Recent Advances in Diagnostic Approaches for Mucormycosis
Abstract
1. Introduction
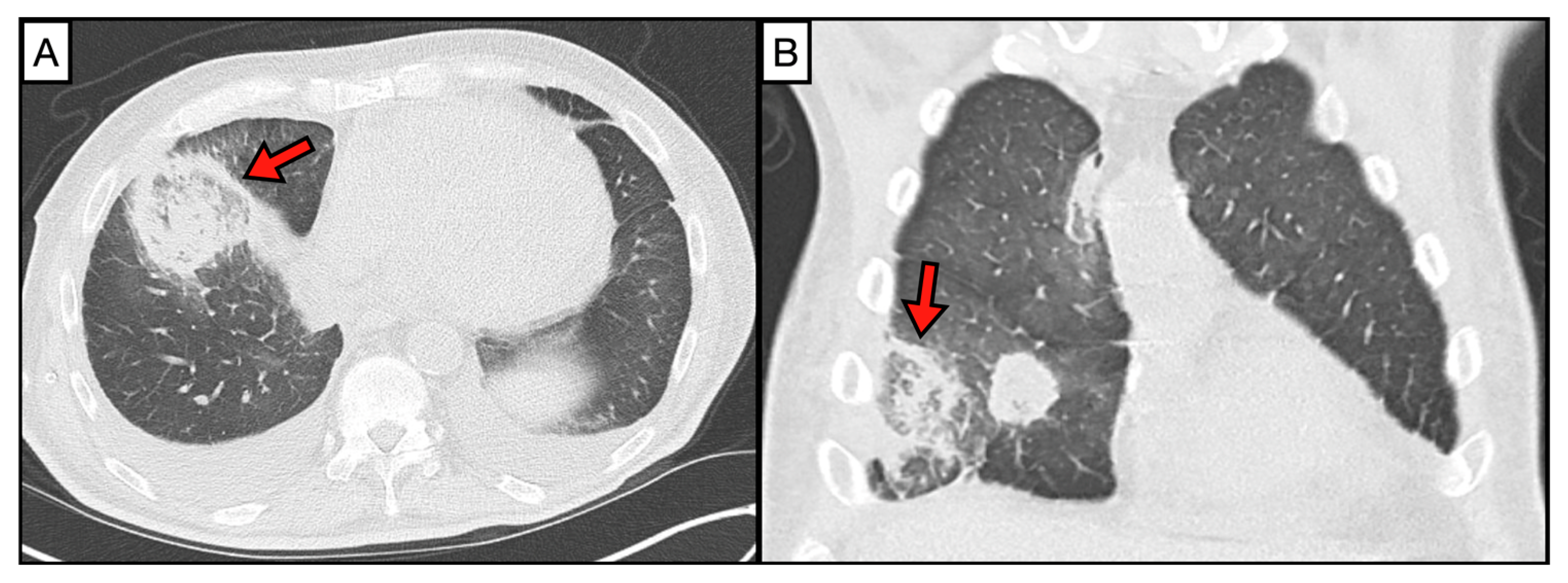
2. Imaging
3. Diagnostic Approaches Based on Morphology
3.1. Direct Microscopy of Clinical Specimens
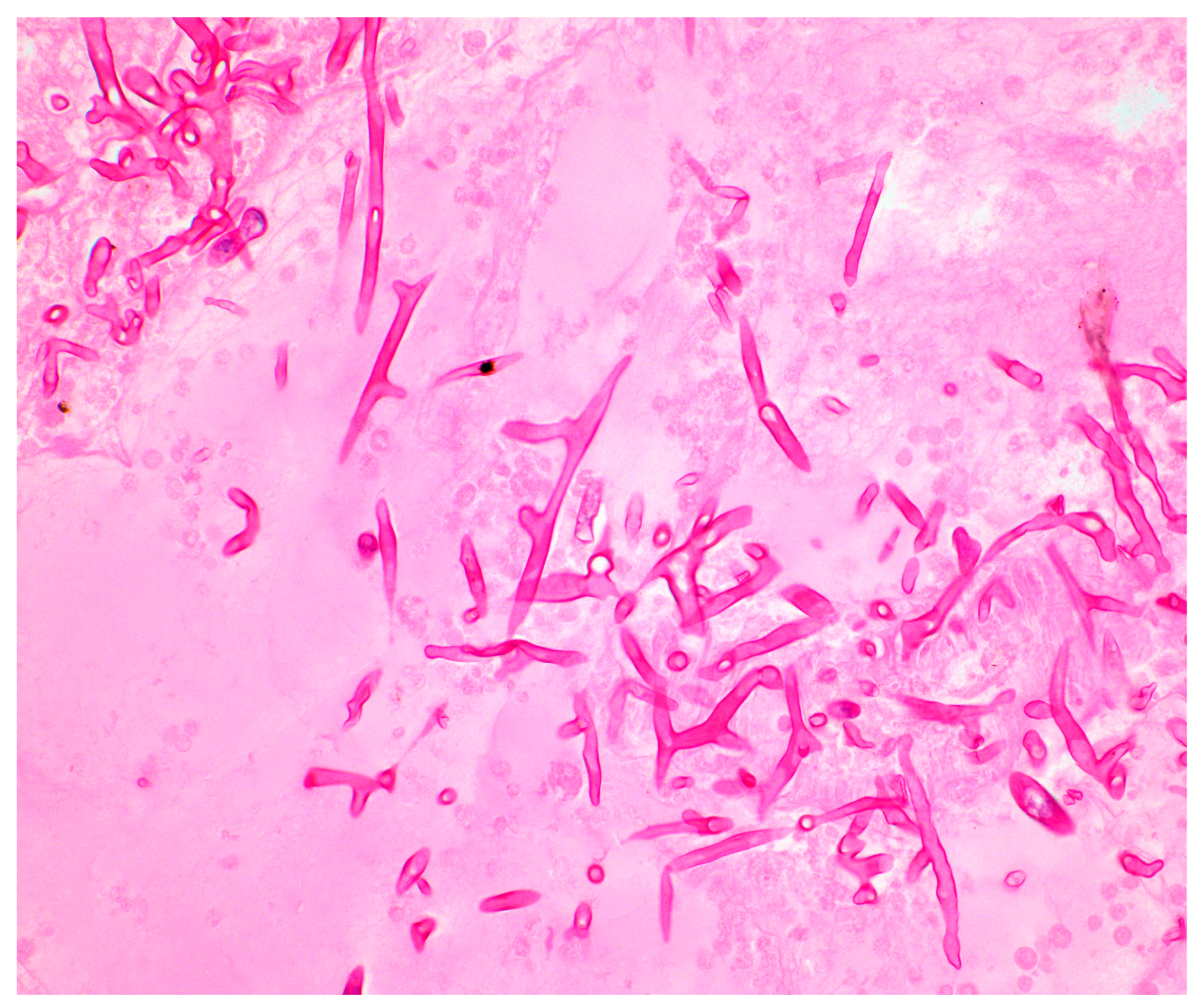
3.2. Histopathology
3.3. Immunohistochemistry
3.4. Fungal Cultures
4. Serology and Biomarkers
5. Molecular Assays
5.1. Molecular Assays in Tissue Samples
5.2. Molecular Diagnosis in Serum/Blood Samples
5.3. Molecular Diagnosis in BAL and Urine Fluids
5.4. Next-Generation Sequencing (NGS)
| Assay Type | Sample Type | Sensitivity (%) | Specificity (%) | Comments |
|---|---|---|---|---|
| Serology | ||||
| ELISA (Rhizopus arrhizus and Rhizomucor pusillus) [50] | Serum | 70–81 | 90–94 | Some cross-reactivity with Aspergillus and Candida. |
| LFIA (α-1,6-mannans) [51] | Serum | 70–90 (experimental) | N/A | Higher sensitivity for Mucorales compared to Aspergillus, Candida, and Fusarium (experimental). |
| LFIA (Fucomannan antigen) [6] | Serum, BAL, Urine | 80–95 (experimental) | N/A | Superior diagnostic performance in murine models. |
| LFIA (15 kDa EPS antigen) [52] | Serum | N/A | N/A | Under investigation for patients with poorly controlled diabetes and on high-dose corticosteroids. |
| Molecular Diagnosis | ||||
| Panfungal PCR (ITS1 region) [56,57] | Fresh tissue | 85–97 | N/A | High sensitivity in fresh tissue. |
| Panfungal PCR (ITS1 region) [56,57] | FFPE tissue | 40–68 | N/A | Lower sensitivity in formalin-fixed paraffin-embedded specimens. |
| Panfungal PCR (18S rRNA sequencing) [62,65] | Tissue | 87–98 | N/A | High sensitivity for 18S rRNA sequencing in clinical samples. |
| In-house panfungal PCR [61,65] | Tissue | 58–90 | 70–80 | Tested on 105 clinical samples for invasive fungal infection. |
| Multiplex qPCR (Mucor/Rhizopus, Lichtheimia, Rhizomucor, Cunninghamella) [70] | Serum | 81–92 | N/A | High-risk patients with hematological malignancy and severe burns. |
| Multiplex qPCR (MODIMUCOR study) [74] | Serum | 85 | 89–90 | Multicenter prospective study qPCR positive before mycological or histopathological examination. |
| qPCR (various assays) [73] | Serum | 79–85 | 85–90 | Different PCR assays on blood samples, high negative predictive value. |
| PCR/HRMA [75] | BAL | 90–100 | 90–93 | High-resolution melt analysis. |
| qPCR [76] | BAL | 90–100 | 95–97 | High sensitivity and specificity for invasive mucormycosis. |
| CotH genes (qPCR) [63] | Urine, BAL, Plasma | 85–90 | 100 | CotH gene target detectable within 24 h of infection in murine models, urine samples particularly reliable. |
