Resynthesis of Damaged Fe-S Cluster Proteins Protects Aspergillus fumigatus Against Oxidative Stress in the Absence of Mn-Superoxide Dismutase
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Culturing Conditions
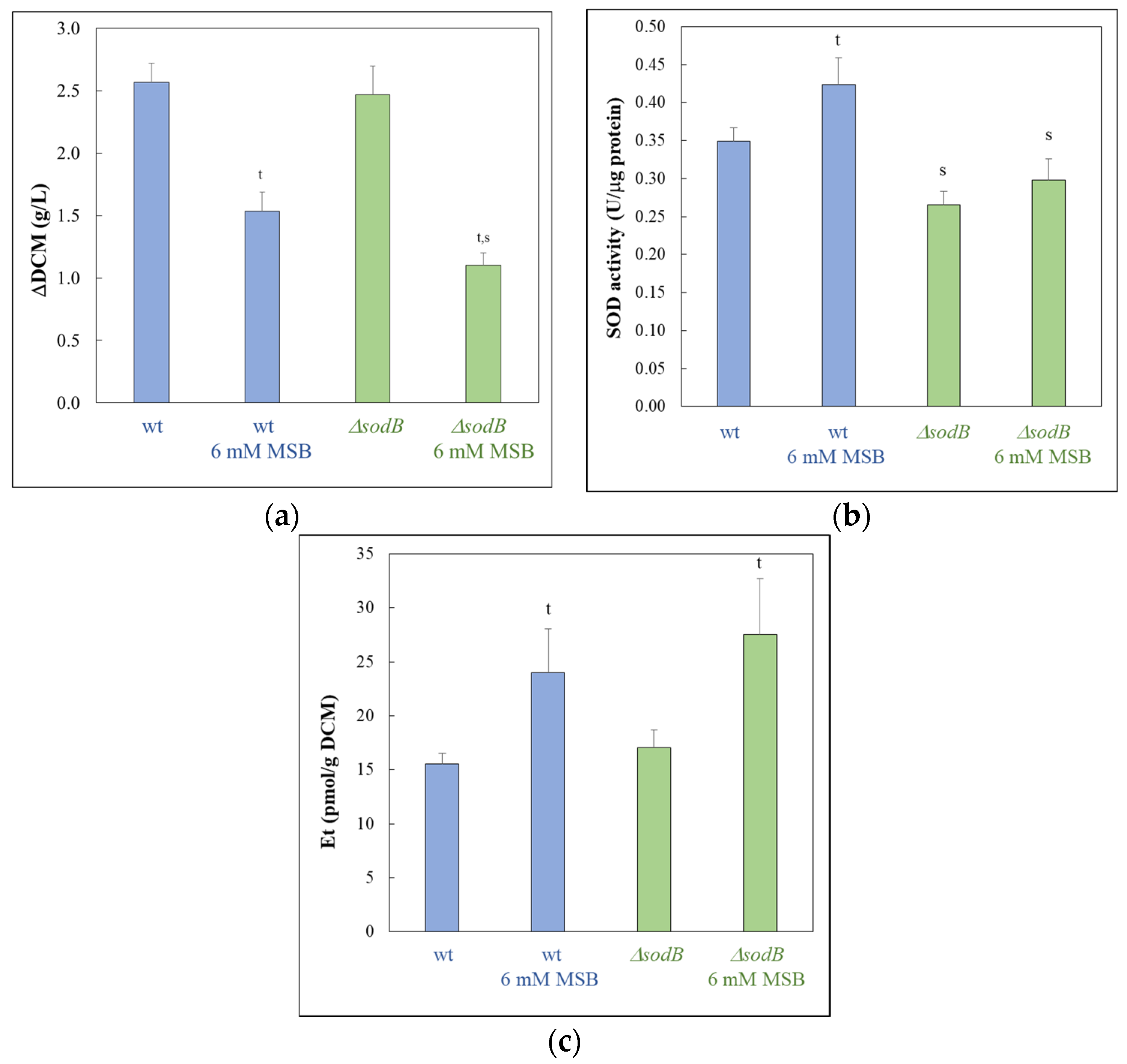
2.2. Determining Formation of Superoxide (Et-Test) and Measuring Specific SOD Activities
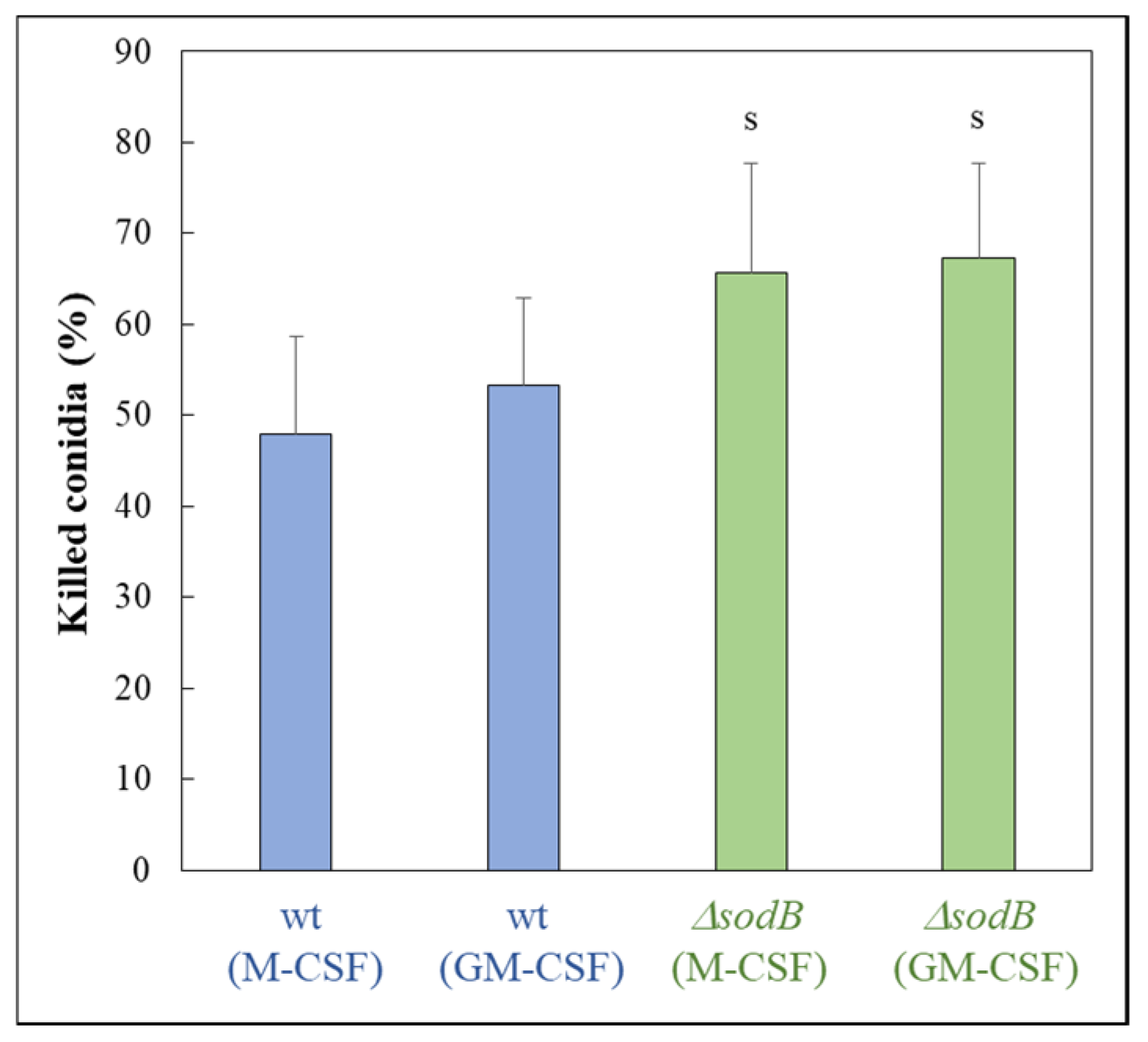
2.3. Generation of Human Macrophages and Testing Susceptibility of Conidia by Macrophage Killing
2.4. Reverse-Transcriptional Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR) Assays
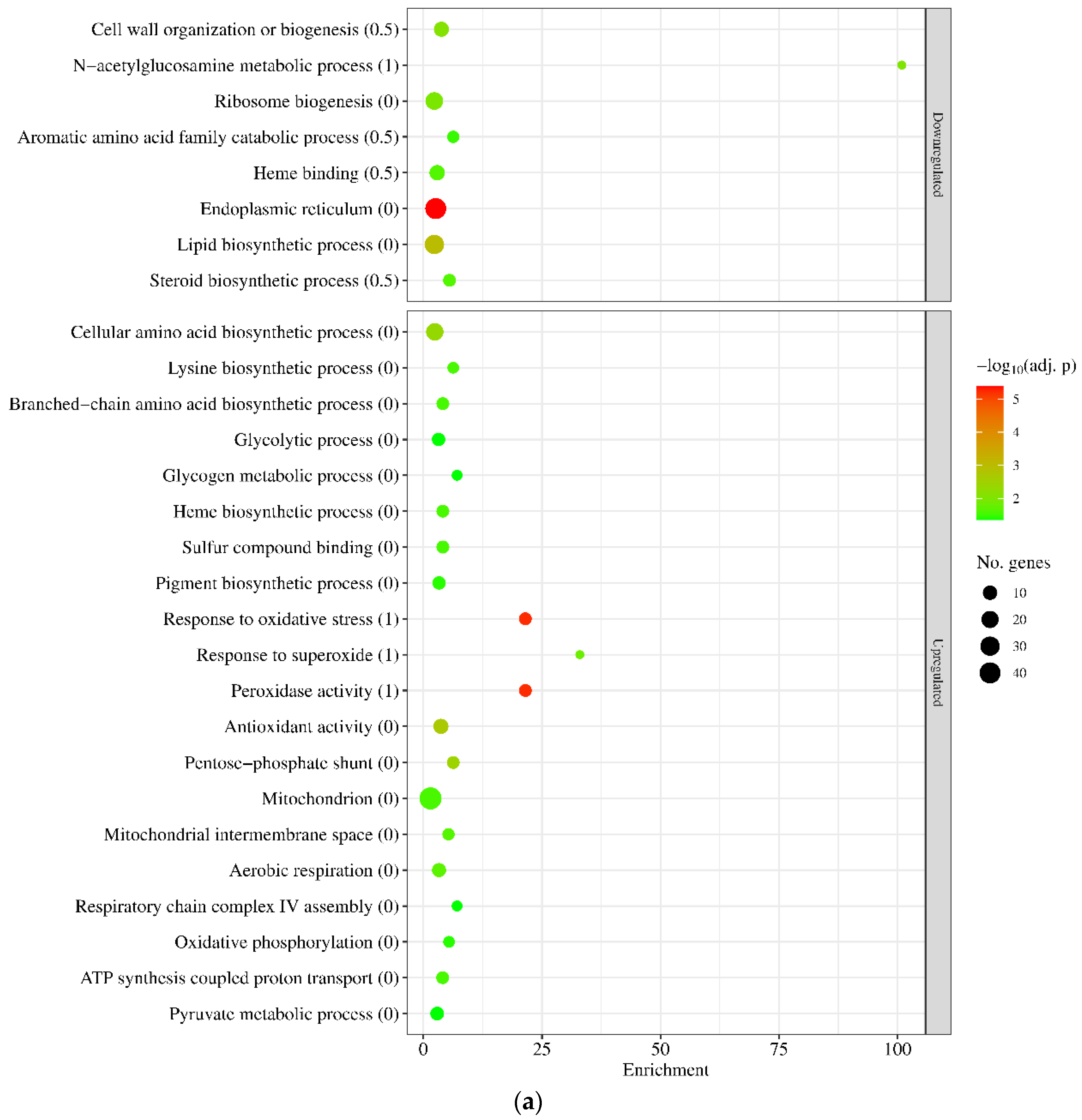
2.5. High-Throughput RNA Sequencing
- Cultures of the A. fumigatus akuBku80 reference strain;
- Cultures of the A. fumigatus IP345 ΔsodB mutant;
- Cultures of the A. fumigatus akuBku80 reference strain treated with 6 mM MSB at 20 h of cultivation;
- Cultures of the A. fumigatus IP345 ΔsodB mutant treated with 6 mM MSB at 20 h of cultivation.
2.6. Evaluation of Transcriptome Data
2.7. List of Abbreviations
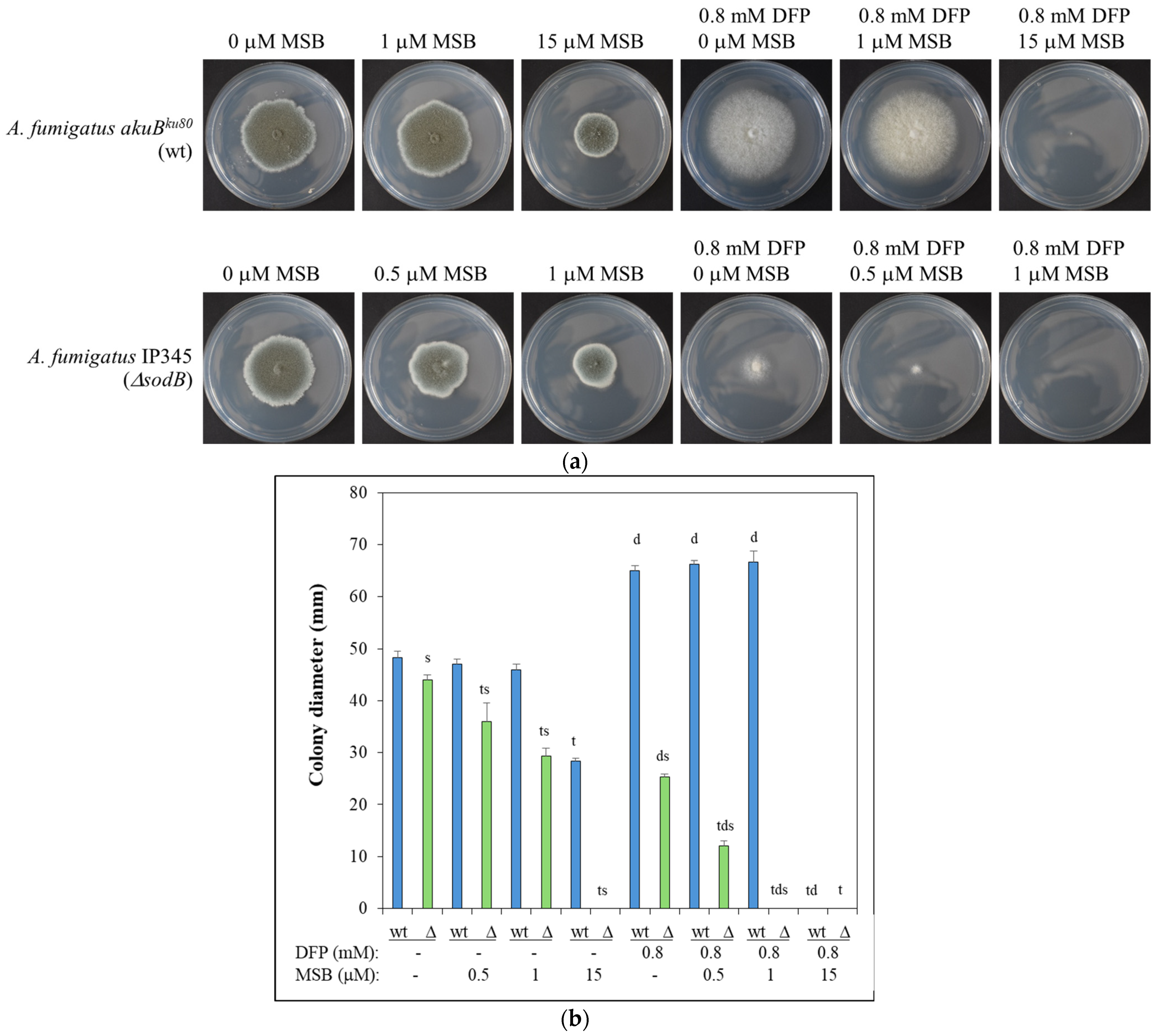
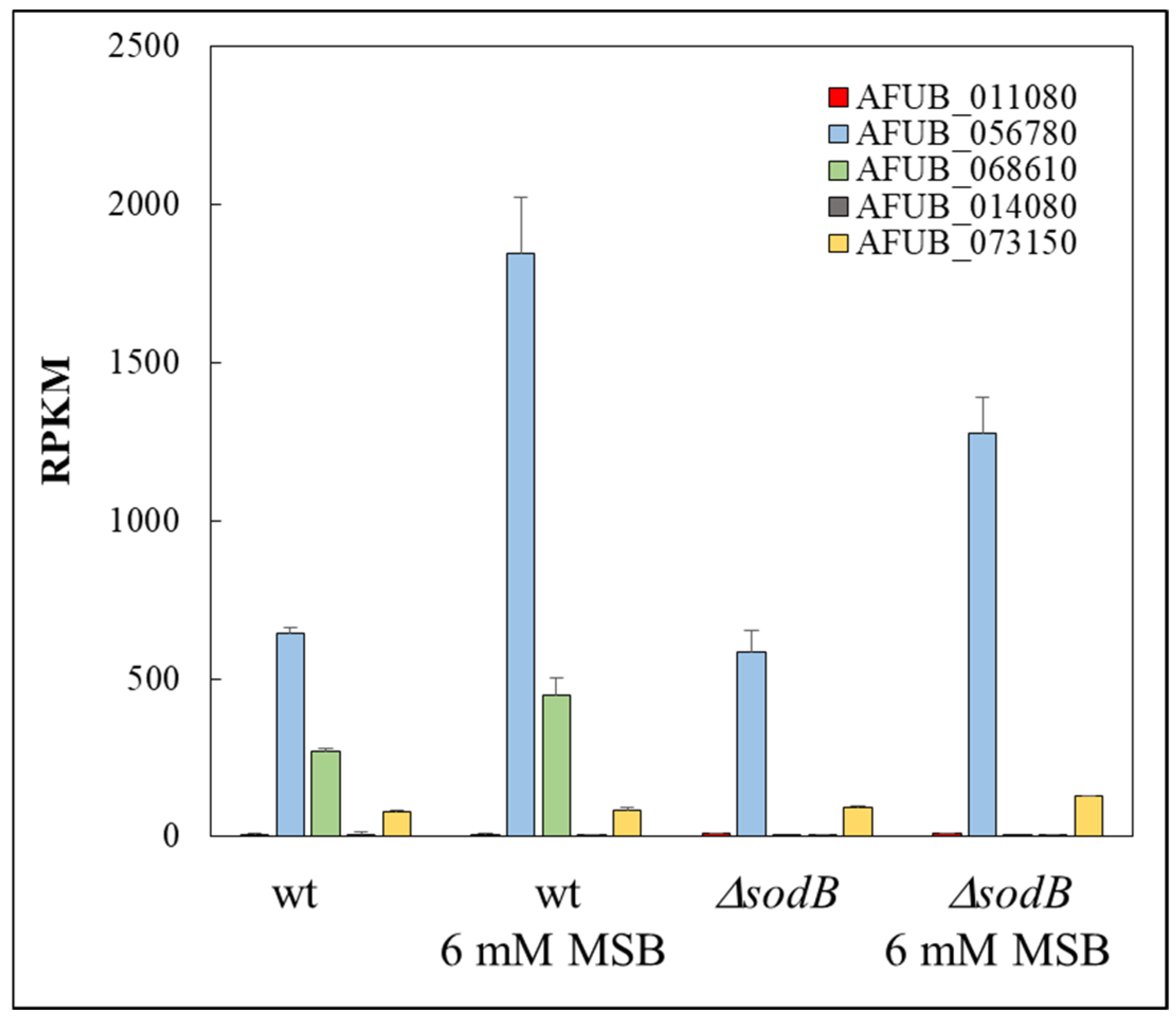
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Brien, K.M.; Dirmeier, R.; Engle, M.; Poyton, R.O. Mitochondrial protein oxidation in yeast mutants lacking manganese-(MnSOD) or copper- and zinc-containing superoxide dismutase (CuZnSOD): Evidence that MnSOD and CuZnSOD have both unique and overlapping functions in protecting mitochondrial proteins from oxidative damage. J. Biol. Chem. 2004, 279, 51817–51827. [Google Scholar] [CrossRef] [PubMed]
- Fréalle, E.; Noël, C.; Nolard, N.; Symoens, F.; Felipe, M.S.; Dei-Cas, E.; Camus, D.; Viscogliosi, E.; Delhaes, L. Manganese superoxide dismutase based phylogeny of pathogenic fungi. Mol. Phylogenet. Evol. 2006, 41, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Leiter, É.; Park, H.S.; Kwon, N.J.; Han, K.H.; Emri, T.; Oláh, V.; Mészáros, I.; Dienes, B.; Vincze, J.; Csernoch, L.; et al. Characterization of the aodA, dnmA, mnSOD and pimA genes in Aspergillus nidulans. Sci. Rep. 2016, 6, 20523. [Google Scholar] [CrossRef] [PubMed]
