Control of Postharvest Green Mold in Citrus by the Antimicrobial Peptide BP15 and Its Lipopeptides
Abstract
1. Introduction
2. Materials and Methods
2.1. Pathogens
2.2. Synthesis of Peptides
2.3. MIC and MFC
2.4. Mycelial Survival Assay
2.5. Scanning Electronic Microscopy (SEM)
2.6. Fluorescence Microscopy
2.7. Measurement of Extracellular Conductivity
2.8. Release of Intracellular Nucleic Acid and Protein
2.9. DNA Binding Assay
2.10. Fruit Decay Test
2.11. Statistical Analysis
3. Results
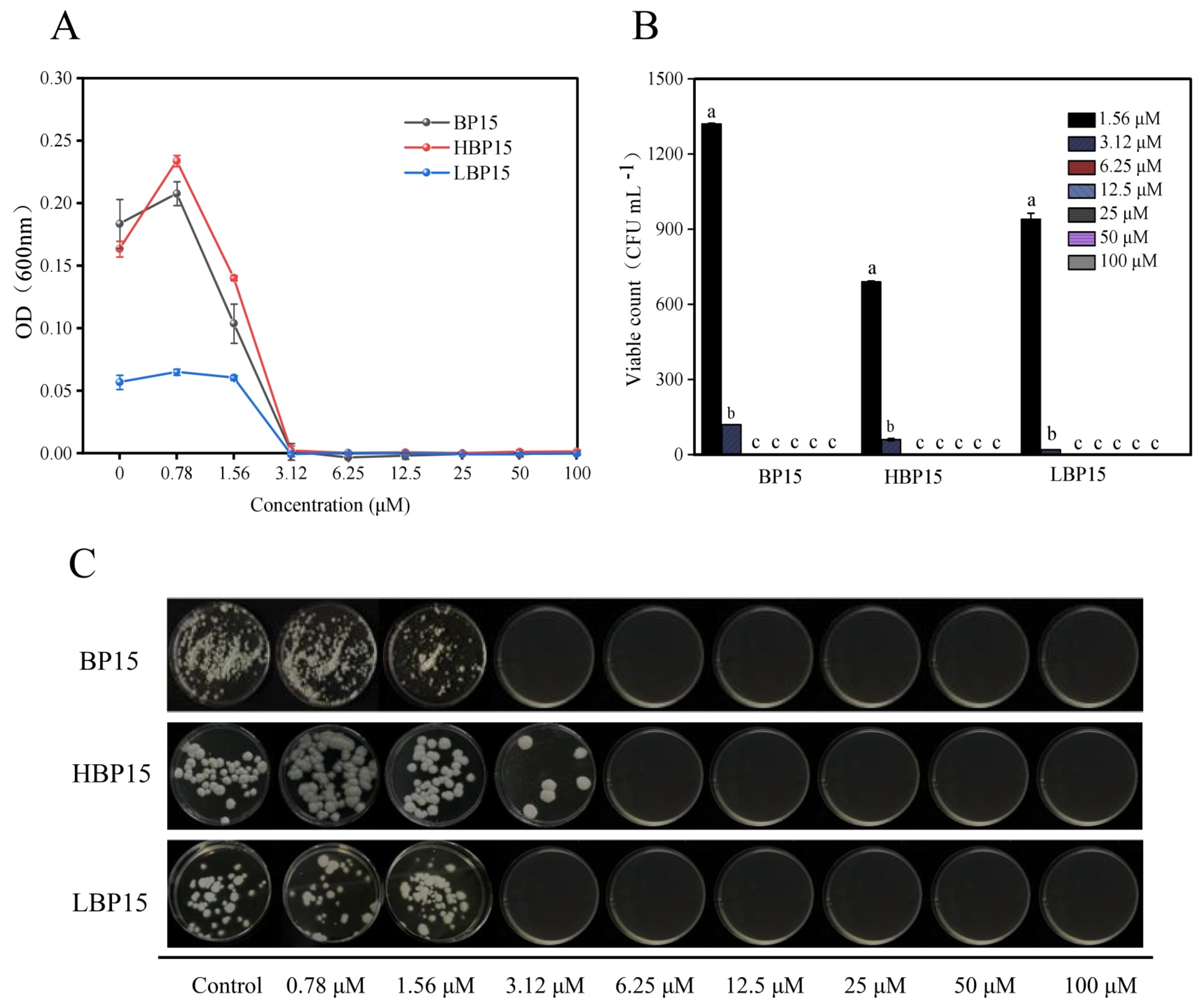
3.1. MIC and MFC
3.2. Mycelial Survival Rate
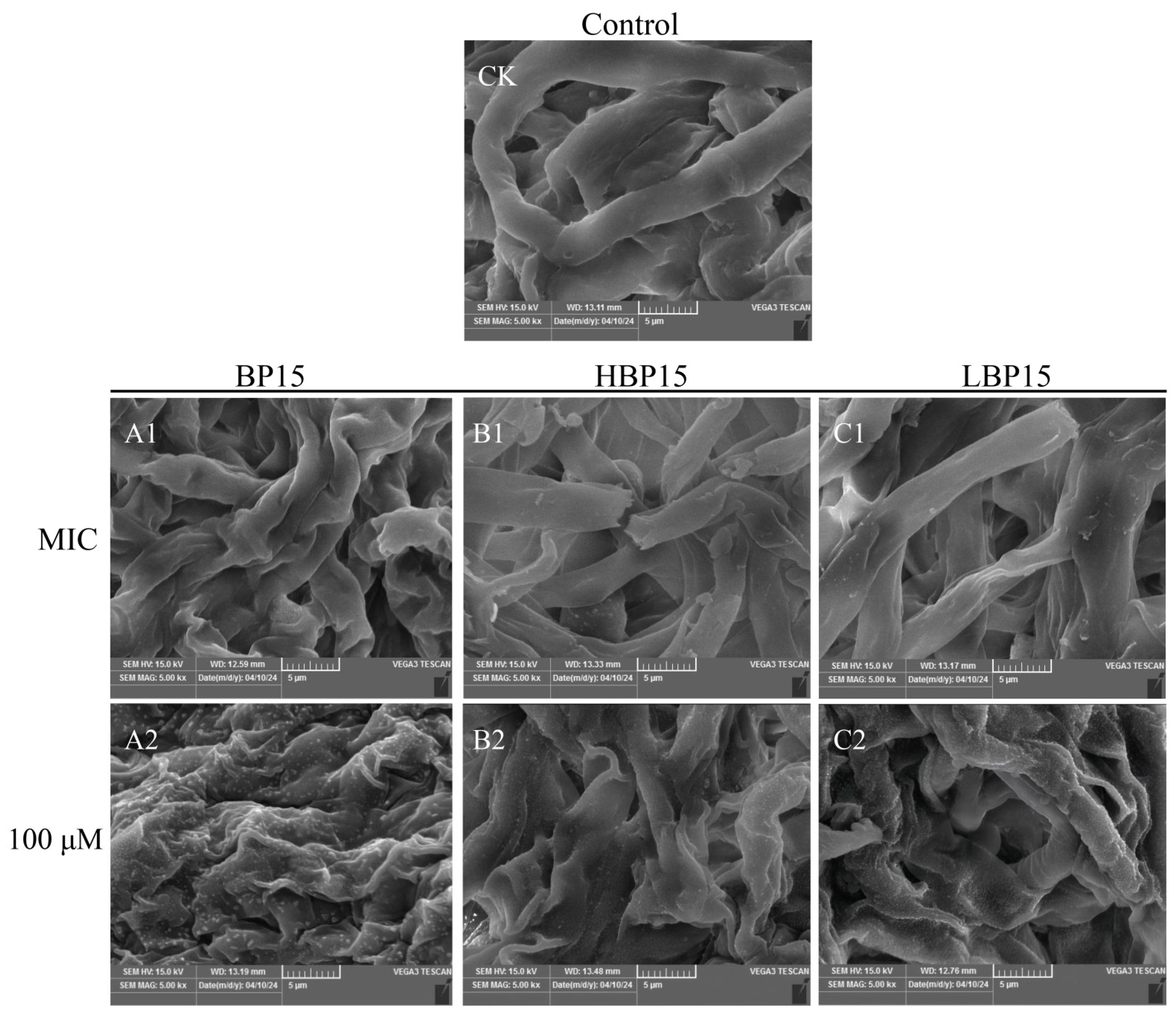
3.3. Scanning Electron Microscopy (SEM)
3.4. Fluorescence Microscopy (FM)
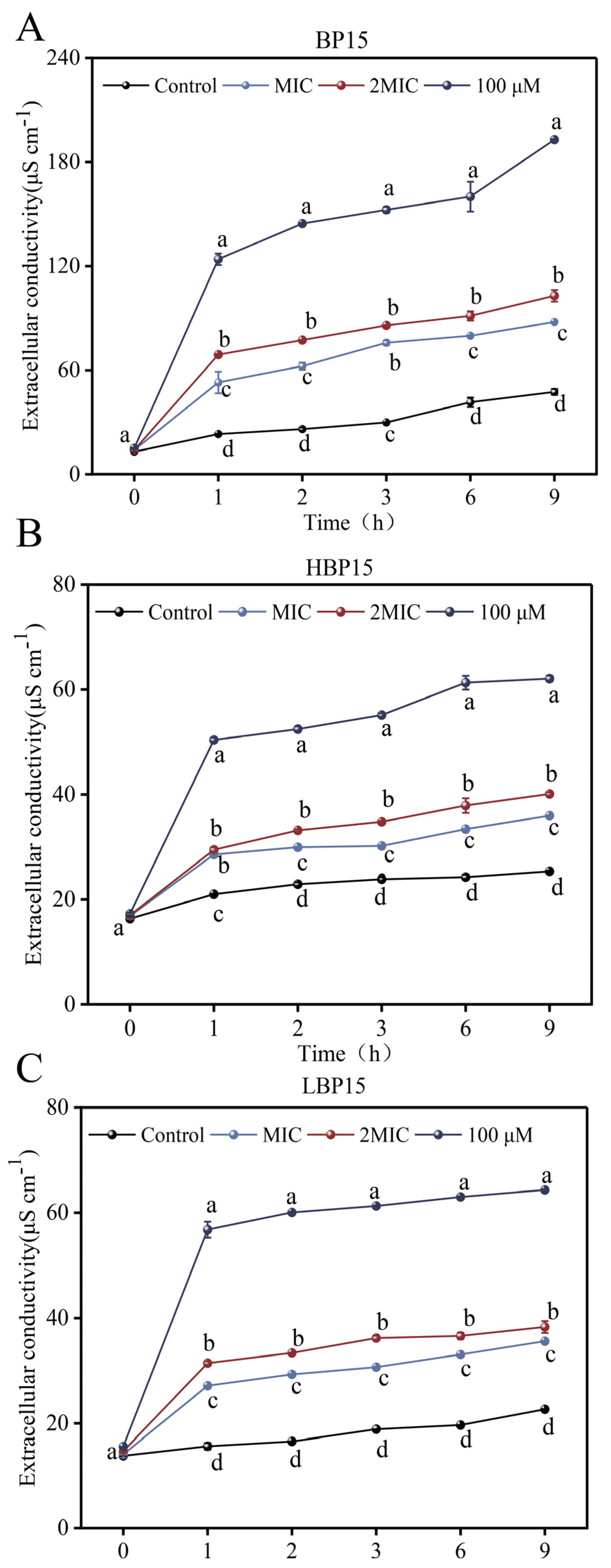
3.5. Extracellular Conductivity
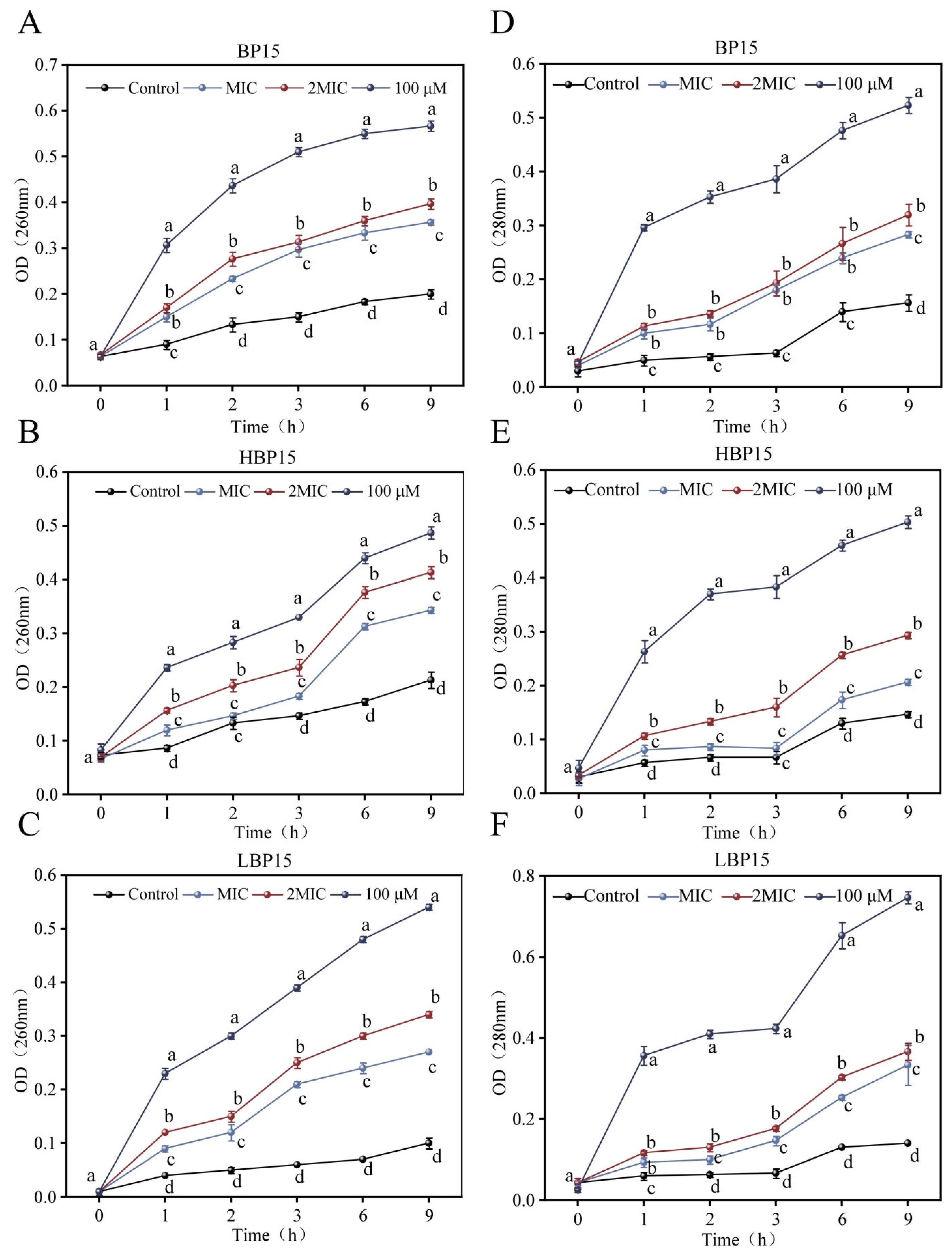
3.6. Intracellular Nucleic Acid and Protein
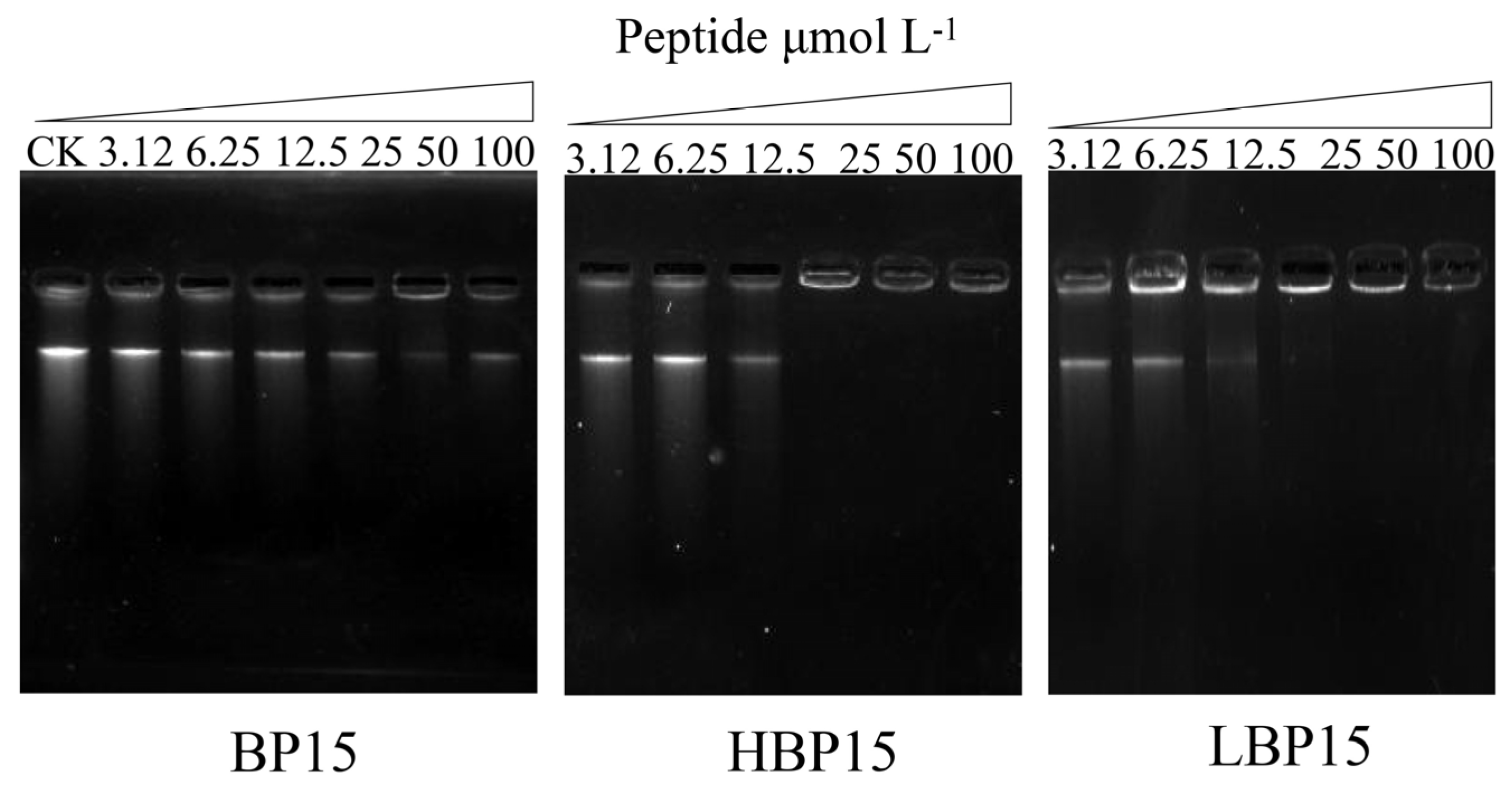
3.7. DNA Binding
3.8. Fruit Decay Test
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rishi, R.; Deepika, K.; Dinesh, V.; Ananya, M.; Bhumika, K.; Anjineyulu, K.; Shruti, R.; Ranjna, S.; Rohitashw, K.; Bindu, N. Citrus fruit: Classification, value addition, nutritional and medicinal values, and relation with pandemic and hidden hunger. J. Agric. Food Res. 2023, 14, 100718. [Google Scholar] [CrossRef]
- Wang, Z.; Sui, Y.; Li, J.; Tian, X.; Wang, Q. Biological control of postharvest fungal decays in citrus: A review. Crit. Rev. Food Sci. Nutr. 2020, 62, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Qian, X.; Routledge, M.N.; Wu, X.; Shi, Y.; Zhu, Q.; Zhang, H. Metabonomics analysis of postharvest citrus response to Penicillium digitatum infection. LWT 2021, 152, 112371. [Google Scholar] [CrossRef]
- Chen, J.; Shen, Y.; Chen, C.; Wan, C. Inhibition of Key Citrus Postharvest Fungal Strains by Plant Extracts In Vitro and In Vivo: A Review. Plants 2019, 8, 26. [Google Scholar] [CrossRef]
