Diversity and Antifungal Susceptibilities of Yeasts from Mangroves in Hong Kong, China—A One Health Aspect
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Sample Purification
2.3. Yeast Isolation and Cultivation
2.4. Identification of Isolated Yeasts
2.4.1. DNA Extraction
2.4.2. Polymerase Chain Reaction (PCR) and DNA Sequencing
2.4.3. TA Cloning and Sequencing
2.4.4. ITS Sequence Analysis
2.4.5. 28S nrDNA Sequence Analysis
2.4.6. Phylogenetic Analyses
2.5. In Vitro Antifungal Susceptibility Test
3. Results
3.1. Isolation of Yeasts from Mangroves in Hong Kong and Their Morphological Characterizations
3.2. Molecular Identification and Phylogenetic Analyses
3.3. In Vitro Antifungal Susceptibility Testing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. One Health. Available online: https://www.who.int/news-room/questions-and-answers/item/one-health (accessed on 21 August 2024).
- World Health Organization. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Liu, F.; Hu, Z.D.; Zhao, X.M.; Zhao, W.N.; Feng, Z.X.; Yurkov, A.; Alwasel, S.; Boekhout, T.; Bensch, K.; Hui, F.L.; et al. Phylogenomic analysis of the Candida auris-Candida haemuli clade and related taxa in the Metschnikowiaceae, and proposal of thirteen new genera, fifty-five new combinations and nine new species. Persoonia 2024, 52, 22–43. [Google Scholar] [CrossRef] [PubMed]
- Rajasingham, R.; Govender, N.P.; Jordan, A.; Loyse, A.; Shroufi, A.; Denning, D.W.; Meya, D.B.; Chiller, T.M.; Boulware, D.R. The global burden of HIV-associated cryptococcal infection in adults in 2020: A modelling analysis. Lancet Infect. Dis. 2022, 22, 1748–1755. [Google Scholar] [CrossRef] [PubMed]
- Raja, N.S. Epidemiology, risk factors, treatment and outcome of Candida bloodstream infections because of Candida albicans and Candida non-albicans in two district general hospitals in the United Kingdom. Int. J. Clin. Pract. 2021, 75, e13655. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Kontoyiannis, D.P.; Robert, V. Environmental Candida auris and the global warming emergence hypothesis. MBio 2021, 12, e00360-21. [Google Scholar] [CrossRef]
- Arora, P.; Singh, P.; Wang, Y.; Yadav, A.; Pawar, K.; Singh, A.; Padmavati, G.; Xu, J.; Chowdhary, A. Environmental isolation of Candida auris from the coastal wetlands of Andaman Islands, India. MBio 2021, 12, e03181-20. [Google Scholar] [CrossRef]
- Mukherjee, J.; Bhowmick, A.R.; Ghosh, P.B.; Ray, S. Impact of environmental factors on the dependency of litter biomass in carbon cycling of Hooghly estuary, India. Ecol. Inform. 2019, 51, 193–200. [Google Scholar] [CrossRef]
- Hoondee, P.; Wattanagonniyom, T.; Weeraphan, T.; Tanasupawat, S.; Savarajara, A. Occurrence of oleaginous yeast from mangrove forest in Thailand. World J. Microbiol. Biotechnol. 2019, 35, 108. [Google Scholar] [CrossRef]
- WorldData.info. Hong Kong. Available online: https://www.worlddata.info/asia/hong-kong/index.php (accessed on 21 August 2024).
- Hong Kong Environmental Protection Department. Western Waters. Available online: https://www.epd.gov.hk/epd/misc/marine_quality/1986-2005/eng/08_western_content.htm#top (accessed on 21 August 2024).
- Hong Kong Environmental Protection Department. Marine Water Quality in Hong Kong in 2022. Available online: https://www.epd.gov.hk/epd/sites/default/files/epd/english/environmentinhk/water/hkwqrc/files/waterquality/annual-report/marinereport2022.pdf (accessed on 21 August 2024).
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press, Inc.: Cambridge, MA, USA, 1990; pp. 315–322. [Google Scholar]
- O’Donnell, K. Fusarium and its near relatives. In The Fungal Holomorph: Mitotic, Meiotic and Pleomorphic Speciation in Fungal Systematics; Reynolds, D.R., Taylor, J.W., Eds.; CAB International: Wallingford, UK, 1993; pp. 225–233. [Google Scholar]
- Zhao, Y.; Tsang, C.-C.; Xiao, M.; Cheng, J.; Xu, Y.; Lau, S.K.P.; Woo, P.C.Y. Intra-genomic internal transcribed spacer region sequence heterogeneity and molecular diagnosis in clinical microbiology. Int. J. Mol. Sci. 2015, 16, 25067–25079. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- O′Leary, N.A.; Wright, M.W.; Brister, J.R.; Ciufo, S.; Haddad, D.; McVeigh, R.; Rajput, B.; Robbertse, B.; Smith-White, B.; Ako-Adjei, D.; et al. Reference sequence (RefSeq) database at NCBI: Current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 2016, 44, D733–D745. [Google Scholar] [CrossRef]
- Schoch, C.L.; Robbertse, B.; Robert, V.; Vu, D.; Cardinali, G.; Irinyi, L.; Meyer, W.; Nilsson, R.H.; Hughes, K.; Miller, A.N.; et al. Finding needles in haystacks: Linking scientific names, reference specimens and molecular data for fungi. Database 2014, 2014, bau061. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-J.; Cao, W.-N.; Ren, Y.-C.; Xu, L.-L.; Yi, Z.-H.; Liu, Z.; Hui, F.-L. Taxonomy and physiological characterisation of Scheffersomyces titanus sp. nov., a new D-xylose-fermenting yeast species from China. Sci. Rep. 2016, 6, 32181. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, H.; Du, H.; Wang, F.; Li, H.; Zhao, X. Identification and characterization of Diutina rugosa SD-17 for potential use as a probiotic. LWT 2019, 109, 283–288. [Google Scholar] [CrossRef]
- Sakpuntoon, V.; Péter, G.; Groenewald, M.; Dlauchy, D.; Limtong, S.; Srisuk, N. Description of Crinitomyces reliqui gen. nov., sp. nov. and reassignment of Trichosporiella flavificans and Candida ghanaensis to the genus Crinitomyces. J. Fungi 2022, 8, 224. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, C.; Marques, M.; Gonçalves, P. Contrasting strategies for sucrose utilization in a floral yeast clade. MSphere 2022, 7, e00035-22. [Google Scholar] [CrossRef]
- Freitas, L.F.D.; Batista, T.M.; Santos, A.R.O.; Hilário, H.O.; Moreira, R.G.; Franco, G.R.; Morais, P.B.; Lachance, M.A.; Rosa, C.A. Yeast communities associated with cacti in Brazil and the description of Kluyveromyces starmeri sp. nov. based on phylogenomic analyses. Yeast 2020, 37, 625–637. [Google Scholar] [CrossRef]