- Szabó, Z.; Pákozdi, K.; Murvai, K.; Kecskeméti, Á.; Oláh, V.; Logrieco, A.F.; Madar, A.; Dienes, B.; Csernoch, L.; Emri, T.; et al. FvmnSOD is involved in oxidative stress defence, mitochondrial stability and apoptosis prevention in Fusarium verticillioides. J. Basic Microbiol. 2020, 60, 994–1003. [Google Scholar] [CrossRef]
- del Valle, A.O.F.; Scheckhuber, C.Q. Superoxide dismutases in eukaryotic microorganisms: Four case studies. Antioxidants 2022, 11, 188. [Google Scholar] [CrossRef] [PubMed]
- Reverberi, M.; Punelli, M.; Scala, V.; Scarpari, M.; Uva, P.; Mentzen, W.I.; Dolezal, A.L.; Woloshuk, C.; Pinzari, F.; Fabbri, A.A.; et al. Genotypic and phenotypic versatility of Aspergillus flavus during maize exploitation. PLoS ONE 2013, 8, e68735. [Google Scholar] [CrossRef]
- Lambou, K.; Lamarre, C.; Beau, R.; Dufour, N.; Latge, J.P. Functional analysis of the superoxide dismutase family in Aspergillus fumigatus. Mol. Microbiol. 2010, 75, 910–923. [Google Scholar] [CrossRef]
- Briard, B.; Bomme, P.; Lechner, B.E.; Mislin, G.L.; Lair, V.; Prévost, M.C.; Latgé, J.P.; Haas, H.; Beauvais, A. Pseudomonas aeruginosa manipulates redox and iron homeostasis of its microbiota partner Aspergillus fumigatus via phenazines. Sci. Rep. 2015, 5, 8220. [Google Scholar] [CrossRef]
- Stuart, G.R.; Humble, M.H.; Strand, M.K.; Copeland, W.C. Transcriptional response to mitochondrial NADH kinase deficiency in Saccharomyces cerevisiae. Mitochondrion 2009, 9, 211–221. [Google Scholar] [CrossRef][Green Version]
- Knuppertz, L.; Warnsmann, V.; Hamann, A.; Grimm, C.; Osiewacz, H.D. Stress-dependent opposing roles for mitophagy in aging of the ascomycete Podospora anserine. Autophagy 2017, 13, 1037–1052. [Google Scholar] [CrossRef]