- Lamberth, C. Latest Research Trends in Agrochemical Fungicides: Any Learnings for Pharmaceutical Antifungals? ACS Med. Chem. Lett. 2022, 13, 895–903. [Google Scholar] [CrossRef]
- Moretta, A.; Scieuzo, C.; Petrone, A.M.; Salvia, R.; Manniello, M.D.; Franco, A.; Lucchetti, D.; Vassallo, A.; Vogel, H.; Sgambato, A.; et al. Antimicrobial Peptides: A New Hope in Biomedical and Pharmaceutical Fields. Front. Cell. Infect. Microbiol. 2021, 11, 668632. [Google Scholar] [CrossRef]
- Van Eijk, M.; Boerefijn, S.; Cen, L.; Rosa, M.; Morren, M.J.; Van Der Ent, C.K.; Kraak, B.; Dijksterhuis, J.; Valdes, I.D.; Haagsman, H.P.; et al. Cathelicidin-inspired antimicrobial peptides as novel antifungal compounds. Med. Mycol. 2020, 58, 1073–1084. [Google Scholar] [CrossRef]
- Li, X.; Zuo, S.; Wang, B.; Zhang, K.; Wang, Y. Antimicrobial Mechanisms and Clinical Application Prospects of Antimicrobial Peptides. Molecules 2022, 27, 2675. [Google Scholar] [CrossRef]
- de Souza, C.M.; da Silva, Á.P.; Júnior, N.G.O.; Martínez, O.F.; Franco, O.L. Peptides as a therapeutic strategy against Klebsiella pneumoniae. Trends Pharmacol. Sci. 2022, 43, 335–348. [Google Scholar] [CrossRef]
- Ji, S.; An, F.; Zhang, T.; Lou, M.; Guo, J.; Liu, K.; Zhu, Y.; Wu, J.; Wu, R. Antimicrobial peptides: An alternative to traditional antibiotics. Eur. J. Med. Chem. 2024, 265, 116072. [Google Scholar] [CrossRef]
- Liu, Y.; Sameen, D.E.; Ahmed, S.; Dai, J.; Qin, W. Antimicrobial peptides and their application in food packaging. Trends Food Sci. Technol. 2021, 112, 471–483. [Google Scholar] [CrossRef]
- Wang, W.; Deng, L.; Yao, S.; Zeng, K. Control of green and blue mold and sour rot in citrus fruits by the cationic antimicrobial peptide PAF56. Postharvest Biol. Technol. 2018, 136, 132–138. [Google Scholar] [CrossRef]