- Valsalan, R.; Mathew, D. Draft genome of Meyerozyma guilliermondii strain vka1: A yeast strain with composting potential. J. Genet. Eng. Biotechnol. 2020, 18, 54. [Google Scholar] [CrossRef]
- Middelhoven, W.J.; Scorzetti, G.; Fell, J.W. Trichosporon porosum comb. nov., an anamorphic basidiomycetous yeast inhabiting soil, related to the loubieri/laibachii group of species that assimilate hemicelluloses and phenolic compounds. FEMS Yeast Res. 2001, 1, 15–22. [Google Scholar] [CrossRef]
- Nundaeng, S.; Suwannarach, N.; Limtong, S.; Khuna, S.; Kumla, J.; Lumyong, S. An updated global species diversity and phylogeny in the genus Wickerhamomyces with addition of two new species from Thailand. J. Fungi 2021, 7, 957. [Google Scholar] [CrossRef]
- Aliyu, H.; Gorte, O.; de Maayer, P.; Neumann, A.; Ochsenreither, K. Genomic insights into the lifestyles, functional capacities and oleagenicity of members of the fungal family Trichosporonaceae. Sci. Rep. 2020, 10, 2780. [Google Scholar] [CrossRef]
- Poomtien, J.; Jindamorakot, S.; Limtong, S.; Pinphanichakarn, P.; Thaniyavarn, J. Two new anamorphic yeasts species, Cyberlindnera samutprakarnensis sp. nov. and Candida thasaenensis sp. nov., isolated from industrial wastes in Thailand. Antonie Van Leeuwenhoek 2013, 103, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Kurata, O.; Kanchan, C.; Wada, S.; Hatai, K.; Miyoshi, Y.; Fukuda, Y. Novel Exophiala infection Iinvolving ulcerative skin lesions in Japanese flounder Paralichthys olivaceus. Fish Pathol. 2008, 43, 35–44. [Google Scholar] [CrossRef]
- Wang, M.; Mao, W.; Wang, X.; Li, F.; Wang, J.; Chi, Z.; Chi, Z.; Liu, G. Efficient simultaneous production of extracellular polyol esters of fatty acids and intracellular lipids from inulin by a deep-sea yeast Rhodotorula paludigena P4R5. Microb. Cell Fact. 2019, 18, 149. [Google Scholar] [CrossRef] [PubMed]
- Khunnamwong, P.; Nualthaisong, P.; Sakolrak, B.; Nutaratat, P.; Limtong, S. Yamadazyma sisaketensis f.a., sp. nov. and Yamadazyma koratensis f.a., sp. nov., two novel ascomycetous yeast species from mushrooms and cocoa leaves in Thailand, and reassignment of Candida andamanensis, Candida jaroonii and Candida songkhlaensis to the genus Yamadazyma. Int. J. Syst. Evol. Microbiol. 2023, 73, 006174. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Castresana, J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Meletiadis, J.; Mouton, J.W.; Lagrou, K.; Hamal, P.; Guinea, J. Method for the Determination of Broth Dilution Minimum Inhibitory Concentrations of Antifungal Agents for Yeasts. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Files/EUCAST_E_Def_7.3.2_Yeast_testing_definitive_revised_2020.pdf (accessed on 21 August 2024).
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs for Antifungal Agents, Version 10.0, Valid from 2020-02-04. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Clinical_breakpoints/AFST_BP_v10.0_200204_updatd_links_200924.pdf (accessed on 21 August 2024).
- Astvad, K.M.T.; Arikan-Akdagli, S.; Arendrup, M.C. A pragmatic approach to susceptibility classification of yeasts without EUCAST clinical breakpoints. J. Fungi 2022, 8, 141. [Google Scholar] [CrossRef]
- Haridy, M.; Abdo, W.; Hashem, M.; Yanai, T. Candida parapsilosis and Candida tropicalis infections in an Okhotsk snailfish (Liparis ochotensis). J. Vet. Med. Sci. 2018, 80, 1676–1680. [Google Scholar] [CrossRef]
- Vidya, P.; Sebastian, C.D. Yeast Diversity in the mangrove sediments of North Kerala, India. Eur. J. Biol. 2022, 81, 50–57. [Google Scholar] [CrossRef]
- O′Brien, C.E.; McCarthy, C.G.P.; Walshe, A.E.; Shaw, D.R.; Sumski, D.A.; Krassowski, T.; Fitzpatrick, D.A.; Butler, G. Genome analysis of the yeast Diutina catenulata, a member of the Debaryomycetaceae/Metschnikowiaceae (CTG-Ser) clade. PLoS ONE 2018, 13, e0198957. [Google Scholar] [CrossRef] [PubMed]
- Guerra, R.S.; do Nascimento, M.M.F.; Miesch, S.; Najafzadeh, M.J.; Ribeiro, R.O.; Ostrensky, A.; de Hoog, G.S.; Vicente, V.A.; Boeger, W.A. Black yeast biota in the mangrove, in search of the origin of the lethargic crab disease (LCD). Mycopathologia 2013, 175, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Araujo, F.V.; Hagler, A.N. Kluyveromyces aestuarii, a potential environmental quality indicator yeast for mangroves in the State of Rio de Janeiro, Brazil. Braz. J. Microbiol. 2011, 42, 954–958. [Google Scholar] [CrossRef]
- Matos, Í.T.S.R.; de Souza, V.A.; D’Angelo, G.d.R.; Astolfi Filho, S.; do Carmo, E.J.; Vital, M.J.S. Yeasts with fermentative potential associated with fruits of camu-camu (Myrciaria dubia, Kunth) from North of Brazilian Amazon. Sci. World J. 2021, 2021, 9929059. [Google Scholar] [CrossRef]
- Leyton, A.; Flores, L.; Mäki-Arvela, P.; Lienqueo, M.E.; Shene, C. Macrocystis pyrifera source of nutrients for the production of carotenoids by a marine yeast Rhodotorula mucilaginosa. J. Appl. Microbiol. 2019, 127, 1069–1079. [Google Scholar] [CrossRef]
- Am-In, S.; Limtong, S.; Yongmanitchai, W.; Jindamorakot, S. Candida andamanensis sp. nov., Candida laemsonensis sp. nov. and Candida ranongensis sp. nov., anamorphic yeast species isolated from estuarine waters in a Thai mangrove forest. Int. J. Syst. Evol. Microbiol. 2011, 61, 454–461. [Google Scholar] [CrossRef]
- Sugita, T.; Nakase, T. Molecular phylogenetic study of the basidiomycetous anamorphic yeast genus Trichosporon and related taxa based on small subunit ribosomal DNA sequences. Mycoscience 1998, 39, 7–13. [Google Scholar] [CrossRef]
- Laurencík, M.; Sulo, P.; Sláviková, E.; Piecková, E.; Seman, M.; Ebringer, L. The diversity of eukaryotic microbiota in the traditional Slovak sheep cheese—Bryndza. Int. J. Food Microbiol. 2008, 127, 176–179. [Google Scholar] [CrossRef]
- Fell, J.W.; Scorzetti, G.; Connell, L.; Craig, S. Biodiversity of micro-eukaryotes in Antarctic Dry Valley soils with <5% soil moisture. Soil Biol. Biochem. 2006, 38, 3107–3119. [Google Scholar] [CrossRef]
- Turin University Culture Collection. MUT Accession Number: MUT00006683. Available online: https://www.tucc-database.unito.it/view_collection_entry/MUT00006683 (accessed on 21 August 2024).