- Pákozdi, K.; Emri, T.; Antal, K.; Pócsi, I. Global transcriptomic changes elicited by sodB deletion and menadione exposure in Aspergillus nidulans. J. Fungi 2023, 9, 1060. [Google Scholar] [CrossRef] [PubMed]
- Long, N.; Xu, X.; Qian, H.; Zhang, S.; Lu, L. A putative mitochondrial iron transporter MrsA in Aspergillus fumigatus plays important roles in azole-, oxidative stress responses and virulence. Front. Microbiol. 2016, 7, 716. [Google Scholar] [CrossRef] [PubMed]
- Misslinger, M.; Lechner, B.E.; Bacher, K.; Haas, H. Iron-sensing is governed by mitochondrial, not by cytosolic iron-sulfur cluster biogenesis in Aspergillus fumigatus. Metallomics 2018, 10, 1687–1700. [Google Scholar] [CrossRef] [PubMed]
- Schrettl, M.; Bignell, E.; Kragl, C.; Sabiha, Y.; Loss, O.; Eisendle, M.; Wallner, A.; Arst, H.N., Jr.; Haynes, K.; Haas, H. Distinct roles for intra- and extracellular siderophores during Aspergillus fumigatus infection. PLoS Pathog. 2007, 3, 1195–1207. [Google Scholar] [CrossRef] [PubMed]
- Schrettl, M.; Kim, H.S.; Eisendle, M.; Kragl, C.; Nierman, W.C.; Heinekamp, T.; Werner, E.R.; Jacobsen, I.; Illmer, P.; Yi, H.; et al. SreA-mediated iron regulation in Aspergillus fumigatus. Mol. Microbiol. 2008, 70, 27–43. [Google Scholar] [CrossRef]
- Blatzer, M.; Binder, U.; Haas, H. The metalloreductase FreB is involved in adaptation of Aspergillus fumigatus to iron starvation. Fungal Genet. Biol. 2011, 48, 1027–1033. [Google Scholar] [CrossRef]
- Schrettl, M.; Haas, H. Iron homeostasis—Achilles’ heel of Aspergillus fumigatus? Curr. Opin. Microbiol. 2011, 14, 400–405. [Google Scholar] [CrossRef]
- Brandon, M.; Howard, B.; Lawrence, C.; Laubenbacher, R. Iron acquisition and oxidative stress response in Aspergillus fumigatus. BMC Syst. Biol. 2015, 9, 19. [Google Scholar] [CrossRef]
- Ben Yaakov, D.; Shadkchan, Y.; Albert, N.; Kontoyiannis, D.P.; Osherov, N. The quinoline bromoquinol exhibits broad-spectrum antifungal activity and induces oxidative stress and apoptosis in Aspergillus fumigatus. J. Antimicrob. Chemother. 2017, 72, 2263–2272. [Google Scholar] [CrossRef]
