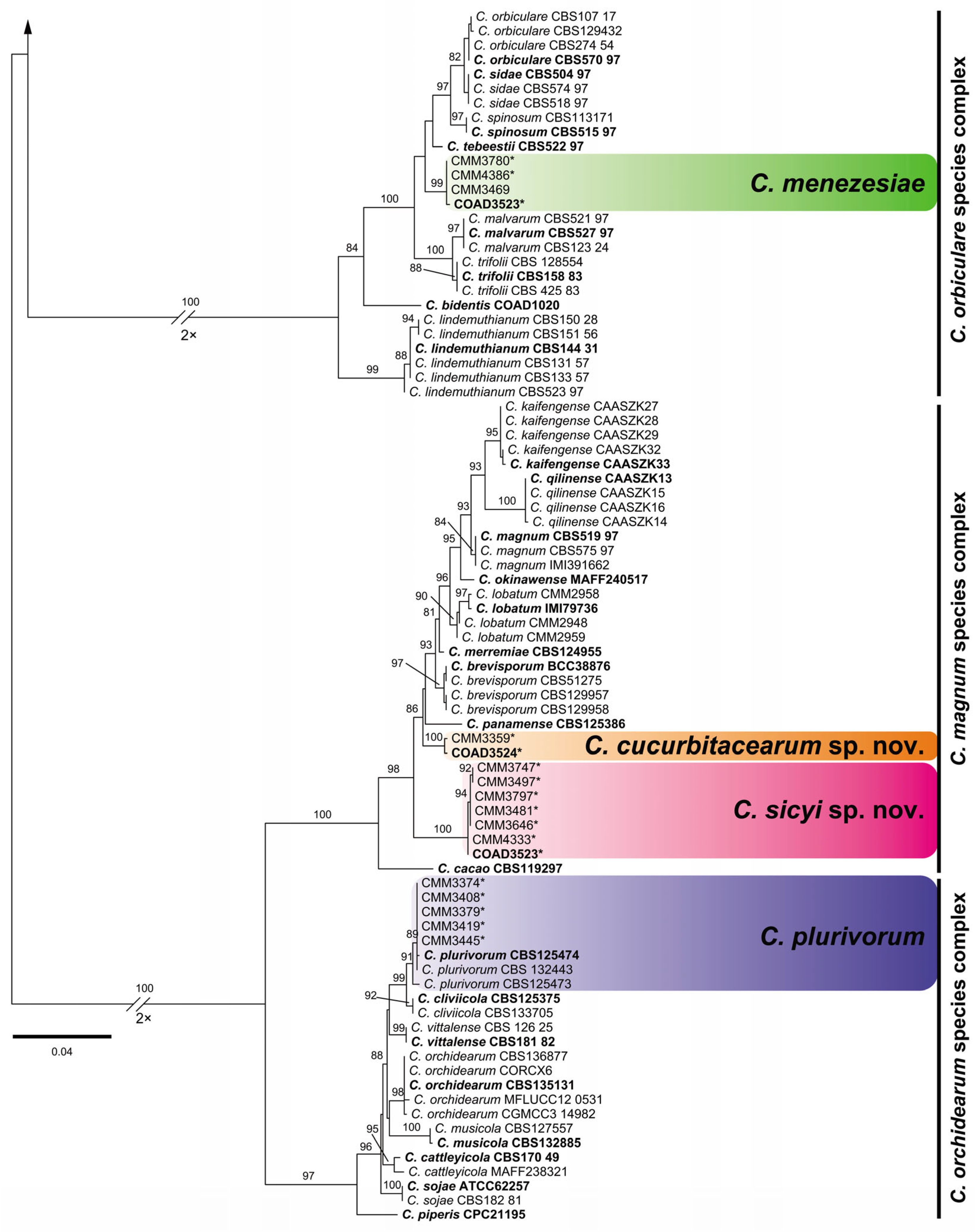
3.1. Phylogenetic Reconstruction and Species Recognition
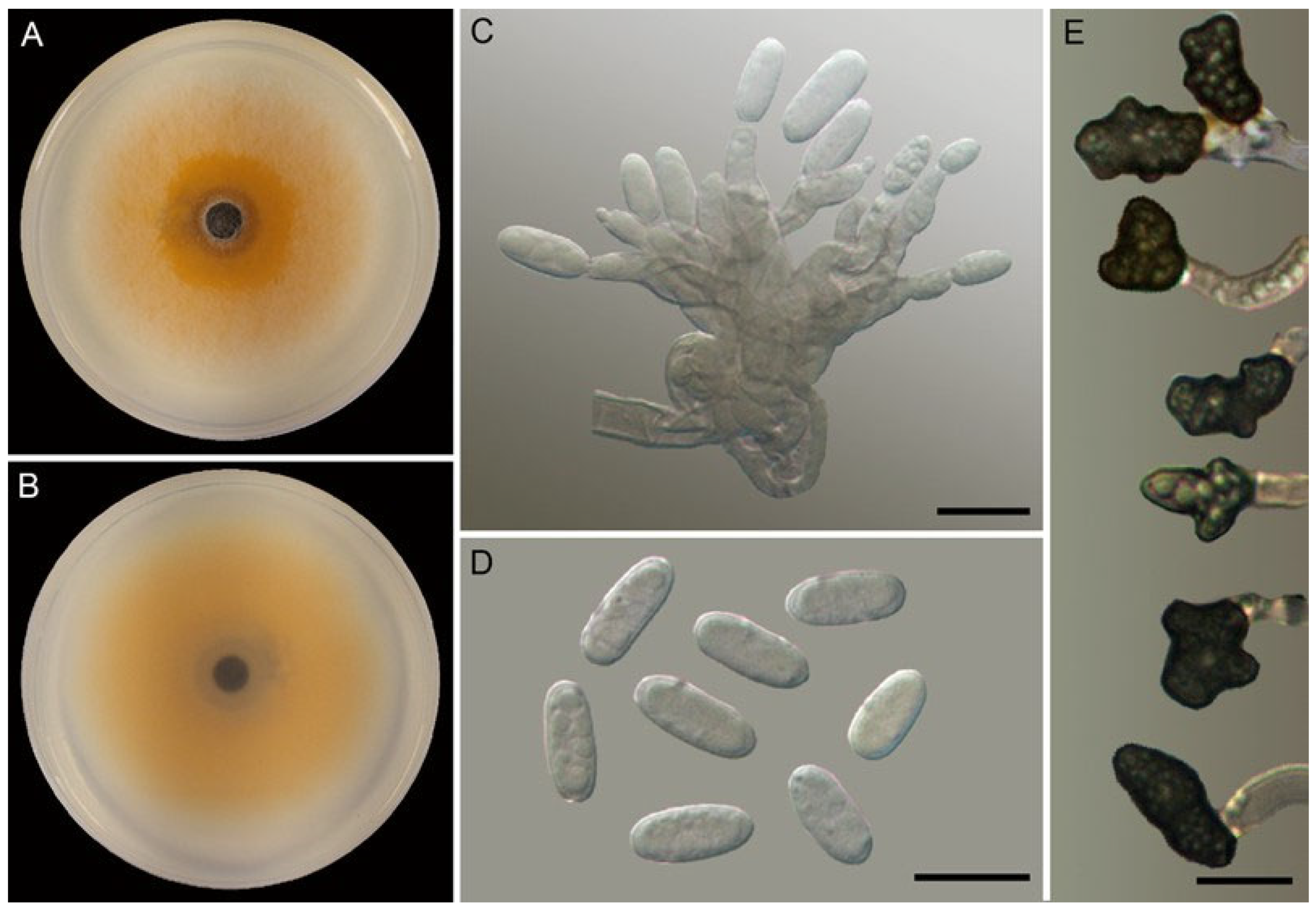
3.2. Taxonomy

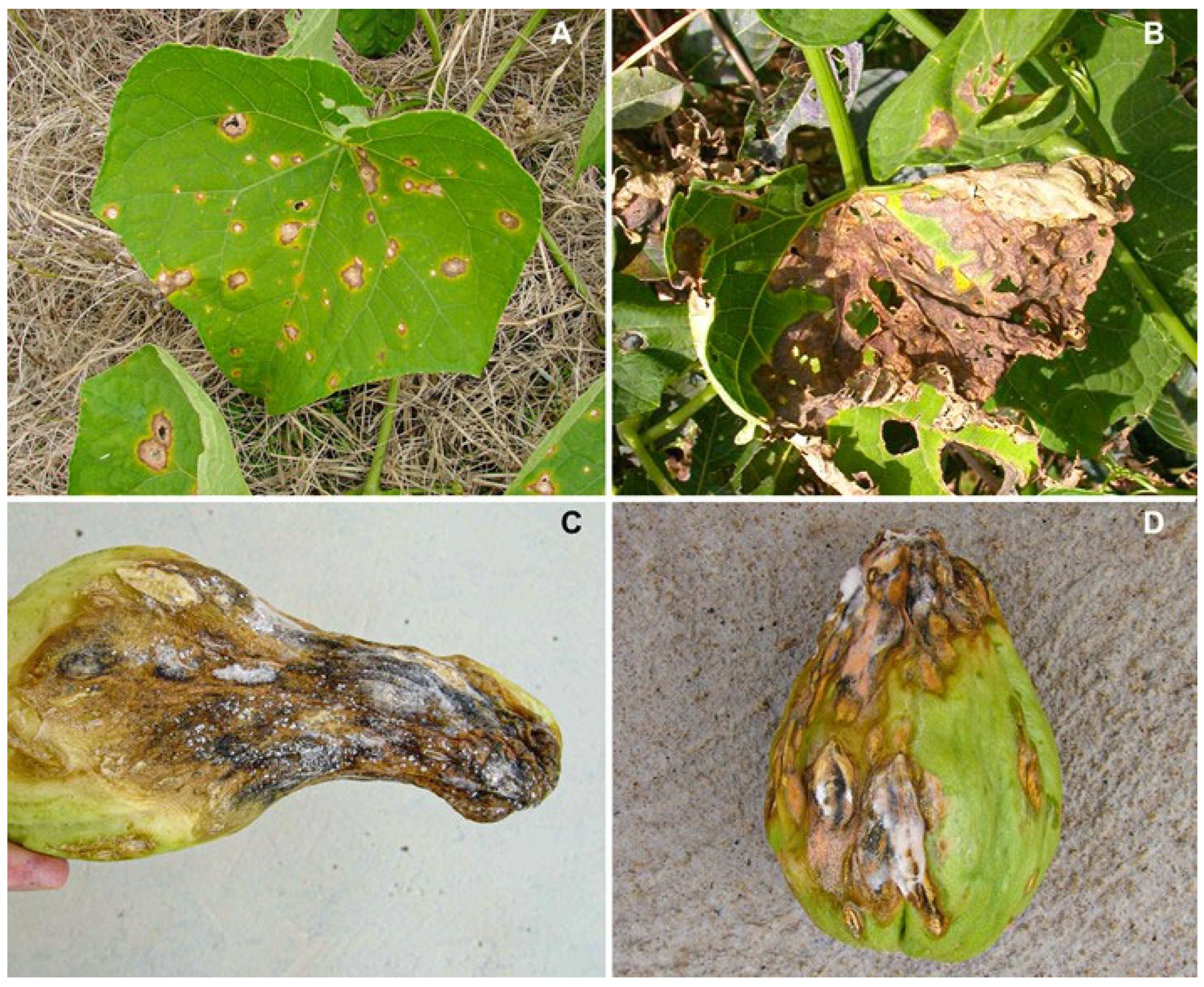
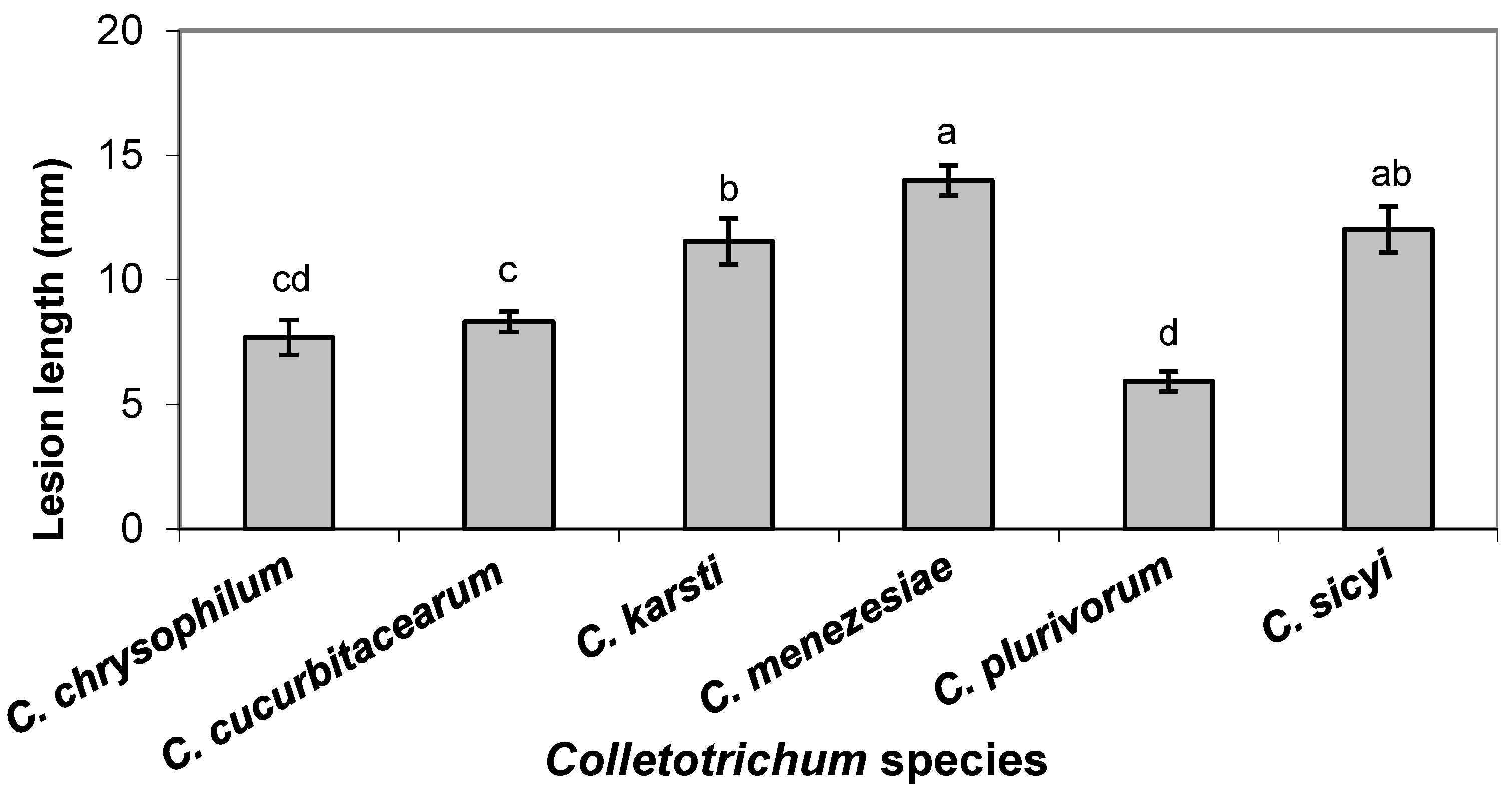
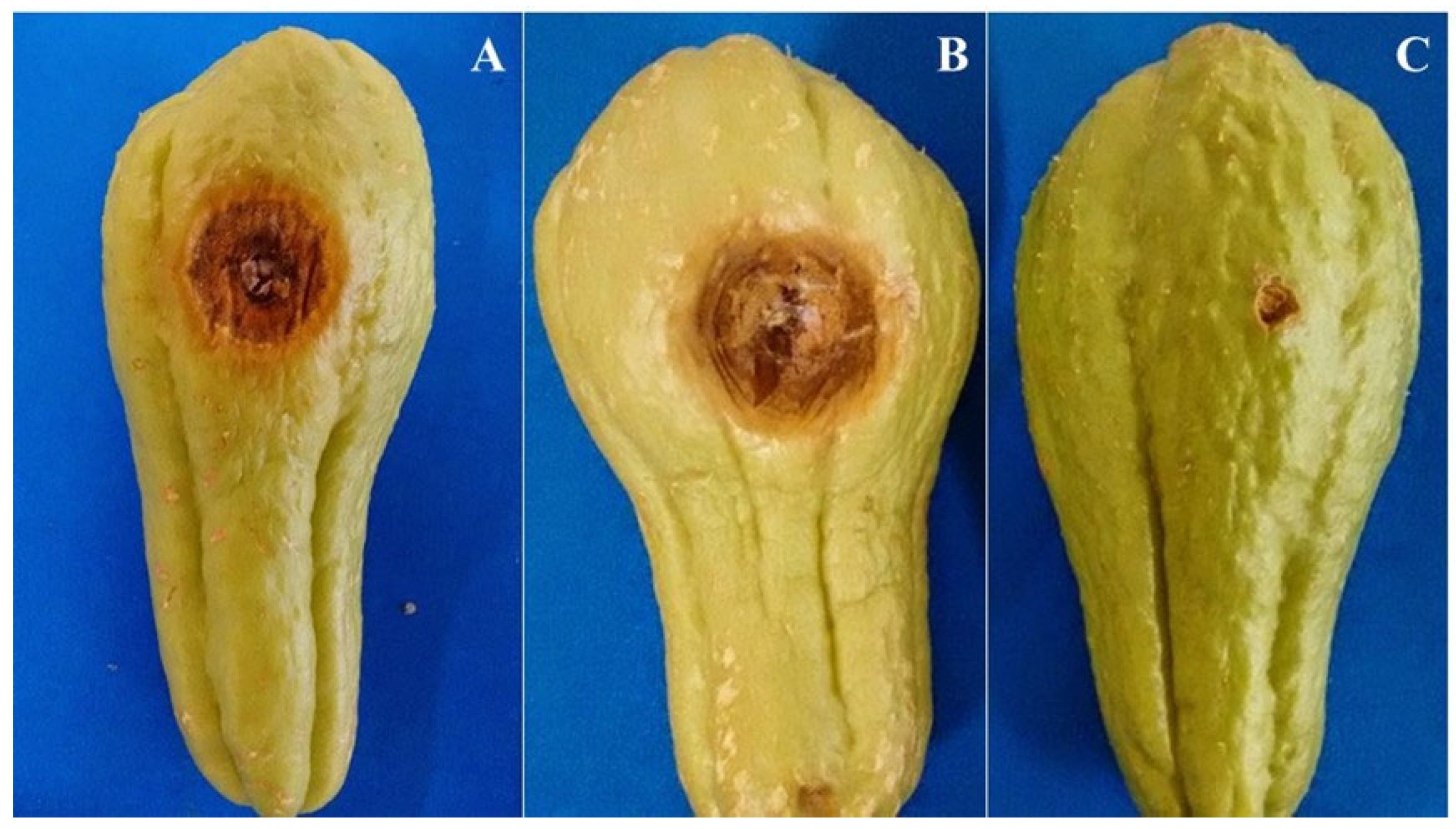
3.3. Pathogenicity and Aggressiveness in Fruit
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lopes, J.F.; Oliveira, C.A.S.; França, F.H.; Charchar, J.M.; Marishima, N.; Fontes, R.R. A Cultura Do Chuchu; Embrapa Hortaliças: Brasília, Brazil, 1994; p. 54. [Google Scholar]
- Montano, H.G.; Davis, R.E.; Dally, E.L.; Pimentel, J.P.; Brioso, P.S.T. Identification and phylogenetic analysis of a new phytoplasma from diseased chayote in Brazil. Plant Dis. 2000, 84, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Díaz-de-Cerio, E.; Verardo, V.; Fernández-Gutiérrez, A.; Gómez-Caravaca, A.M. New insight into phenolic composition of chayote (Sechium edule (Jacq.) Sw.). Food Chem. 2019, 295, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Domingues, R.J.; Töfoli, J.G.; Ferrari, J.T.; Azevedo Filho, J.A. Principais doenças fúngicas do chuchuzeiro (Sechium edule) no estado de São Paulo. Biológico 2011, 73, 5–9. [Google Scholar]
- Santi, A.; Scaramuzza, W.L.M.P.; Soares, D.M.J.; Scaramuzza, J.F.; Dallacort, R.; Krause, W.; Tieppo, R.C. Desempenho e orientação do crescimento do pepino japonês em ambiente protegido. Hortic. Bras. 2013, 31, 649–653. [Google Scholar] [CrossRef]
- Reis, A.; Henz, G.P.; Brune, S. Principais Doenças Do Chuchuzeiro no Brasil; Embrapa Hortaliças: Brasília, Brazil, 2008; p. 5. [Google Scholar]