- Ganesan, N.; Mishra, B.; Felix, L.; Mylonakis, E. Antimicrobial Peptides and Small Molecules Targeting the Cell Membrane of Staphylococcus aureus. Microbiol. Mol. Biol. Rev. MMBR 2023, 87, e0003722. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Zeng, P.; Liu, J.; Wong, K.-Y.; Chan, E.W.-C.; Lin, Y.; Chan, K.-F.; Chen, S. Antimicrobial peptide zp37 inhibits Escherichia coli O157:H7 in alfalfa sprouts by inflicting damage in cell membrane and binding to DNA. LWT 2021, 146, 111392. [Google Scholar] [CrossRef]
- Dou, Y.; Routledge, M.N.; Gong, Y.; Godana, E.A.; Dhanasekaran, S.; Yang, Q.; Zhang, X.; Zhang, H. Efficacy of epsilon-poly-L-lysine inhibition of postharvest blue mold in apples and potential mechanisms. Postharvest Biol. Technol. 2021, 171, 111346. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Jiang, H.; Song, R.; Liu, Y.; Guo, H.; Meng, D. Antimicrobial peptide CB-M exhibits direct antifungal activity against Botrytis cinerea and induces disease resistance to gray mold in cherry tomato fruit. Postharvest Biol. Technol. 2023, 196, 112184. [Google Scholar] [CrossRef]
- Huang, Y.; Gao, L.; Lin, M.; Yu, T. Recombinant expression of antimicrobial peptides in Pichia pastoris: A strategy to inhibit the Penicillium expansum in pears. Postharvest Biol. Technol. 2021, 171, 111298. [Google Scholar] [CrossRef]
- Muñoz, A.; López-García, B.; Marcos, J.F. Comparative study of antimicrobial peptides to control citrus postharvest decay caused by Penicillium digitatum. J. Agric. Food Chem. 2007, 55, 8170–8176. [Google Scholar] [CrossRef]
- Rosés, C.; Carbajo, D.; Sanclimens, G.; Farrera-Sinfreu, J.; Blancafort, A.; Oliveras, G.; Cirac, A.D.; Bardají, E.; Puig, T.; Planas, M.; et al. Cell-penetrating Î3-peptide/antimicrobial undecapeptide conjugates with anticancer activity. Tetrahedron 2012, 68, 4406–4412. [Google Scholar] [CrossRef]
- Badosa, E.; Ferré, R.; Francés, J.; Bardají, E.; Feliu, L.; Planas, M.; Montesinos, E. Sporicidal Activity of Synthetic Antifungal Undecapeptides and Control of Penicillium Rot of Apples. Appl. Environ. Microbiol. 2009, 75, 5563–5569. [Google Scholar] [CrossRef]