- Kajadpai, N.; Angchuan, J.; Khunnamwong, P.; Srisuk, N. Diversity of duckweed (Lemnaceae) associated yeasts and their plant growth promoting characteristics. AIMS Microbiol. 2023, 9, 486–517. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.-L.; Li, Y.; Chai, C.-Y.; Yan, Z.-L.; Hui, F.-L. New species of Yamadazyma from rotting wood in China. MycoKeys 2021, 83, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.V.G.; Nguyen, H.H.N.; Vo, T.-H.; Le, M.-T.; Tran-Nguyen, V.-K.; Vu, T.T.; Nguyen, P.-V. Prevalence and drug susceptibility of clinical Candida species in nasopharyngeal cancer patients in Vietnam. One Health 2024, 18, 100659. [Google Scholar] [CrossRef] [PubMed]
- Borman, A.M.; Fraser, M.; Johnson, E.M. CHROMagarTM Candida Plus: A novel chromogenic agar that permits the rapid identification of Candida auris. Med. Mycol. 2021, 59, 253–258. [Google Scholar] [CrossRef]
- Marathe, A.; Zhu, Y.; Chaturvedi, V.; Chaturvedi, S. Utility of CHROMagar™ Candida Plus for presumptive identification of Candida auris from surveillance samples. Mycopathologia 2022, 187, 527–534. [Google Scholar] [CrossRef]
- Mulet Bayona, J.V.; Salvador García, C.; Tormo Palop, N.; Valentín Martín, A.; González Padrón, C.; Colomina Rodríguez, J.; Pemán, J.; Gimeno Cardona, C. Novel chromogenic medium CHROMagarTM Candida Plus for detection of Candida auris and other Candida species from surveillance and environmental samples: A multicenter study. J. Fungi 2022, 8, 281. [Google Scholar] [CrossRef]
- Tóth, R.; Nosek, J.; Mora-Montes, H.M.; Gabaldon, T.; Bliss, J.M.; Nosanchuk, J.D.; Turner, S.A.; Butler, G.; Vágvölgyi, C.; Gácser, A. Candida parapsilosis: From genes to the bedside. Clin. Microbiol. Rev. 2019, 32, e00111-18. [Google Scholar] [CrossRef]
- Megri, Y.; Arastehfar, A.; Boekhout, T.; Daneshnia, F.; Hörtnagl, C.; Sartori, B.; Hafez, A.; Pan, W.; Lass-Flörl, C.; Hamrioui, B. Candida tropicalis is the most prevalent yeast species causing candidemia in Algeria: The urgent need for antifungal stewardship and infection control measures. Antimicrob. Resist. Infect. Control 2020, 9, 50. [Google Scholar] [CrossRef]
- Marcos-Zambrano, L.J.; Escribano, P.; Bouza, E.; Guinea, J. Production of biofilm by Candida and non-Candida spp. isolates causing fungemia: Comparison of biomass production and metabolic activity and development of cut-off points. Int. J. Med. Microbiol. 2014, 304, 1192–1198. [Google Scholar] [CrossRef]
- Orsi, C.F.; Colombari, B.; Blasi, E. Candida metapsilosis as the least virulent member of the ‘C. parapsilosis’ complex. Med. Mycol. 2010, 48, 1024–1033. [Google Scholar] [CrossRef]
- Silva, S.; Henriques, M.; Martins, A.; Oliveira, R.; Williams, D.; Azeredo, J. Biofilms of non-Candida albicans Candida species: Quantification, structure and matrix composition. Med. Mycol. 2009, 47, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Nobrega de Almeida, J.; de Souza, L.B.; Motta, A.L.; Rossi, F.; Romano Di Gioia, T.S.; Benard, G.; Del Negro, G.M.B. Evaluation of the MALDI-TOF VITEK MS™ system for the identification of Candida parapsilosis, C. orthopsilosis and C. metapsilosis from bloodstream infections. J. Microbiol. Methods 2014, 105, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Shan, Y.; Fan, S.; Li, J.; Liu, X. Candida parapsilosis sensu stricto and the closely related species Candida orthopsilosis and Candida metapsilosis in vulvovaginal candidiasis. Mycopathologia 2015, 179, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, J. Lodderomyces elongisporus: An emerging human fungal pathogen. PLoS Pathog. 2023, 19, e1011613. [Google Scholar] [CrossRef]
- Yadav, A.; Jain, P.; Jain, K.; Wang, Y.; Singh, A.; Singh, A.; Xu, J.; Chowdhary, A. Genomic analyses of a fungemia outbreak caused by Lodderomyces elongisporus in a neonatal intensive care unit in Delhi, India. MBio 2023, 14, e00636-23. [Google Scholar] [CrossRef]
- Silva, S.; Negri, M.; Henriques, M.; Oliveira, R.; Williams, D.W.; Azeredo, J. Candida glabrata, Candida parapsilosis and Candida tropicalis: Biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol. Rev. 2012, 36, 288–305. [Google Scholar] [CrossRef]
- Guo, L.-N.; Xiao, M.; Cao, B.; Qu, F.; Zhan, Y.-L.; Hu, Y.-J.; Wang, X.-R.; Liang, G.-W.; Gu, H.-T.; Qi, J.; et al. Epidemiology and antifungal susceptibilities of yeast isolates causing invasive infections across urban Beijing, China. Future Microbiol. 2017, 12, 1075–1086. [Google Scholar] [CrossRef]
- Daneshnia, F.; de Almeida Júnior, J.N.; Ilkit, M.; Lombardi, L.; Perry, A.M.; Gao, M.; Nobile, C.J.; Egger, M.; Perlin, D.S.; Zhai, B.; et al. Worldwide emergence of fluconazole-resistant Candida parapsilosis: Current framework and future research roadmap. Lancet Microbe. 2023, 4, e470–e480. [Google Scholar] [CrossRef]
- Sugita, T.; Ikeda, R.; Nishikawa, A. Analysis of Trichosporon isolates obtained from the houses of patients with summer-type hypersensitivity pneumonitis. J. Clin. Microbiol. 2004, 42, 5467–5471. [Google Scholar] [CrossRef]
- Alcoba-Florez, J.; Laich, F.; Pérez-Roth, E.; Ode-Febles, J.; Méndez-Álvarez, S. First reported case of catheter-related fungemia due to Candida mengyuniae. J. Clin. Microbiol. 2011, 49, 3429–3431. [Google Scholar] [CrossRef]
- Radosavljevic, M.; Koenig, H.; Letscher-Bru, V.; Waller, J.; Maloisel, F.; Lioure, B.; Herbrecht, R. Candida catenulata fungemia in a cancer patient. J. Clin. Microbiol. 1999, 37, 475–477. [Google Scholar] [CrossRef] [PubMed]
- Borman, A.M.; Muller, J.; Walsh-Quantick, J.; Szekely, A.; Patterson, Z.; Palmer, M.D.; Fraser, M.; Johnson, E.M. Fluconazole resistance in isolates of uncommon pathogenic yeast species from the United Kingdom. Antimicrob. Agents Chemother. 2019, 63, e00211-19. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-F.; Zhang, W.; Fan, X.; Hou, X.; Liu, X.-Y.; Huang, J.-J.; Kang, W.; Zhang, G.; Zhang, H.; Yang, W.-H.; et al. Antifungal susceptibility profiles and resistance mechanisms of clinical Diutina catenulata isolates with high MIC values. Front. Cell Infect. Microbiol. 2021, 11, 739496. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Zhao, Y.; Tang, M.; Xia, H.; Li, D.; Lu, G. Concurrent infection of Exophiala dermatitidis and Angiostrongylus cantonensis in central nervous system of a child with inherited CARD9 deficiency: A case report and literature review. J. Mycol. Med. 2024, 34, 101455. [Google Scholar] [CrossRef] [PubMed]