- Leal, S.M., Jr.; Vareechon, C.; Cowden, S.; Cobb, B.A.; Latgé, J.P.; Momany, M.; Pearlman, E. Fungal antioxidant pathways promote survival against neutrophils during infection. J. Clin. Investig. 2012, 122, 2482–2498. [Google Scholar] [CrossRef]
- Leal, S.M., Jr.; Roy, S.; Vareechon, C.; Carrion, S.d.; Clark, H.; Lopez-Berges, M.S.; Di Pietro, A.; Schrettl, M.; Beckmann, N.; Redl, B.; et al. Targeting iron acquisition blocks infection with the fungal pathogens Aspergillus fumigatus and Fusarium oxysporum. PLoS Pathog. 2013, 9, e1003436. [Google Scholar] [CrossRef]
- Berdoukas, V.; Farmaki, K.; Carson, S.; Wood, J.; Coates, T. Treating thalassemia major-related iron overload: The role of deferiprone. J. Blood Med. 2012, 3, 119–129. [Google Scholar] [CrossRef]
- Glickstein, H.; El, R.B.; Shvartsman, M.; Cabantchik, Z.I. Intracellular labile iron pools as direct targets of iron chelators: A fluorescence study of chelator action in living cells. Blood 2005, 106, 3242–3250. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J. The vital role played by deferiprone in the transition of thalassaemia from a fatal to a chronic disease and challenges in its repurposing for use in non-iron-loaded diseases. Pharmaceuticals 2023, 16, 1016. [Google Scholar] [CrossRef] [PubMed]
- Nazik, H.; Penner, J.C.; Ferreira, J.A.; Haagensen, J.A.; Cohen, K.; Spormann, A.M.; Martinez, M.; Chen, V.; Hsu, J.L.; Clemons, K.V.; et al. Effects of iron chelators on the formation and development of Aspergillus fumigatus biofilm. Antimicrob. Agents Chemother. 2015, 59, 6514–6520. [Google Scholar] [CrossRef]
- Sass, G.; Ansari, S.R.; Dietl, A.M.; Déziel, E.; Haas, H.; Stevens, D.A. Intermicrobial interaction: Aspergillus fumigatus siderophores protect against competition by Pseudomonas aeruginosa. PLoS ONE 2019, 14, e0216085. [Google Scholar] [CrossRef] [PubMed]