| NGS [77,78,81] | Blood, BAL, Urine | 81.3% (BAL), 25% (blood) | N/A | More sensitive in BAL fluid than in blood for pulmonary mucormycosis. |
6. Metabolomics
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Roden, M.M.; Zaoutis, T.E.; Buchanan, W.L.; Knudsen, T.A.; Sarkisova, T.A.; Schaufele, R.L.; Sein, M.; Sein, T.; Chiou, C.C.; Chu, J.H.; et al. Epidemiology and outcome of zygomycosis: A review of 929 reported cases. Clin. Infect. Dis. 2005, 41, 634–653. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.; Keighley, C.; Wolfe, R.; Lee, W.L.; Slavin, M.A.; Kong, D.C.M.; Chen, S.C.-A. The epidemiology and clinical manifestations of mucormycosis: A systematic review and meta-analysis of case reports. Clin. Microbiol. Infect. 2019, 25, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Slavin, M.; van Hal, S.; Sorrell, T.C.; Lee, A.; Marriott, D.J.; Daveson, K.; Kennedy, K.; Hajkowicz, K.; Halliday, C.; Athan, E.; et al. Invasive infections due to filamentous fungi other than Aspergillus: Epidemiology and determinants of mortality. Clin. Microbiol. Infect. 2015, 21, e1–e10. [Google Scholar] [CrossRef] [PubMed]
- Lanternier, F.; Dannaoui, E.; Morizot, G.; Elie, C.; Garcia-Hermoso, D.; Huerre, M.; Bitar, D.; Dromer, F.; Lortholary, O. A Global Analysis of Mucormycosis in France: The RetroZygo Study (2005–2007). Clin. Infect. Dis. 2012, 54, S35–S43. [Google Scholar] [CrossRef] [PubMed]
- Kontoyiannis, D.P.; Yang, H.; Song, J.; Kelkar, S.S.; Yang, X.; Azie, N.; Harrington, R.; Fan, A.; Lee, E.; Spalding, J.R. Prevalence, clinical and economic burden of mucormycosis-related hospitalizations in the United States: A retrospective study. BMC Infect. Dis. 2016, 16, 730. [Google Scholar] [CrossRef]
- Skiada, A.; Pavleas, I.; Drogari-Apiranthitou, M. Epidemiology and Diagnosis of Mucormycosis: An Update. J. Fungi 2020, 6, 265. [Google Scholar] [CrossRef]
- Pasquier, G. COVID-19-associated mucormycosis in India: Why such an outbreak? J. Mycol. Med. 2023, 33, 101393. [Google Scholar] [CrossRef]
- Özbek, L.; Topçu, U.; Manay, M.; Esen, B.H.; Bektas, S.N.; Aydın, S.; Özdemir, B.; Khostelidi, S.N.; Klimko, N.; Cornely, O.; et al. COVID-19–associated mucormycosis: A systematic review and meta-analysis of 958 cases. Clin. Microbiol. Infect. 2023, 29, 722–731. [Google Scholar] [CrossRef]
- Vaughan, C.; Bartolo, A.; Vallabh, N.; Leong, S.C. A meta-analysis of survival factors in rhino-orbital-cerebral mucormycosis—Has anything changed in the past 20 years? Clin. Otolaryngol. 2018, 43, 1454–1464. [Google Scholar] [CrossRef]