- Puig, M.; Moragrega, C.; Ruz, L.; Calderón, C.E.; Cazorla, F.M.; Montesinos, E.; Llorente, I. Interaction of antifungal peptide BP15 with Stemphylium vesicarium, the causal agent of brown spot of pear. Fungal Biol. 2016, 120, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, N.; Teng, D.; Mao, R.; Hao, Y.; Ma, X.; Wang, X.; Wang, J. Fatty acid modified-antimicrobial peptide analogues with potent antimicrobial activity and topical therapeutic efficacy against Staphylococcus hyicus. Appl. Microbiol. Biotechnol. 2021, 105, 5845–5859. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, X.; Yin, K.; Xu, Q.; Ren, S.; Wang, X.; Wang, Z.; Yi, Y. Fatty acid modification of antimicrobial peptide CGA-N9 and the combats against Candida albicans infection. Biochem. Pharmacol. 2023, 211, 115535. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Wang, J.; Peng, J.; Zhao, P.; Kong, Z.; Wang, K.; Yan, W.; Wang, R. The in vitro, in vivo antifungal activity and the action mode of Jelleine-I against Candida species. Amino Acids 2017, 50, 229–239. [Google Scholar] [CrossRef]
- Van Der Weerden, N.L.; Hancock, R.E.; Anderson, M.A. Permeabilization of Fungal Hyphae by the Plant Defensin NaD1 Occurs through a Cell Wall-dependent Process. J. Biol. Chem. 2010, 285, 37513–37520. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, R.; Wang, X. Evaluation of fungal infection in peaches based on optical and microstructural properties. Postharvest Biol. Technol. 2020, 165, 111181. [Google Scholar] [CrossRef]
- Li, X.; Wang, W.; Liu, S.; Ruan, C.; Yi, L.; Deng, L.; Yao, S.; Zeng, K. Effects of the peptide H-OOWW-NH2 and its derived lipopeptide C12-OOWW-NH2 on controlling of citrus postharvest green mold. Postharvest Biol. Technol. 2019, 158, 110979. [Google Scholar] [CrossRef]