- Emri, T.; Antal, K.; Varga, K.; Gila, B.C.; Pócsi, I. The oxidative stress response highly depends on glucose and iron availability in Aspergillus fumigatus. J. Fungi 2024, 10, 221. [Google Scholar] [CrossRef]
- Barratt, R.W.; Johnson, G.B.; Ogata, W.N. Wild type and mutant stocks of Aspergillus nidulans. Genetics 1965, 52, 233–246. [Google Scholar] [CrossRef]
- Emri, T.; Pócsi, I.; Szentirmai, A. Analysis of the oxidative stress response of Penicillium chrysogenum to menadione. Free Radic. Res. 1999, 30, 125–132. [Google Scholar] [CrossRef]
- Oberley, L.W.; Spitz, D.R. Assay of superoxide dismutase activity in tumour tissue. Meth. Enzymol. 1984, 105, 457–464. [Google Scholar] [CrossRef]
- Chomczynski, P.A. Reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. BioTechniques 1993, 15, 532–534, 536–537. [Google Scholar] [PubMed]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and 169 HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome. Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Flipphi, M.; Sun, J.; Robellet, X.; Karaffa, L.; Fekete, E.; Zeng, A.P.; Kubicek, C.P. Biodiversity and evolution of primary carbon metabolism in Aspergillus nidulans and other Aspergillus spp. Fungal Genet. Biol. 2009, 46, S19–S44. [Google Scholar] [CrossRef]
- Emri, T.; Antal, K.; Gila, B.; Jónás, A.P.; Pócsi, I. Stress responses elicited by glucose withdrawal in Aspergillus fumigatus. J. Fungi 2022, 8, 1226. [Google Scholar] [CrossRef] [PubMed]
- Inglis, D.O.; Binkley, J.; Skrzypek, M.S.; Arnaud, M.B.; Cerqueira, G.C.; Shah, P.; Wymore, F.; Wortman, J.R.; Sherlock, G. Comprehensive annotation of secondary metabolite biosynthetic genes and gene clusters of Aspergillus nidulans, A. fumigatus, A. niger and A. oryzae. BMC Microbiol. 2013, 13, 91. [Google Scholar] [CrossRef]
- Enright, A.J.; Van Dongen, S.; Ouzounis, C.A. An efficient algorithm for large-scale detection of protein families. Nucleic Acids Res. 2002, 30, 1575–1584. [Google Scholar] [CrossRef]