- Maraki, S.; Katzilakis, N.; Neonakis, I.; Stafylaki, D.; Meletiadis, J.; Hamilos, G.; Stiakaki, E. Exophiala dermatitidis central line-associated bloodstream infection in a child with Ewing’s sarcoma: Case report and literature review on paediatric infections. Mycopathologia 2022, 187, 595–602. [Google Scholar] [CrossRef]
- Salvador, A.; Veiga, F.F.; Svidzinski, T.I.E.; Negri, M. Case of mixed infection of toenail caused by Candida parapsilosis and Exophiala dermatitidis and in vitro effectiveness of propolis extract on mixed biofilm. J. Fungi 2023, 9, 581. [Google Scholar] [CrossRef]
- Setoguchi, D.; Iwanaga, N.; Ito, Y.; Ashizawa, N.; Hirayama, T.; Takeda, K.; Ide, S.; Takemoto, S.; Tashiro, M.; Hosogaya, N.; et al. Pulmonary phaeohyphomycosis due to Exophiala dermatitidis in a patient with pulmonary non-tuberculous mycobacterial infection. J. Infect. Chemother. 2023, 29, 615–619. [Google Scholar] [CrossRef]
- Yoshinouchi, T.; Yamamoto, K.; Migita, M.; Yokoyama, T.; Nakamura, T.; Matsuoka, M. Diagnosis and clinical management of Exophiala dermatitidis pneumonia in a patient with anorexia nervosa: A case report. Med. Mycol. Case Rep. 2023, 42, 100617. [Google Scholar] [CrossRef]
- Yu, H.-Y.; Qu, T.-T.; Yang, Q.; Hu, J.-H.; Sheng, J.-F. A fatal case of Exophiala dermatitidis meningoencephalitis in an immunocompetent host: A case report and literature review. J. Infect. Chemother. 2021, 27, 1520–1524. [Google Scholar] [CrossRef]
- Diekema, D.J.; Messer, S.A.; Boyken, L.B.; Hollis, R.J.; Kroeger, J.; Tendolkar, S.; Pfaller, M.A. In vitro activity of seven systemically active antifungal agents against a large global collection of rare Candida Species as determined by CLSI broth microdilution methods. J. Clin. Microbiol. 2009, 47, 3170–3177. [Google Scholar] [CrossRef]
- Alfouzan, W.; Dhar, R.; Ashkanani, H.; Gupta, M.; Rachel, C.; Khan, Z.U. Species spectrum and antifungal susceptibility profile of vaginal isolates of Candida in Kuwait. J. Mycol. Med. 2015, 25, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.H.P.; Miranda, E.T.; Zaia, J.E.; Giannini, M.J.S.M. Species diversity of yeast in oral colonization of insulin-treated diabetes mellitus patients. Mycopathologia 2006, 162, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Shin, J.M.; Lee, K.W.; Kim, Y.S.; Rao, B.; Lee, Y. Kaposi sarcoma-like lesions caused by Candida guilliermondii infection in a kidney transplant patient. Ann. Dermatol. 2021, 33, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.A.; Pathmanathan, S.G.; Hussin, H.; Zaini, A.B. Distribution and antifungal susceptibility pattern of Candida species at a tertiary hospital in Malaysia. J. Infect. Dev. Ctries. 2018, 12, 102–108. [Google Scholar] [CrossRef]
- Zhang, M.-j.; Liang, G.-z.; Mei, H.; Song, G.; Liu, W.-d. Onychomycosis caused by Pichia guilliermondii: A case report and mini-review. Med. Mycol. Case Rep. 2020, 27, 72–76. [Google Scholar] [CrossRef]
- Cabral, A.M.; da Siveira Rioja, S.; Brito-Santos, F.; Peres da Silva, J.R.; MacDowell, M.L.; Melhem, M.S.C.; Mattos-Guaraldi, A.L.; Hirata Junior, R.; Damasco, P.V. Endocarditis due to Rhodotorula mucilaginosa in a kidney transplanted patient: Case report and review of medical literature. JMM Case Rep. 2017, 4, e005119. [Google Scholar] [CrossRef]
- Ferreira, A.I.; Cruz, H.; Ranchor, R.; Silva, B.S.; Serôdio, J.; Lopes, V.; Ramos, M.H. Pleural empyema due to Rhodotorula mucilaginosa: A rare yet severe complication of a previously undiagnosed cancer patient. IDCases 2022, 28, e01469. [Google Scholar] [CrossRef]
- Garcia-Gutiérrez, C.A.; Cuétara-García, M.S.; Moragues, M.D.; Ligero, J.; Quevedo, S.M.; Buitrago, M.J. Low sensitivity of conventional fungal agars in fungemia by Rhodotorula mucilaginosa: Description of two cases. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 21. [Google Scholar] [CrossRef]
- Ge, G.; Li, D.; Mei, H.; Lu, G.; Zheng, H.; Liu, W.; Shi, D. Different toenail onychomycosis due to Rhodotorula mucilaginosa and Candida parapsilosis in an immunocompetent young adult. Med. Mycol. Case Rep. 2019, 24, 69–71. [Google Scholar] [CrossRef]
- Goravey, W.; Ali, G.A.; Abid, F.; Ibrahim, E.B.; Al Maslamani, M.A.; Abdel Hadi, H. Central line-associated Rhodotorula mucilaginosa fungemia in an immunocompetent host: Case report and review of the literature. Clin. Case Rep. 2021, 9, 2158–2161. [Google Scholar] [CrossRef]
- Hirano, R.; Mitsuhashi, T.; Osanai, K. Rhodotorula mucilaginosa fungemia, a rare opportunistic infection without central venous catheter implantation, successfully treated by liposomal amphotericin B. Case Rep. Infect. Dis. 2022, 2022, 7830126. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.A.; Hyun, M.; Ryu, S.-Y. Catheter-associated Rhodotorula mucilaginosa fungemia in an immunocompetent host. Infect. Chemother. 2013, 45, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Noni, M.; Stathi, A.; Velegraki, A.; Malamati, M.; Kalampaliki, A.; Zachariadou, L.; Michos, A. Rare invasive yeast infections in Greek neonates and children, a retrospective 12-year study. J. Fungi 2020, 6, 194. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.C.; Correia, A.F.; da Silva, Z.D.L.; de Resende, C.N.; Brandão, F.; Almeida, R.M.; de Medeiros Nóbrega, Y.K. Vulvovaginal candidiasis and current perspectives: New risk factors and laboratory diagnosis by using MALDI TOF for identifying species in primary infection and recurrence. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 1681–1693. [Google Scholar] [CrossRef]
- Simon, M.S.; Somersan, S.; Singh, H.K.; Hartman, B.; Wickes, B.L.; Jenkins, S.G.; Walsh, T.J.; Schuetz, A.N. Endocarditis caused by Rhodotorula infection. J. Clin. Microbiol. 2014, 52, 374–378. [Google Scholar] [CrossRef]
- Guo, L.-N.; Yu, S.-Y.; Hsueh, P.-R.; Al-Hatmi Abdullah, M.S.; Meis Jacques, F.; Hagen, F.; Xiao, M.; Wang, H.; Barresi, C.; Zhou, M.-L.; et al. Invasive infections due to Trichosporon: Species distribution, genotyping, and antifungal susceptibilities from a multicenter study in China. J. Clin. Microbiol. 2019, 57, e01505-18. [Google Scholar] [CrossRef]
- Li, T.; Huang, Y.; Chen, X.; Wang, Z.; Xu, Y. Urinary tract infections caused by fluconazole-resistant Trichosporon japonicum in 2 kidney transplant patients and analysis of their homology. Open Forum Infect. Dis. 2020, 7, ofaa365. [Google Scholar] [CrossRef]