- Tao, N.; Jia, L.; Zhou, H. Antifungal activity of Citrus reticulata Blanco essential oil against Penicillium italicum and Penicillium digitatum. Food Chem. 2014, 153, 265–271. [Google Scholar] [CrossRef]
- Paul, S.; Dubey, R.C.; Maheswari, D.K.; Kang, S.C. Trachyspermum ammi (L.) fruit essential oil influencing on membrane permeability and surface characteristics in inhibiting food-borne pathogens. Food Control 2011, 22, 725–731. [Google Scholar] [CrossRef]
- Miao, J.; Zhou, J.; Liu, G.; Chen, F.; Chen, Y.; Gao, X.; Dixon, W.; Song, M.; Xiao, H.; Cao, Y. Membrane disruption and DNA binding of Staphylococcus aureus cell induced by a novel antimicrobial peptide produced by Lactobacillus paracasei subsp. tolerans FX-6. Food Control 2016, 59, 609–613. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, J.; Xie, J.; Deng, L.; Yao, S.; Zeng, K. Transcriptomic and biochemical analysis of highlighted induction of phenylpropanoid pathway metabolism of citrus in response to salicylic acid, Pichia membranaefaciens, and oligochitosan. Postharvest Biol. Technol. 2018, 142, 81–92. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, S.; Yi, L.; Zeng, K. Tryptophan enhances biocontrol efficacy of Metschnikowia citriensis FL01 against postharvest fungal diseases of citrus fruit by increasing pulcherriminic acid production. Int. J. Food Microbiol. 2023, 386, 110013. [Google Scholar] [CrossRef] [PubMed]
- Benov, L. Improved Formazan Dissolution for Bacterial MTT Assay. ASM J. 2021, 9, e0163721. [Google Scholar] [CrossRef] [PubMed]
- Lammertink, B.H.A.; Deckers, R.; Derieppe, M.; De Cock, I.; Lentacker, I.; Storm, G.; Moonen, C.T.W.; Bos, C. Dynamic Fluorescence Microscopy of Cellular Uptake of Intercalating Model Drugs by Ultrasound-Activated Microbubbles. Mol. Imaging Biol. 2017, 19, 683–693. [Google Scholar] [CrossRef]