- van Dongen, S. Graph clustering via a discrete uncoupling process. SIAM J. Matrix Anal. Appl. 2008, 30, 121–141. [Google Scholar] [CrossRef]
- Emri, T.; Vékony, V.; Gila, B.; Nagy, F.; Forgács, K.; Pócsi, I. Autolytic hydrolases affect sexual and asexual development of Aspergillus nidulans. Folia Microbiol. 2018, 63, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Haas, H. Iron—A key nexus in the virulence of Aspergillus fumigatus. Front. Microbiol. 2012, 3, 28. [Google Scholar] [CrossRef] [PubMed]
- Drummond, R.A.; Gaffen, S.L.; Hise, A.G.; Brown, G.D. Innate Defense against Fungal Pathogens. Cold Spring Harb. Perspect. Med. 2014, 5, a019620. [Google Scholar] [CrossRef] [PubMed]
- Saffer, C.; Timme, S.; Rudolph, P.; Figge, M.T. Surrogate infection model predicts optimal alveolar macrophage number for clearance of Aspergillus fumigatus infections. NPJ Syst. Biol. Appl. 2023, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.J.; Rafiq, M.; Radosa, L.; Hortschansky, P.; Cunha, C.; Cseresnyés, Z.; Krüger, T.; Schmidt, F.; Heinekamp, T.; Straßburger, M.; et al. Aspergillus fumigatus hijacks human p11 to redirect fungal-containing phagosomes to non-degradative pathway. Cell Host Microbe 2023, 31, 373–388.e10. [Google Scholar] [CrossRef]
- Verburg, K.; van Neer, J.; Duca, M.; de Cock, H. Novel Treatment Approach for Aspergilloses by Targeting Germination. J. Fungi 2022, 8, 758. [Google Scholar] [CrossRef]
- Emri, T.; Sümegi-Győri, V.M.; Páll, K.; Gila, B.C.; Pócsi, I. Effect of the combinatorial iron-chelation and oxidative stress on the growth of Aspergillus species. Res. Microbiol. 2022, 173, 103969. [Google Scholar] [CrossRef]
- Hallsworth, J.E.; Magan, N. Manipulation of intracellular glycerol and erythritol enhances germination of conidia at low water availability. Microbiology 1995, 141, 1109–1115. [Google Scholar] [CrossRef]