- Muthu, V.; Agarwal, R.; Dhooria, S.; Sehgal, I.S.; Prasad, K.T.; Aggarwal, A.N.; Chakrabarti, A. Has the mortality from pulmonary mucormycosis changed over time? A systematic review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 538–549. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Spellberg, B.; Avanessian, V.; Fu, Y.; Edwards, J.E. Rhizopus oryzae Adheres to, Is Phagocytosed by, and Damages Endothelial Cells In Vitro. Infect. Immun. 2005, 73, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Gebremariam, T.; Liu, M.; Luo, G.; Bruno, V.; Phan, Q.T.; Waring, A.J.; Edwards, J.E.; Filler, S.G.; Yeaman, M.R.; Ibrahim, A.S. CotH3 mediates fungal invasion of host cells during mucormycosis. J. Clin. Investig. 2014, 124, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Gebremariam, T.; Lin, L.; Liu, M.; Kontoyiannis, D.P.; French, S.; Edwards, J.E.; Filler, S.G.; Ibrahim, A.S. Bicarbonate correction of ketoacidosis alters host-pathogen interactions and alleviates mucormycosis. J. Clin. Investig. 2016, 126, 2280–2294. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Spellberg, B.; Phan, Q.T.; Fu, Y.; Fu, Y.; Lee, A.S.; Edwards, J.E.; Filler, S.G.; Ibrahim, A.S. The endothelial cell receptor GRP78 is required for mucormycosis pathogenesis in diabetic mice. J. Clin. Investig. 2010, 120, 1914–1924. [Google Scholar] [CrossRef]
- Chamilos, G.; Lewis, R.E.; Kontoyiannis, D.P. Delaying Amphotericin B–Based Frontline Therapy Significantly Increases Mortality among Patients with Hematologic Malignancy Who Have Zygomycosis. Clin. Infect. Dis. 2008, 47, 503–509. [Google Scholar] [CrossRef]
- Bartrum, R.J.; Watnick, M.; Herman, P.G. Roentgenographic findings in pulmonary mucormycosis. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1973, 117, 810–815. [Google Scholar] [CrossRef]
- Chamilos, G.; Marom, E.M.; Lewis, R.E.; Lionakis, M.S.; Kontoyiannis, D.P. Predictors of pulmonary zygomycosis versus invasive pulmonary aspergillosis in patients with cancer. Clin. Infect. Dis. 2005, 41, 60–66. [Google Scholar] [CrossRef]
- Dykhuizen, R.S.; Kerr, K.N.; Soutar, R.L. Air crescent sign and fatal haemoptysis in pulmonary mucormycosis. Scand. J. Infect. Dis. 1994, 26, 498–501. [Google Scholar] [CrossRef]
- Wahba, H.; Truong, M.T.; Lei, X.; Kontoyiannis, D.P.; Marom, E.M. Reversed halo sign in invasive pulmonary fungal infections. Clin. Infect. Dis. 2008, 46, 1733–1737. [Google Scholar] [CrossRef]
- Legouge, C.; Caillot, D.; Chrétien, M.L.; Lafon, I.; Ferrant, E.; Audia, S.; Pagès, P.B.; Roques, M.; Estivalet, L.; Martin, L.; et al. The reversed halo sign: Pathognomonic pattern of pulmonary mucormycosis in leukemic patients with neutropenia? Clin. Infect. Dis. 2014, 58, 672–678. [Google Scholar] [CrossRef]