- Mortimer, F.C.; Mason, D.J.; Gant, V.A. Flow cytometric monitoring of antibiotic-induced injury in Escherichia coli using cell-impermeant fluorescent probes. Antimicrob. Agents Chemother. 2000, 44, 676–681. [Google Scholar] [CrossRef]
- Li, T.; Liu, Q.; Wang, D.; Li, J. Characterization and antimicrobial mechanism of CF-14, a new antimicrobial peptide from the epidermal mucus of catfish. Fish Shellfish Immunol. 2019, 92, 881–888. [Google Scholar] [CrossRef]
- Cheng, Y.; Lin, Y.; Cao, H.; Li, Z. Citrus Postharvest Green Mold: Recent Advances in Fungal Pathogenicity and Fruit Resistance. Microorganisms 2020, 8, 449. [Google Scholar] [CrossRef]
- Zhang, M.; Li, S.; Zhao, J.; Shuang, Q.; Xia, Y.; Zhang, F. A novel endogenous antimicrobial peptide MP-4 derived from koumiss of Inner Mongolia by peptidomics, and effects on Staphylococcus aureus. LWT 2024, 191, 115595. [Google Scholar] [CrossRef]
- Mao, F.; Bao, Y.; Wong, N.-K.; Huang, M.; Liu, K.; Zhang, X.; Yang, Z.; Yi, W.; Shu, X.; Xiang, Z.; et al. Large-Scale Plasma Peptidomic Profiling Reveals a Novel, Nontoxic, Crassostrea hongkongensis-Derived Antimicrobial Peptide against Foodborne Pathogens. Mar. Drugs 2021, 19, 420. [Google Scholar] [CrossRef]
- Oliva, R.; Vecchio, P.D.; Grimaldi, A.; Notomista, E.; Cafaro, V.; Pane, K.; Schuabb, V.; Winter, R.; Petraccone, L. Membrane disintegration by the antimicrobial peptide (P)GKY20: Lipid segregation and domain formation. Phys. Chem. Chem. Phys. PCCP 2019, 21, 3989–3998. [Google Scholar] [CrossRef]
- Zhu, X.; Shi, Z.; Mao, Y.; Lächelt, U.; Huang, R. Cell Membrane Perforation: Patterns, Mechanisms and Functions. Small 2024, 20, 2310605. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shi, J.; Tong, Z.; Jia, Y.; Yang, B.; Wang, Z. The revitalization of antimicrobial peptides in the resistance era. Pharmacol. Res. 2021, 163, 105276. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, W.; Deng, L.; Ming, J.; Yao, S.; Zeng, K. Control of sour rot in citrus fruit by three insect antimicrobial peptides. Postharvest Biol. Technol. 2019, 149, 200–208. [Google Scholar] [CrossRef]