- Rangel, D.E.N.; Fernandes, E.K.; Braga, G.U.; Roberts, D.W. Visible light during mycelial growth and conidiation of Metarhizium robertsii produces conidia with increased stress tolerance. FEMS Microbiol. Lett. 2011, 315, 81–86. [Google Scholar] [CrossRef]
- Rangel, D.E.N.; ·Braga, G.U.L.; Fernandes, É.K.K.; Keyser, C.A.; Hallsworth, J.E.; Roberts, D.W. Stress tolerance and virulence of insect-pathogenic fungi are determined by environmental conditions during conidial formation. Curr. Genet. 2015, 61, 383–404. [Google Scholar] [CrossRef]
- Kocsis, B.; Lee, M.-K.; Yu, J.-H.; Nagy, T.; Daróczi, L.; Batta, G.; Pócsi, I.; Leiter, É. Functional analysis of the bZIP-type transcription factors AtfA and AtfB in Aspergillus nidulans. Front. Microbiol. 2022, 13, 1003709. [Google Scholar] [CrossRef] [PubMed]
- Goffart, S.; Tikkanen, P.; Michell, C.; Wilson, T.; Pohjoismäki, J. LOLO. The type and source of reactive oxygen species influences the outcome of oxidative stress in cultured cells. Cells 2021, 10, 1075. [Google Scholar] [CrossRef]
- Imlay, J.A. Iron-sulphur clusters and the problem with oxygen. Mol. Microbiol. 2006, 59, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Healy, C.; Munoz-Wolf, N.; Strydom, J.; Faherty, L.; Williams, N.C.; Kenny, S.; Donnelly, S.C.; Cloonan, S.M. Nutritional immunity: The impact of metals on lung immune cells and the airway microbiome during chronic respiratory disease. Respir. Res. 2021, 22, 133. [Google Scholar] [CrossRef]
- Kurucz, V.; Krüger, T.; Antal, K.; Dietl, A.M.; Haas, H.; Pócsi, I.; Kniemeyer, O.; Emri, T. Additional oxidative stress reroutes the global response of Aspergillus fumigatus to iron depletion. BMC Genom. 2018, 19, 357. [Google Scholar] [CrossRef] [PubMed]
- Sugui, J.A.; Kim, H.S.; Zarember, K.A.; Chang, Y.C.; Gallin, J.I.; Nierman, W.C.; Kwon-Chung, K.J. Genes differentially expressed in conidia and hyphae of Aspergillus fumigatus upon exposure to human neutrophils. PLoS ONE 2008, 3, e2655. [Google Scholar] [CrossRef]