- Menu, E.; Kabtani, J.; Roubin, J.; Ranque, S.; L’Ollivier, C. Pericardial effusion due to Trichosporon japonicum: A case report and review of the literature. Pathogens 2022, 11, 598. [Google Scholar] [CrossRef]
- Bilal, H.; Shafiq, M.; Hou, B.; Islam, R.; Khan, M.N.; Khan, R.U.; Zeng, Y. Distribution and antifungal susceptibility pattern of Candida species from mainland China: A systematic analysis. Virulence 2022, 13, 1573–1589. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, M.; Qiao, D.; Shen, H.; Wang, L.; Wang, D.; Li, L.; Liu, Y.; Lu, H.; Wang, C.; et al. Prevalence and antifungal susceptibility of Candida parapsilosis species complex in eastern China: A 15-year retrospective study by ECIFIG. Front. Microbiol. 2021, 12, 644000. [Google Scholar] [CrossRef]
- Song, Y.; Chen, X.; Yan, Y.; Wan, Z.; Liu, W.; Li, R. Prevalence and antifungal susceptibility of pathogenic yeasts in China: A 10-year retrospective study in a teaching hospital. Front. Microbiol. 2020, 11, 1401. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, C.; Li, Z.; Ji, B.; Man, S.; Yi, M.; Li, R.; Hao, M.; Wang, S. Epidemiology and antifungal susceptibility of fungal infections from 2018 to 2021 in Shandong, eastern China: A report from the SPARSS program. Indian J. Med. Microbiol. 2024, 47, 100518. [Google Scholar] [CrossRef]
- Cavassin, F.B.; Baú-Carneiro, J.L.; Vilas-Boas, R.R.; Queiroz-Telles, F. Sixty years of amphotericin B: An overview of the main antifungal agent used to treat invasive fungal infections. Infect. Dis. Ther. 2021, 10, 115–147. [Google Scholar] [CrossRef]
- Assress, H.A.; Selvarajan, R.; Nyoni, H.; Ogola, H.J.O.; Mamba, B.B.; Msagati, T.A.M. Azole antifungal resistance in fungal isolates from wastewater treatment plant effluents. Environ. Sci. Pollut. Res. Int. 2021, 28, 3217–3229. [Google Scholar] [CrossRef]
- Monapathi, M.E.; Oguegbulu, J.C.; Adogo, L.; Klink, M.; Okoli, B.; Mtunzi, F.; Modise, J.S. Pharmaceutical pollution: Azole antifungal drugs and resistance of opportunistic pathogenic yeasts in wastewater and environmental water. Appl. Environ. Soil Sci. 2021, 2021, 9985398. [Google Scholar] [CrossRef]
- Richter, E.; Wick, A.; Ternes, T.A.; Coors, A. Ecotoxicity of climbazole, a fungicide contained in antidandruff shampoo. Environ. Toxicol. Chem. 2013, 32, 2816–2825. [Google Scholar] [CrossRef]
- Kwong, I.H.Y.; Wong, F.K.K.; Fung, T. Automatic mapping and monitoring of marine water quality parameters in Hong Kong using Sentinel-2 image time-series and Google Earth Engine cloud computing. Front. Mar. Sci. 2022, 9, 871470. [Google Scholar] [CrossRef]
- Hafeez, S.; Wong, M.S. Measurement of coastal water quality indicators using Sentinel-2; An evaluation over Hong Kong and the Pearl River estuary. In Proceedings of the IGARSS 2019—2019 IEEE International Geoscience and Remote Sensing Symposium, Yokohama, Japan, 28 July–2 August 2019; pp. 8249–8252. [Google Scholar]
- Chen, X.; Li, Y.; Liu, Z.; Yin, K.D.; Li, Z.; Wai, O.; King, B. Integration of multi-source data for water quality classification in the Pearl River estuary and its adjacent coastal waters of Hong Kong. Cont. Shelf Res. 2004, 24, 1827–1843. [Google Scholar] [CrossRef]
- Wroński, M.; Trawiński, J.; Skibiński, R. Antifungal drugs in the aquatic environment: A review on sources, occurrence, toxicity, health effects, removal strategies and future challenges. J. Hazard. Mater. 2024, 465, 133167. [Google Scholar] [CrossRef]
- Angeles, L.F.; Singh, R.R.; Vikesland, P.J.; Aga, D.S. Increased coverage and high confidence in suspect screening of emerging contaminants in global environmental samples. J. Hazard. Mater. 2021, 414, 125369. [Google Scholar] [CrossRef]
- da Silva, F.A.; Medeiros, S.; da Costa-Junior, S.D.; Roberto, A.E.M.; Palácio, S.B.; de Lima-Neto, R.G.; Neves, R.P.; Magalhães, C.P.; Garcia, J.E.; Cavalcanti, I.M.F. Antimicrobial resistance profile and biofilm production of microorganisms isolated from oropharynx of Rupornis magnirostris (Gmelin, 1788) and Caracara plancus (Miller, 1777). Vet. Med. Int. 2020, 2020, 8888618. [Google Scholar] [CrossRef]
- Lord, A.T.; Mohandas, K.; Somanath, S.; Ambu, S. Multidrug resistant yeasts in synanthropic wild birds. Ann. Clin. Microbiol. Antimicrob. 2010, 9, 11. [Google Scholar] [CrossRef]
- Rosario Medina, I.; Román Fuentes, L.; Batista Arteaga, M.; Real Valcárcel, F.; Acosta Arbelo, F.; Padilla del Castillo, D.; Déniz Suárez, S.; Ferrer Quintana, O.; Vega Gutiérrez, B.; Silva Sergent, F.; et al. Pigeons and their droppings as reservoirs of Candida and other zoonotic yeasts. Rev. Iberoam. Micol. 2017, 34, 211–214. [Google Scholar] [CrossRef]
- Sudhadham, M.; Prakitsin, S.; Sivichai, S.; Chaiyarat, R.; Dorrestein, G.M.; Menken, S.B.; de Hoog, G.S. The neurotropic black yeast Exophiala dermatitidis has a possible origin in the tropical rain forest. Stud. Mycol. 2008, 61, 145–155. [Google Scholar] [CrossRef]
- Mendes, J.F.; Albano, A.P.N.; Coimbra, M.A.A.; Ferreira, G.F.d.; Gonçalves, C.L.; Nascente, P.d.S.; Mello, J.R.B.d. Fungi isolated from the excreta of wild birds in screening centers in Pelotas, RS, Brazil. Rev. Inst. Med. Trop. Sao Paulo 2014, 56, 525–528. [Google Scholar] [CrossRef]
- Subramanya, S.H.; Sharan, N.K.; Baral, B.P.; Hamal, D.; Nayak, N.; Prakash, P.Y.; Sathian, B.; Bairy, I.; Gokhale, S. Diversity, in-vitro virulence traits and antifungal susceptibility pattern of gastrointestinal yeast flora of healthy poultry, Gallus gallus domesticus. BMC Microbiol. 2017, 17, 113. [Google Scholar] [CrossRef]
- Levison, M.E. Diseases transmitted by birds. Microbiol. Spectr. 2015, 3, 16. [Google Scholar] [CrossRef]
- Akter, M.; Islam, M.S.; Islam, M.A.; Sobur, M.A.; Jahan, M.S.; Rahman, S.; Nazmul Hussain Nazir, K.H.M.; Rahman, M.T. Migratory birds as the potential source for the transmission of Aspergillus and other fungus to Bangladesh. J. Adv. Vet. Anim. Res. 2020, 7, 338–344. [Google Scholar] [CrossRef]
- Wu, Y.; Fan, X.; Yu, J.; Liu, T.; Cui, R.; Xiang, X. Characteristics of cross transmission of gut fungal pathogens between wintering Hooded Cranes and sympatric Domestic Geese. Avian Res. 2023, 14, 100142. [Google Scholar] [CrossRef]
- Agriculture, Fisheries and Conservation Department, the Government of the Hong Kong Special Asministrative Region. Birds. Available online: https://www.afcd.gov.hk/english/conservation/hkbiodiversity/speciesgroup/speciesgroup_birds.html (accessed on 21 August 2024).