- Cytryńska, M.; Rahnamaeian, M.; Zdybicka-Barabas, A.; Dobslaff, K.; Züchner, T.; Sacheau, G.; Innis, C.A.; Vilcinskas, A. Proline-Rich Antimicrobial Peptides in Medicinal Maggots of Lucilia sericata Interact With Bacterial DnaK But Do Not Inhibit Protein Synthesis. Front. Pharmacol. 2020, 11, 532. [Google Scholar] [CrossRef]
- Luo, Y.; Song, Y. Mechanism of Antimicrobial Peptides: Antimicrobial, Anti-Inflammatory and Antibiofilm Activities. Int. J. Mol. Sci. 2021, 22, 11401. [Google Scholar] [CrossRef]
- Ma, L.; Xie, X.; Liu, H.; Huang, Y.; Wu, H.; Jiang, M.; Xu, P.; Ye, X.; Zhou, C. Potent antibacterial activity of MSI-1 derived from the magainin 2 peptide against drug-resistant bacteria. Theranostics 2020, 10, 1373–1390. [Google Scholar] [CrossRef]
- Jia, B.; Wang, Y.; Zhang, Y.; Wang, Z.; Wang, X.; Muhammad, I.; Kong, L.; Pei, Z.; Ma, H.; Jiang, X. High Cell Selectivity and Bactericidal Mechanism of Symmetric Peptides Centered on d-Pro–Gly Pairs. Int. J. Mol. Sci. 2020, 21, 1140. [Google Scholar] [CrossRef]
- Lourenço, A.L.; Rios, T.B.; da Silva, Á.P.; Franco, O.L.; Ramada, M.H. Peptide Stapling Applied to Antimicrobial Peptides. Antibiotics 2023, 12, 1400. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, Y.; Zhang, H.; Du, X.; Cao, Z.; Wu, Y.; Liu, C.; Sun, Y. Antibacterial Activity and Mechanisms of TroHepc2-22, a Derived Peptide of Hepcidin2 from Golden Pompano (Trachinotus ovatus). Int. J. Mol. Sci. 2023, 24, 9251. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, H.; Gao, C.; Chen, R.; Li, C. Antimicrobial Mechanism of pBD2 against Staphylococcus aureus. Molecules 2020, 25, 3513. [Google Scholar] [CrossRef]



| Incidence Rate (%) | Spot Diameter (mm) | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | BP15 | HBP15 | LBP15 | Control | BP15 | HBP15 | LBP15 | |