- DelGaudio, J.M.; Swain, R.E.; Kingdom, T.T.; Muller, S.; Hudgins, P.A. Computed tomographic findings in patients with invasive fungal sinusitis. Arch. Otolaryngol. Head. Neck Surg. 2003, 129, 236–240. [Google Scholar] [CrossRef]
- Shrikrishna, B.H.; Kumbhalkar, S.; Selvi, K.; Deepa, G.; Bidkar, V.; Dabhekar, S.; Prathipati, K.; Sawal, A. Sinonasal and Orbital Imaging Findings in COVID-Associated Rhino-Orbito-Cerebral Mucormycosis During the Second Wave of COVID-19: A Retrospective Cohort Study in a Tertiary Hospital in Central India. Cureus 2023, 15, e42674. [Google Scholar]
- Patil, C.; Kumar, A.; Battula, V.; Kumar, P.; Kollu, R.; Kotamraju, S.; Nethi Balingari, B.L.; Reddy, S.; Ravula, S.; Reddy, A.R. Radiological Manifestations of Rhino-Orbito-Cranial Mucormycosis in COVID-19 Patients Correlated with Pathological and Clinical Outcomes and Emphasis on Magnetic Resonance Imaging-Based Scoring System. Cureus 2023, 15, e35745. [Google Scholar] [CrossRef] [PubMed]
- Hot, A.; Maunoury, C.; Poiree, S.; Lanternier, F.; Viard, J.P.; Loulergue, P.; Coignard, H.; Bougnoux, M.E.; Suarez, F.; Rubio, M.T.; et al. Diagnostic contribution of positron emission tomography with [18F]fluorodeoxyglucose for invasive fungal infections. Clin. Microbiol. Infect. 2011, 17, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Altini, C.; Niccoli Asabella, A.; Ferrari, C.; Rubini, D.; Dicuonzo, F.; Rubini, G. (18)F-FDG PET/CT contribution to diagnosis and treatment response of rhino-orbital-cerebral mucormycosis. Hell. J. Nucl. Med. 2015, 18, 68–70. [Google Scholar] [PubMed]
- Liu, Y.; Wu, H.; Huang, F.; Fan, Z.; Xu, B. Utility of 18F-FDG PET/CT in Diagnosis and Management of Mucormycosis. Clin. Nucl. Med. 2013, 38, e370–e371. [Google Scholar] [CrossRef] [PubMed]
- Song, B.-I. F-18 fluorodeoxyglucose positron emission tomography/computed tomography image of gastric mucormycosis mimicking advanced gastric cancer: A case report. World J. Clin. Cases 2019, 7, 1155–1160. [Google Scholar] [CrossRef]
- Gallo, F.; Vija, L.; Le Grand, S.; Moukarbel, N.; Mortele, K.; Gabiache, E.; Courbon, F.; Tavitian, S.; Dierickx, L.O. Diagnosis of an intestinal mucormycosis ‘fungus ball’ located with PET/CT with [18F] FDG-PET/CT. Eur. J. Hybrid Imaging 2019, 3, 21. [Google Scholar] [CrossRef]
- Dang, C.-J.; Li, Y.-J.; Zhan, F.-H.; Shang, X.-M. The Appearance of Pulmonary Mucormycosis on FDG PET/CT. Clin. Nucl. Med. 2012, 37, 801–803. [Google Scholar] [CrossRef]
- Kung, V.L.; Chernock, R.D.; Burnham, C.-A.D. Diagnostic accuracy of fungal identification in histopathology and cytopathology specimens. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 157–165. [Google Scholar] [CrossRef]