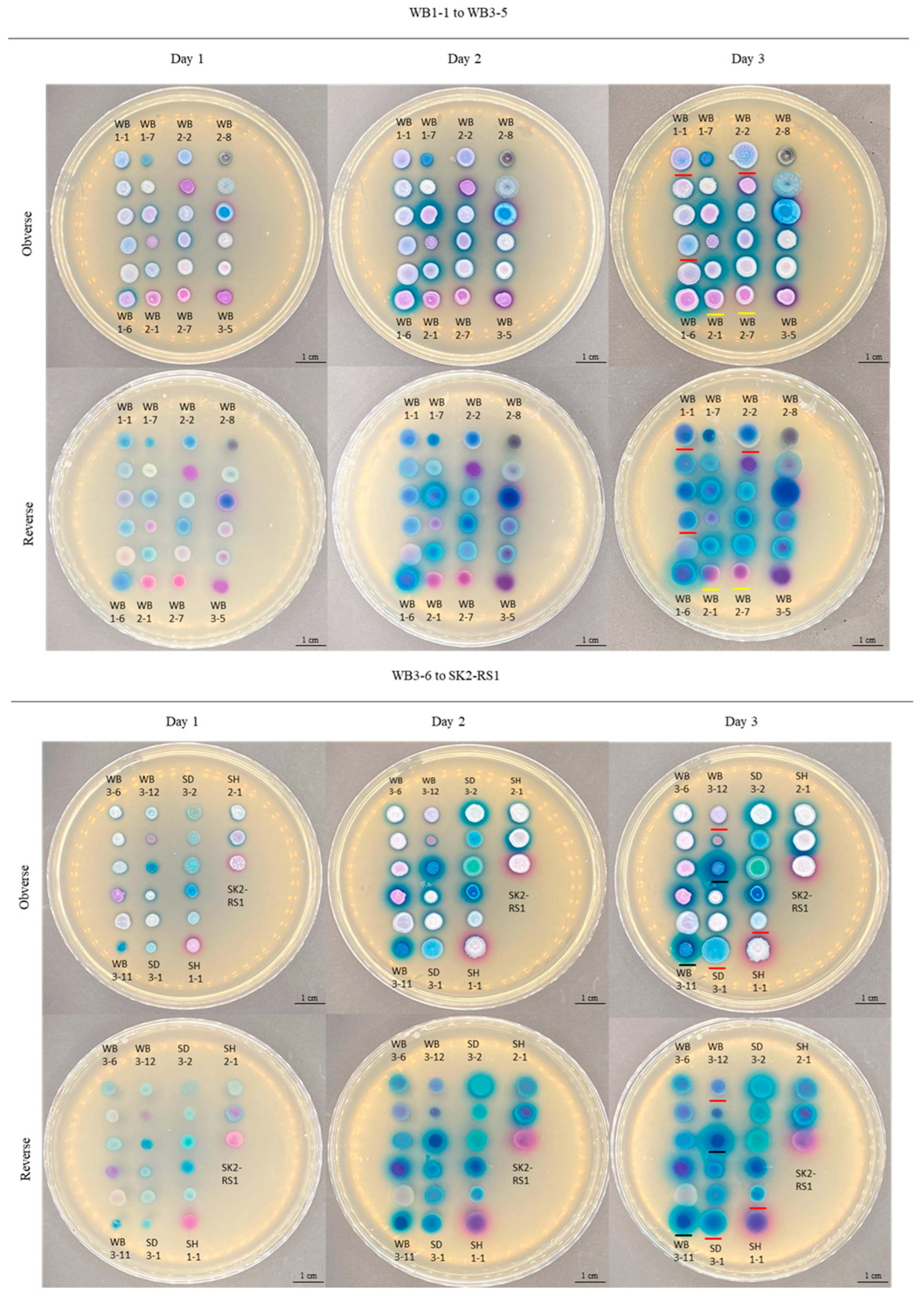
| Location | Sample Name | Colony Color | Presence of Halo and Its Color | Identification by CHROMagar™ Candida Plus | Species Identification by DNA Sequencing | Genus/Clade |
|---|---|---|---|---|---|---|
| Lau Fau Shan | WB1-1 | Light blue | No | Unidentified | Diutina catenulata | Diutina |
| WB1-2 | White or pink | Blue | Unidentified | Candida parapsilosis | Lodderomyces | |
| WB1-3 | White | Blue | Unidentified | Candida metapsilosis | Lodderomyces | |
| WB1-4 | Light blue | No | Unidentified | Diutina catenulata | Diutina | |
| WB1-5 | Pink | No | Unidentified | Candida parapsilosis | Lodderomyces | |
| WB1-6 | Purple | Blue | Unidentified | Candida metapsilosis | Lodderomyces | |
| WB1-7 | Blue | No | Unidentified | Apiotrichum domesticum | Apiotrichum | |
| WB1-8 | White | Blue | Unidentified | Candida parapsilosis | Lodderomyces | |
| WB1-9 | Pink | Blue | Unidentified | Candida metapsilosis | Lodderomyces | |
| WB1-10 | Purple | No | Unidentified | Candida parapsilosis | Lodderomyces | |
| WB1-11 | Pink | Blue | Unidentified | Candida metapsilosis | Lodderomyces | |
| WB2-1 | Pink | No | Unidentified | Wickerhamomyces onychis | Wickerhamomyces | |
| WB2-2 | Blue | No | Unidentified | Diutina catenulata | Diutina | |
| WB2-3 | Purple | Blue | Unidentified | Wickerhamiella tropicalis | Wickerhamiella | |
| WB2-4 | White | Blue | Unidentified | Candida parapsilosis | Lodderomyces | |
| WB2-5 | Purple and blue | Blue | Unidentified | Candida metapsilosis | Lodderomyces | |
| WB2-6 | White or pink | Blue | Unidentified | Candida parapsilosis | Lodderomyces | |
| WB2-7 | Purple | No | Unidentified | Wickerhamomyces onychis | Wickerhamomyces | |
| WB2-8 | Black | No | Unidentified | Exophiala dermatitidis | Exophiala | |
| WB3-1 | Blue | No | Unidentified | Crinitomyces flavificans | Crinitomyces | |
| WB3-2 | Blue | Purple | Candida tropicalis | Candida tropicalis | Lodderomyces | |
| WB3-3 | White | Blue | Unidentified | Candida parapsilosis | Lodderomyces | |
| WB3-4 | White | Blue | Unidentified | Candida parapsilosis | Lodderomyces | |
| WB3-5 | Pink and purple | Purple | Unidentified | Wickerhamiella tropicalis | Wickerhamiella | |
| WB3-6 | White | Blue | Unidentified | Candida parapsilosis | Lodderomyces | |
| WB3-7 | White | Blue | Unidentified | Candida parapsilosis | Lodderomyces | |
| WB3-8 | Pink | Blue | Unidentified | Candida parapsilosis | Lodderomyces | |
| WB3-9 | Purple | Blue | Unidentified | Wickerhamiella martinezcruziae | Wickerhamiella | |
| WB3-10 | White | No | Unidentified | [Candida] mengyuniae | Cyberlindnera | |
| WB3-11 | Blue | Blue | Unidentified | Trichosporon japonicum | Trichosporon | |
| WB3-12 | Light blue | No | Unidentified | Diutina catenulata | Diutina | |
| WB3-13 | Purple | Blue | Unidentified | Rhodotorula mucilaginosa | Rhodotorula | |
| WB3-14 | Blue | Blue | Unidentified | Trichosporon japonicum | Trichosporon | |
| WB3-15 | White | Blue | Unidentified | Candida parapsilosis | Lodderomyces | |
| WB3-16 | White | Blue | Unidentified | Candida parapsilosis | Lodderomyces | |
| Ma Wan | SD3-1 | Blue | No | Unidentified | Diutina catenulata | Diutina |
| SD3-2 | White | Blue | Unidentified | Kluyveromyces aestuarii | Kluyveromyces | |
| Nai Chung | SE1-1 | Blue | No | Unidentified | Crinitomyces ghanaensis | Crinitomyces |