| 5 d | 100.00 ± 0.00 a | 0.00 ± 0.00 d | 12.50 ± 0.00 c | 62.50 ± 14.43 d | 24.43 ± 3.21 a | 0.00 ± 0.00 c | 1.41 ± 0.80 c | 10.80 ± 4.93 b |
| 6 d | 100.00 ± 0.00 a | 4.17 ± 7.22 c | 20.83 ± 7.22 c | 70.83 ± 19.09 b | 36.61 ± 2.88 a | 0.43 ± 0.74 c | 4.45 ± 0.80 c | 21.23 ± 4.46 b |
| 7 d | 100.00 ± 0.00 a | 25.00 ± 12.50 c | 37.50 ± 0.00 c | 80.83 ± 11.54 b | 51.25 ± 2.88 a | 4.33 ± 2.49 c | 9.76 ± 0.84 c | 34.00 ± 10.39 b |
| 8 d | 100.00 ± 0.00 a | 29.17 ± 7.22 c | 37.50 ± 0.00 c | 80.83 ± 11.54 b | 66.67 ± 3.55 a | 8.13 ± 4.24 c | 14.81 ± 1.19 c | 45.60 ± 13.19 b |
| 9 d | 100.00 ± 0.00 a | 29.17 ± 7.22 c | 37.50 ± 0.00 c | 80.83 ± 11.54 b | 80.33 ± 3.21 a | 12.73 ± 5.65 c | 19.72 ± 1.95 c | 54.08 ± 12.44 b |
| 10 d | 100.00 ± 0.00 a | 37.50 ± 12.50 b | 41.67 ± 7.22 b | 87.50 ± 0.00 a | 91.04 ± 4.59 a | 17.24 ± 9.03 c | 23.27 ± 3.61 c | 69.6 ± 8.37 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, Y.; Lyu, A.; Pan, M.; Shi, Q.; Xu, H.; Li, D.; Deng, M. Control of Postharvest Green Mold in Citrus by the Antimicrobial Peptide BP15 and Its Lipopeptides. J. Fungi 2024, 10, 837. https://doi.org/10.3390/jof10120837
Lei Y, Lyu A, Pan M, Shi Q, Xu H, Li D, Deng M. Control of Postharvest Green Mold in Citrus by the Antimicrobial Peptide BP15 and Its Lipopeptides. Journal of Fungi. 2024; 10(12):837. https://doi.org/10.3390/jof10120837
Chicago/Turabian StyleLei, Yu, Aiyuan Lyu, Mengjuan Pan, Qingxia Shi, Haowan Xu, Dong Li, and Mengsheng Deng. 2024. "Control of Postharvest Green Mold in Citrus by the Antimicrobial Peptide BP15 and Its Lipopeptides" Journal of Fungi 10, no. 12: 837. https://doi.org/10.3390/jof10120837
APA StyleLei, Y., Lyu, A., Pan, M., Shi, Q., Xu, H., Li, D., & Deng, M. (2024). Control of Postharvest Green Mold in Citrus by the Antimicrobial Peptide BP15 and Its Lipopeptides. Journal of Fungi, 10(12), 837. https://doi.org/10.3390/jof10120837