- Sitterly, W.R.; Keinath, A.P. Anthracnose. In Compendium of Cucurbit Diseases; Zitter, T.A., Hopkins, D.L., Thomas, C.E., Eds.; APS Press: St. Paul, MN, USA, 1996; pp. 24–25. [Google Scholar]
- Damm, U.; Cannon, P.F.; Liu, F.; Barreto, R.W.; Guatimosim, E.; Crous, P.W. The Colletotrichum orbiculare species complex: Important pathogens of field crops and weeds. Fungal Divers. 2013, 61, 29–59. [Google Scholar] [CrossRef]
- Talhinhas, P.; Baroncelli, R. Colletotrichum species and complexes: Geographic distribution, host range and conservation status. Fungal Divers. 2021, 110, 109–198. [Google Scholar] [CrossRef]
- Cannon, P.F.; Damm, U.; Johnston, P.R.; Weir, B.S. Colletotrichum—Current status and future directions. Stud. Mycol. 2012, 73, 181–213. [Google Scholar] [CrossRef]
- Damm, U.; Cannon, P.F.; Woudenberg, J.H.C.; Johnston, P.R.; Weir, B. The Colletotrichum boninense species complex. Stud. Mycol. 2012, 73, 1–36. [Google Scholar] [CrossRef]
- Damm, U.; Cannon, P.F.; Woudenberg, J.H.C.; Crous, P.W. The Colletotrichum acutatum species complex. Stud. Mycol. 2012, 73, 37–113. [Google Scholar] [CrossRef]
- Damm, U.; Sato, T.; Alizadeh, A.; Groenewald, J.Z.; Crous, P.W. The Colletotrichum dracaenophilum, C. magnum and C. orchidearum species complexes. Stud. Mycol. 2019, 92, 1–46. [Google Scholar] [CrossRef]
- Hyde, K.D.; Nilsson, R.H.; Alias, S.A.; Ariyawansa, H.A.; Blair, J.E.; Cai, L.; de Cock, A.W.A.M.; Dissanayake, A.J.; Glockling, S.L.; Goonasekara, I.D.; et al. One stop shop: Backbones trees for important pytopathogenic genera: I (2014). Fungal Divers. 2014, 67, 21–125. [Google Scholar] [CrossRef]