| SE3-1 | Green | Blue | Unidentified | Crinitomyces flavificans | Crinitomyces | |
| Pak Tam Chung | SF2-1 | Blue | Blue | Unidentified | Yamadazyma sp. | Yamadazyma |
| Shui Hau | SG2-1 | Light blue | No | Unidentified | Diutina catenulata | Diutina |
| San Tau | SH1-1 | White and pale pink | Purple | Unidentified | Meyerozyma carpophila * | Meyerozyma |
| SH2-1 | White | Blue | Unidentified | Candida parapsilosis | Lodderomyces | |
| Tai Tam | SJ1-RS1 | White and pale pink | Blue | Unidentified | Kluyveromyces aestuarii | Kluyveromyces |
| Luk Keng | SK2-RS1 | White | Purple | Unidentified | Meyerozyma caribbica * | Meyerozyma |
| Isolates | Identified Species | Minimum Inhibitory Concentration (mg/L) after 24–48 h of Incubation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Polyene | Triazole | Echinocandins | Antimetabolite | ||||||||
| AMB | FLZ | ISZ | ITZ | PSZ | VRZ | ANF | CSF | MFC | 5-FC | ||
| WB1-1 | Diutina catenulata | 0.5 | 2 | 0.008 | 0.06 | 0.03 | 0.06 | 0.03 | 0.5 | 0.03 | 0.25 |
| WB1-2 | Candida parapsilosis | 0.25 | 2 | 0.016 | 0.06 | 0.125 | 0.03 | 2 | 2 | 1 | 0.06 |
| WB1-3 | Candida metapsilosis | 0.25 | 1 | 0.016 | 0.03 | 0.03 | 0.016 | 0.06 | 0.5 | 0.125 | 0.125 |
| WB1-4 | Diutina catenulata | >4 | 2 | 0.008 | 0.06 | 0.016 | 0.06 | 0.03 | 0.25 | 0.016 | 0.25 |
| WB1-5 | Candida parapsilosis | 0.25 | 1 | 0.008 | 0.06 | 0.125 | 0.016 | 2 | 2 | 2 | 0.06 |
| WB1-6 | Candida metapsilosis | 0.25 | 2 | 0.03 | 0.125 | 0.125 | 0.03 | 0.25 | 1 | 0.5 | 0.06 |
| WB1-7 | Apiotrichum domesticum | >4 | 2 | 0.03 | 0.25 | 0.25 | 0.25 | 2 | 0.5 | >4 | 64 |
| WB1-8 | Candida parapsilosis | 0.5 | 2 | 0.03 | 0.06 | 0.125 | 0.03 | 2 | 4 | 2 | 0.125 |
| WB1-9 | Candida metapsilosis | 0.5 | 2 | 0.06 | 0.125 | 0.125 | 0.06 | 0.5 | 0.5 | 0.5 | 0.125 |
| WB1-10 | Candida parapsilosis | 0.25 | 1 | 0.03 | 0.125 | 0.125 | 0.06 | 4 | 1 | 1 | 0.125 |
| WB1-11 | Candida metapsilosis | 0.25 | 1 | 0.016 | 0.125 | 0.125 | 0.03 | 1 | 0.5 | 0.5 | 0.125 |
| WB2-1 | Wickerhamomyces onychis | 0.25 | 4 | 0.25 | 0.25 | 0.5 | 0.06 | 0.06 | 0.125 | 0.125 | 0.125 |
| WB2-2 | Diutina catenulata | 0.25 | 2 | 0.008 | 0.03 | 0.06 | 0.06 | 0.016 | 1 | 0.016 | 0.125 |
| WB2-3 | Wickerhamiella tropicalis | 0.25 | 4 | 0.03 | 0.125 | 0.25 | 0.03 | 0.25 | 0.5 | 0.06 | 0.125 |
| WB2-4 | Candida parapsilosis | 1 | 0.5 | 0.008 | 0.25 | 0.06 | 0.008 | 0.125 | 2 | 1 | 0.125 |
| WB2-5 | Candida metapsilosis | >4 | 1 | 0.016 | 0.25 | 0.25 | 0.03 | 0.25 | 1 | 0.5 | 0.125 |
| WB2-6 | Candida parapsilosis | 0.5 | 0.5 | 0.008 | 0.125 | 0.125 | 0.016 | 0.06 | 2 | 2 | 0.125 |
| WB2-7 | Wickerhamomyces onychis | 0.25 | 2 | 0.125 | 0.5 | 1 | 0.06 | 0.06 | 0.125 | 0.125 | 0.125 |
| WB2-8 | Exophiala dermatitidis | >4 | 8 | 0.25 | 0.25 | 0.06 | 0.06 | >4 | 4 | 4 | >64 |
| WB3-1 | Crinitomyces flavificans | >4 | >32 | 0.008 | 0.016 | 0.5 | 0.03 | 0.06 | 0.25 | 0.125 | 0.25 |
| WB3-2 | Candida tropicalis | 0.5 | 0.5 | 1 | 0.06 | 0.5 | 0.008 | 0.06 | 2 | 1 | 0.125 |
| WB3-3 | Candida parapsilosis | 0.25 | 0.25 | 0.008 | 0.03 | 0.03 | 0.016 | 0.125 | 0.125 | 0.03 | 0.125 |
| WB3-4 | Candida parapsilosis | 0.5 | 0.5 | 0.016 | 0.06 | 0.125 | 0.016 | 2 | 1 | 1 | 0.125 |
| WB3-5 | Wickerhamiella tropicalis | 0.125 | 4 | 0.06 | 0.25 | 0.25 | 0.06 | 1 | 2 | 0.125 | 0.125 |
| WB3-6 | Candida parapsilosis | 0.5 | 0.5 | 0.008 | 0.125 | 0.06 | 0.008 | >4 | 2 | 1 | 0.125 |