- Cornely, O.A.; Alastruey-Izquierdo, A.; Arenz, D.; Chen, S.C.A.; Dannaoui, E.; Hochhegger, B.; Hoenigl, M.; Jensen, H.E.; Lagrou, K.; Lewis, R.E.; et al. Mucormycosis ECMM MSG Global Guideline Writing Group. Global guideline for the diagnosis and management of mucormycosis: An initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect. Dis. 2019, 19, e405–e421. [Google Scholar] [PubMed]
- Lass-Flörl, C. Zygomycosis: Conventional laboratory diagnosis. Clin. Microbiol. Infect. 2009, 15, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Frater, J.L.; Hall, G.S.; Procop, G.W. Histologic Features of Zygomycosis: Emphasis on Perineural Invasion and Fungal Morphology. Arch. Pathol. Lab. Med. 2001, 125, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Guarner, J.; Brandt, M.E. Histopathologic diagnosis of fungal infections in the 21st century. Clin. Microbiol. Rev. 2011, 24, 247–280. [Google Scholar] [CrossRef]
- Sangoi, A.R.; Rogers, W.M.; Longacre, T.A.; Montoya, J.G.; Baron, E.J.; Banaei, N. Challenges and pitfalls of morphologic identification of fungal infections in histologic and cytologic specimens: A Ten-Year retrospective review at a single institution. Am. J. Clin. Pathol. 2009, 131, 364–375. [Google Scholar] [CrossRef]
- Pagano, L.; Dragonetti, G.; de Carolis, E.; Veltri, G.; Del Principe, M.I.; Busca, A. Developments in identifying and managing mucormycosis in hematologic cancer patients. Expert. Rev. Hematol. 2020, 13, 895–905. [Google Scholar] [CrossRef]
- Kamo, Y.K.; Igarashi, H.; Sugimura, H. Modification of Grocott’s staining procedure with heat treatment and oxidation by periodic acid for mucormycosis in tissue: A method to detect Mucor spp. Biotechniques 2023, 74, 143–147. [Google Scholar] [CrossRef]
- Tome, Y.; Hayashi, I.; Matsuoka, S.; Yamamoto, U.; Upton, M.P.; Hirohashi, S.; Shimosato, Y. A simple and highly reproducible staining method for fungi and polysaccharide-rich microorganisms in animal tissues. Stain Technol. 1988, 63, 53–57. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, S.W.; Hwang, S.H. Usefulness of intraoperative frozen section for diagnosing acute invasive fungal rhinosinusitis: A systematic review and meta-analysis. Int. Forum Allergy Rhinol. 2021, 11, 1347–1354. [Google Scholar] [CrossRef]
- Choi, S.; Song, J.S.; Kim, J.Y.; Cha, H.H.; Yun, J.H.; Park, J.W.; Jung, K.H.; Jo, K.M.; Jung, J.; Kim, M.J.; et al. Diagnostic performance of immunohistochemistry for the aspergillosis and mucormycosis. Mycoses 2019, 62, 1006–1014. [Google Scholar] [CrossRef]