- Liu, F.L.; Cai, L.; Crous, P.W.; Damm, U. The Colletotrichum gigasporum species complex. Persoonia 2014, 33, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Weir, B.S.; Johnston, P.R.; Damm, U. The Colletotrichum gloeosporioides species complex. Stud. Mycol. 2012, 73, 115–180. [Google Scholar] [CrossRef] [PubMed]
- Sussel, A.A.B. Caracterização de Isolados de Colletrotrichum lagenarium, Agente Causal da Antracnose das Cucurbitáceas. Master’s Thesis, University of São Paulo, Piracicaba, Brazil, 2005. [Google Scholar]
- Bezerra, J.P.; Ferreira, P.V.; Barbosa, L.F.; Ramos-Sobrinho, R.; Pinho, D.B.; Reis, A.; Assunção, I.P.; Lima, G.S.A. First report anthracnose chayote fruits (Sechium edule) caused by Colletotrichum brevisporum. Plant Dis. 2016, 100, 217. [Google Scholar] [CrossRef]
- Chomnunti, P.; Schoch, C.L.; Aguirre-Hudson, B.; Ko-Ko, T.W.; Hongsanan, S.; Jones, E.B.G.; Kodsueb, R.; Phookamsak, R.; Chukeatirote, E.; Bahkali, A.H.; et al. Capnodiaceae. Fungal Divers. 2011, 51, 103–134. [Google Scholar] [CrossRef] [PubMed]
- Sutton, B.C. The Coelomycetes: Fungi Imperfecti with Pycnidia Acervuli and Stromata; Commonwealth Mycological Institute: Kew, UK, 1980; p. 696. [Google Scholar]
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Rozas, J.; Sánchez-DelBarrio, J.C.; Messeguer, X.; Rozas, R. DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics 2003, 19, 2496–2497. [Google Scholar] [CrossRef]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Templeton, M.D.; Rikkerink, E.H.A.; Solon, S.L.; Crowhurst, R.N. Cloning and molecular characterization of the glyceraldehyde-3-phosphate dehydrogenase encoding gene and cDNA from the plant pathogenic fungus Glomerella cingulata. Gene 1992, 122, 225–230. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Application; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, Y.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- O’Donnell, K.; Cigelnik, E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol. Phylogenet. Evol. 1997, 7, 103–116. [Google Scholar] [CrossRef]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Kuraku, S.; Zmasek, C.M.; Nishimura, O.; Katoh, K. aLeaves facilitates on-demand exploration of metazoan gene family trees on MAFFT sequence alignment server with enhanced interactivity. Nucleic Acids Res. 2013, 41, W22–W28. [Google Scholar] [CrossRef]
- Vaidya, G.; Lohman, D.J.; Meier, R. SequenceMatrix: Concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistic 2011, 27, 171–180. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Shimodaira, H. An approximately unbiased test of phylogenetic tree selection. Syst. Biol. 2002, 51, 492–508. [Google Scholar] [CrossRef]
- Taylor, J.W.; Jacobson, D.J.; Kroken, S.; Kasuga, T.; Geiser, D.M.; Hibbett, D.S.; Fisher, M.C. Phylogenetic species recognition and species concepts in fungi. Fungal Genet. Biol. 2000, 31, 21–32. [Google Scholar] [CrossRef]
- Dettman, J.R.; Jacobson, D.J.; Taylor, J.W. A multilocus genealogical approach to phylogenetic species recognition in the model eukaryote Neurospora. Evolution 2003, 57, 2703–2720. [Google Scholar] [PubMed]
- Johnston, P.R.; Jones, D. Relationship among Colletotrichum isolates from fruit rots assessed using rDNA sequences. Mycologia 1997, 89, 420–430. [Google Scholar] [CrossRef]