- Hwang, C.S.; Baek, Y.U.; Yim, H.S.; Kang, S.O. Protective roles of mitochondrial manganese-containing superoxide dismutase against various stresses in Candida albicans. Yeast 2003, 20, 929–941. [Google Scholar] [CrossRef]
- Giles, S.S.; Batinic-Haberle, I.; Perfect, J.R.; Cox, G.M. Cryptococcus neoformans mitochondrial superoxide dismutase: An essential link between antioxidant function and high-temperature growth. Eukaryot. Cell 2005, 4, 46–54. [Google Scholar] [CrossRef]
- Misslinger, M.; Hortschansky, P.; Brakhage, A.A.; Haas, H. Fungal iron homeostasis with a focus on Aspergillus fumigatus. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118885. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pákozdi, K.; Antal, K.; Pázmándi, K.; Miskei, M.; Szabó, Z.; Pócsi, I.; Emri, T. Resynthesis of Damaged Fe-S Cluster Proteins Protects Aspergillus fumigatus Against Oxidative Stress in the Absence of Mn-Superoxide Dismutase. J. Fungi 2024, 10, 823. https://doi.org/10.3390/jof10120823
Pákozdi K, Antal K, Pázmándi K, Miskei M, Szabó Z, Pócsi I, Emri T. Resynthesis of Damaged Fe-S Cluster Proteins Protects Aspergillus fumigatus Against Oxidative Stress in the Absence of Mn-Superoxide Dismutase. Journal of Fungi. 2024; 10(12):823. https://doi.org/10.3390/jof10120823
Chicago/Turabian StylePákozdi, Klaudia, Károly Antal, Kitti Pázmándi, Márton Miskei, Zsuzsa Szabó, István Pócsi, and Tamás Emri. 2024. "Resynthesis of Damaged Fe-S Cluster Proteins Protects Aspergillus fumigatus Against Oxidative Stress in the Absence of Mn-Superoxide Dismutase" Journal of Fungi 10, no. 12: 823. https://doi.org/10.3390/jof10120823
APA StylePákozdi, K., Antal, K., Pázmándi, K., Miskei, M., Szabó, Z., Pócsi, I., & Emri, T. (2024). Resynthesis of Damaged Fe-S Cluster Proteins Protects Aspergillus fumigatus Against Oxidative Stress in the Absence of Mn-Superoxide Dismutase. Journal of Fungi, 10(12), 823. https://doi.org/10.3390/jof10120823