| WB3-7 | Candida parapsilosis | 0.5 | 2 | 0.016 | 0.06 | 0.25 | 0.03 | 1 | 2 | 1 | 0.125 |
| WB3-8 | Candida parapsilosis | 0.5 | 1 | 0.03 | 0.125 | 0.125 | 0.016 | 0.5 | 2 | 1 | 0.125 |
| WB3-9 | Wickerhamiella martinezcruziae | 0.25 | 4 | 0.06 | 0.25 | 0.25 | 0.25 | 0.06 | 2 | 0.125 | 0.125 |
| WB3-10 | Candida mengyuniae | 0.25 | 1 | 0.06 | 0.125 | 0.25 | 0.03 | 0.008 | 0.125 | 0.016 | 0.125 |
| WB3-11 | Trichosporon japonicum | >4 | 1 | 0.125 | 0.03 | 0.125 | 0.03 | >4 | >4 | >4 | 4 |
| WB3-12 | Diutina catenulata | 0.25 | 2 | 0.008 | 0.03 | 0.016 | 0.03 | 0.008 | 1 | 0.016 | 0.125 |
| WB3-13 | Rhodotorula mucilaginosa | 0.25 | >32 | 0.06 | 0.06 | 0.25 | 0.06 | 2 | >4 | 0.016 | 0.125 |
| WB3-14 | Trichosporon japonicum | >4 | 1 | 0.03 | 0.25 | 0.125 | 0.016 | >4 | >4 | 0.008 | 1 |
| WB3-15 | Candida parapsilosis | 0.5 | 0.25 | 0.25 | 0.25 | 0.25 | 0.016 | 1 | 1 | 1 | 0.125 |
| WB3-16 | Candida parapsilosis | 0.5 | 0.5 | 0.008 | 0.25 | 0.125 | 0.016 | 1 | 1 | 1 | 0.125 |
| SD3-1 | Diutina catenulata | 0.5 | 4 | 0.016 | 0.06 | 0.06 | 0.06 | 0.008 | 0.5 | 0.03 | 0.125 |
| SD3-2 | Kluyveromyces aestuarii | 0.06 | 1 | 0.016 | 0.25 | 0.06 | 0.016 | 0.03 | 0.125 | 0.008 | 0.125 |
| SE1-1 | Crinitomyces ghanaensis | 0.25 | 16 | 0.125 | 0.125 | 0.125 | 0.06 | 0.016 | 0.125 | 0.008 | 0.125 |
| SE3-1 | Crinitomyces flavificans | >4 | >32 | 0.125 | 0.25 | 0.5 | 0.06 | 0.008 | 0.25 | 0.03 | 0.125 |
| SF2-1 | Yamadazyma sp. | 0.25 | 2 | 0.03 | 0.06 | 0.06 | >4 | 0.003 | 1 | 2 | 0.06 |
| SG2-1 | Diutina catenulata | 0.5 | 4 | 0.008 | 0.03 | 0.016 | 0.03 | 0.008 | 1 | 0.016 | 0.06 |
| SH1-1 | Meyerozyma carpophila | 0.5 | 2 | 0.06 | 0.25 | 0.125 | 0.03 | 1 | 1 | 0.5 | 0.06 |
| SH2-1 | Candida parapsilosis | 0.5 | 1 | 0.016 | 0.125 | 0.125 | 0.03 | 1 | 1 | 1 | 0.125 |
| SJ1-RS1 | Kluyveromyces aestuarii | 0.5 | 2 | 0.03 | 0.016 | 0.016 | 0.016 | 0.016 | 0.125 | 0.03 | 0.06 |
| SK2-RS1 | Meyerozyma caribbica | 1 | 2 | 0.008 | 0.016 | 0.016 | 0.016 | 1 | 0.125 | 0.03 | 0.125 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hau, P.-T.; Shiu, A.; Tam, E.W.-T.; Chau, E.C.-T.; Murillo, M.; Humer, E.; Po, W.-W.; Yu, R.C.-W.; Fung, J.; Seto, S.-W.; et al. Diversity and Antifungal Susceptibilities of Yeasts from Mangroves in Hong Kong, China—A One Health Aspect. J. Fungi 2024, 10, 728. https://doi.org/10.3390/jof10100728
Hau P-T, Shiu A, Tam EW-T, Chau EC-T, Murillo M, Humer E, Po W-W, Yu RC-W, Fung J, Seto S-W, et al. Diversity and Antifungal Susceptibilities of Yeasts from Mangroves in Hong Kong, China—A One Health Aspect. Journal of Fungi. 2024; 10(10):728. https://doi.org/10.3390/jof10100728
Chicago/Turabian StyleHau, Pak-Ting, Anson Shiu, Emily Wan-Ting Tam, Eddie Chung-Ting Chau, Michaela Murillo, Eva Humer, Wai-Wai Po, Ray Chun-Wai Yu, Joshua Fung, Sai-Wang Seto, and et al. 2024. "Diversity and Antifungal Susceptibilities of Yeasts from Mangroves in Hong Kong, China—A One Health Aspect" Journal of Fungi 10, no. 10: 728. https://doi.org/10.3390/jof10100728
APA StyleHau, P.-T., Shiu, A., Tam, E. W.-T., Chau, E. C.-T., Murillo, M., Humer, E., Po, W.-W., Yu, R. C.-W., Fung, J., Seto, S.-W., Tsang, C.-C., & Chow, F. W.-N. (2024). Diversity and Antifungal Susceptibilities of Yeasts from Mangroves in Hong Kong, China—A One Health Aspect. Journal of Fungi, 10(10), 728. https://doi.org/10.3390/jof10100728