- Vieira, W.A.S.; Costa, C.A.; Câmara, M.P.S.; Michereff, S.J.; Reis, A. Colletotrichum menezesiae sp. nov.: A novel species causing anthracnose on chayote in Brazil. Phytotaxa 2023, 597, 80–86. [Google Scholar] [CrossRef]
- Jenkins, S.F.; Winstead, N.N. Glomerella magna, cause of a new anthracnose of cucurbits. Phytopathology 1964, 54, 452–454. [Google Scholar]
- Liu, F.; Cai, L.; Crous, P.W.; Damm, U. Circumscription of the anthracnose pathogens Colletotrichum lindemuthianum and C. nigrum. Mycologia 2013, 105, 844–860. [Google Scholar] [CrossRef] [PubMed]
- Tsay, J.G.; Chen, R.S.; Wang, W.L.; Weng, B.C. First report of anthracnose on cucurbitaceous crops caused by Glomerella magna in Taiwan. Plant Dis. 2010, 94, 787. [Google Scholar] [CrossRef]
- Yang, Y.L.; Cai, L.; Yu, Z.N.; Liu, Z.Y.; Hyde, K.D. Colletotrichum species on Orchidaceae in southwest China. Cryptog. Mycol. 2011, 32, 229–253. [Google Scholar]
- Cao, X.; Xu, X.; Che, H.; West, J.S.; Luo, D. Eight Colletotrichum species, including a novel species, are associated with areca palm anthracnose in Hainan, China. Plant Dis. 2020, 104, 1369–1377. [Google Scholar] [CrossRef]
- Li, Y.L.; Yan, Z.B.; Wang, Y.H.; Lin, Q.K.; Wang, S.B.; Zhou, Z. First report of Colletotrichum karstii causing anthracnose on lotus bamboo (Dracaena sanderiana) in China. Plant Dis. 2018, 102, 2641. [Google Scholar] [CrossRef]
- Zhao, Q.; Xia, C.J.; Wang, J.; Lv, H.K.; Wang, J.; Yang, J.G.; Chen, X.; Qian, Y.M.; Li, M.; Sohail, M.A. First report of Colletotrichum karstii causing anthracnose on cigar tobacco in Hainan, China. Plant Dis. 2020, 104, 2025. [Google Scholar] [CrossRef]
- Belisário, R.; Borges, L.S.; Furtado, G.Q. First report of Colletotrichum karstii causing anthracnose on Annona muricata in Brazil. Plant Dis. 2018, 102, 2372. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.F.O.; Kamei, S.H.; Rossane, J.A.S.; Miranda, A.R.G.S.; Netto, M.B.; Silva, S.J.C.; Correia, K.C.; Lima, G.S.A.; Assunção, I.P. Species diversity of Colletotrichum infecting Annona spp. in Brazil. Eur. J. Plant Pathol. 2018, 153, 1119–1130. [Google Scholar] [CrossRef]
- Lima, N.B.; Batista, M.V.A.; Morais, M.A., Jr.; Barbosa, M.A.G.; Michereff, S.J.; Hyde, K.D.; Câmara, M.P.S. Five Colletotrichum species are responsible for mango anthracnose in northeastern Brazil. Fungal Divers. 2013, 61, 75–88. [Google Scholar] [CrossRef]
- Vieira, W.A.S.; Michereff, S.J.; Morais, M.A., Jr.; Hyde, K.D.; Câmara, M.P.S. Endophytic species of Colletotrichum associated with mango in northeastern Brazil. Fungal Divers. 2014, 67, 181–202. [Google Scholar] [CrossRef]
- Vieira, W.A.S.; Lima, W.G.; Nascimento, E.S.; Michereff, S.J.; Câmara, M.P.S.; Doyle, V.P. The impact of phenotypic and molecular data on the inference of Colleotrichum diversity associated with Musa. Mycologia 2018, 109, 912–934. [Google Scholar] [CrossRef]
- Veloso, J.S.; Câmara, M.P.S.; Lima, W.G.; Michereff, S.J.; Doyle, V.P. Why species delimitation matters for fungal ecology: Colletotrichum diversity on wild and cultivated cashew in Brazil. Fungal Biol. 2018, 122, 677–691. [Google Scholar] [CrossRef]
- Cavalcante, G.R.S.; Vieira, W.A.S.; Michereff, S.J.; Barguil, B.M.; Doyle, V.P.; Câmara, M.P.S. First report of anthracnose caused by Colletotrichum sichuanensis on Phaseolus lunatus in Brazil. Plant Dis. 2018, 102, 680. [Google Scholar] [CrossRef]
- Cavalcante, G.R.S.; Barguil, B.M.; Vieira, W.A.S.; Lima, W.G.; Michereff, S.J.; Doyle, V.P.; Câmara, M.P.S. Diversity, prevalence, and virulence of Colletotrichum species associated with lima bean in Brazil. Plant Dis. 2019, 103, 1961–1966. [Google Scholar] [CrossRef]
- Liu, F.; Tang, G.; Zheng, X.; Li, Y.; Sun, X.; Qi, X.; Zhou, Y.; Xu, J.; Chen, H.; Chang, X.; et al. Molecular and phenotypic characterization of Colletotrichum species associated with anthracnose disease in peppers from Sichuan Province, China. Sci. Rep. 2016, 6, e32761. [Google Scholar] [CrossRef]
- Sun, Y.C.; Damm, U.; Huang, C.J. Colletotrichum plurivorum, the causal agent of anthracnose fruit rot of papaya in Taiwan. Plant Dis. 2019, 103, 1040. [Google Scholar] [CrossRef]
- USDA. USDA Fungal Databases. Available online: https://fungi.ars.usda.gov/ (accessed on 21 May 2024).
| Genes | Primer | Sequence (5’–3’) | Reference |
|---|---|---|---|
| ACT | ACT-512F | ATG TGC AAG GCC GGT TTC GC | Carbone and Kohn (1999) [23] |
| ACT-783R | TAC GAG TCC TTC TGG CCC AT | Carbone and Kohn (1999) [23] | |
| GAPDH | GDF | GCC GTC AAC GAC CCC TTC ATT GA | Templeton et al. (1992) [24] |
| GDR | GGG TGG AGT CGT ACT TGA GCA TGT | Templeton et al. (1992) [24] | |
| ITS | ITS1 | CTT GGT CAT TTA GAG GAA GTA A | White et al. (1990) [25] |
| ITS4 | TCC TCC GCT TAT TGA TAT GC | White et al. (1990) [25] | |
| TUB2 | T1 | AACATGCGTGAGATTGTAAGT | O’Donnell and Cigelnik (1997) [26] |
| Bt2a | GGT AAC CAA ATC GGT GCT GCT TTC | Glass and Donaldson (1995) [27] | |
| Bt2b | ACC CTC AGT GTA GTG ACC CTT GGC | Glass and Donaldson (1995) [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, W.A.d.S.; Costa, C.A.d.; Veloso, J.S.; Lima, W.G.; Correia, K.C.; Michereff, S.J.; Pinho, D.B.; Câmara, M.P.S.; Reis, A. Diversity of Colletotrichum Species Causing Anthracnose in Chayote in Brazil, with a Description of Two New Species in the C. magnum Complex. J. Fungi 2024, 10, 847. https://doi.org/10.3390/jof10120847
Vieira WAdS, Costa CAd, Veloso JS, Lima WG, Correia KC, Michereff SJ, Pinho DB, Câmara MPS, Reis A. Diversity of Colletotrichum Species Causing Anthracnose in Chayote in Brazil, with a Description of Two New Species in the C. magnum Complex. Journal of Fungi. 2024; 10(12):847. https://doi.org/10.3390/jof10120847
Chicago/Turabian StyleVieira, Willie Anderson dos Santos, Christiane Almeida da Costa, Josiene Silva Veloso, Waléria Guerreiro Lima, Kamila Câmara Correia, Sami Jorge Michereff, Danilo Batista Pinho, Marcos Paz Saraiva Câmara, and Ailton Reis. 2024. "Diversity of Colletotrichum Species Causing Anthracnose in Chayote in Brazil, with a Description of Two New Species in the C. magnum Complex" Journal of Fungi 10, no. 12: 847. https://doi.org/10.3390/jof10120847
APA StyleVieira, W. A. d. S., Costa, C. A. d., Veloso, J. S., Lima, W. G., Correia, K. C., Michereff, S. J., Pinho, D. B., Câmara, M. P. S., & Reis, A. (2024). Diversity of Colletotrichum Species Causing Anthracnose in Chayote in Brazil, with a Description of Two New Species in the C. magnum Complex. Journal of Fungi, 10(12), 847. https://doi.org/10.3390/jof10120847