- Son, H.J.; Song, J.S.; Choi, S.; Jung, J.; Kim, M.J.; Chong, Y.P.; Lee, S.O.; Choi, S.H.; Kim, Y.S.; Woo, J.H.; et al. A comparison of histomorphologic diagnosis with culture- and immunohistochemistry-based diagnosis of invasive aspergillosis and mucormycosis. Infect. Dis. 2020, 52, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Dalimot, J.J.; Smith, I.M.C.; Gerkrath, J.; Hartmann, S.; Cornely, O.A.; Lee, S.C.; Heitman, J.; Rickerts, V. Identification of Mucormycosis by Fluorescence In Situ Hybridization Targeting Ribosomal RNA in Tissue Samples. J. Fungi 2022, 8, 289. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Song, Y.; Li, R. The use of combined PCR, fluorescence in situ hybridisation and immunohistochemical staining to diagnose mucormycosis from formalin-fixed paraffin-embedded tissues. Mycoses 2021, 64, 1460–1470. [Google Scholar] [CrossRef] [PubMed]
- Branscomb, R. An Overview of Mucormycosis. Lab. Med. 2002, 33, 453–455. [Google Scholar] [CrossRef]
- Chayakulkeeree, M.; Ghannoum, M.A.; Perfect, J.R. Zygomycosis: The re-emerging fungal infection. Eur. J. Clin. Microbiol. Infect. Dis. 2006, 25, 215–229. [Google Scholar] [CrossRef]
- Morrissey, C.O.; Kim, H.Y.; Garnham, K.; Dao, A.; Chakrabarti, A.; Perfect, J.R.; Alastruey-Izquierdo, A.; Harrison, T.S.; Bongomin, F.; Galas, M.; et al. Mucorales: A systematic review to inform the World Health Organization priority list of fungal pathogens. Med. Mycol. 2024, 62, myad130. [Google Scholar] [CrossRef]
- Lewis, J.S., 2nd; Wiederhold, N.P.; Hakki, M.; Thompson, G.R., 3rd. New Perspectives on Antimicrobial Agents: Isavuconazole. Antimicrob. Agents Chemother. 2022, 66, e00177-22. [Google Scholar] [CrossRef]
- Ostrosky-Zeichner, L.; Alexander, B.D.; Kett, D.H.; Vazquez, J.; Pappas, P.G.; Saeki, F.; Ketchum, P.A.; Wingard, J.; Schiff, R.; Tamura, H.; et al. Multicenter Clinical Evaluation of the (1→3) beta-D-Glucan Assay as an Aid to Diagnosis of Fungal Infections in Humans. Clin. Infect. Dis. 2005, 41, 654–659. [Google Scholar] [CrossRef]
- Kędzierska, A.; Kochan, P.; Pietrzyk, A.; Kȩdzierska, J. Current status of fungal cell wall components in the immunodiagnostics of invasive fungal infections in humans: Galactomannan, mannan and (1→3) beta-D-glucan antigens. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 755–766. [Google Scholar] [CrossRef]
- Jensen, H.E.; Salonen, J.; Ekfors, T.O. The use of immunohistochemistry to improve sensitivity and specificity in the diagnosis of systemic mycoses in patients with haematological malignancies. J. Pathol. 1997, 181, 100–105. [Google Scholar] [CrossRef]
- Burnham-Marusich, A.R.; Hubbard, B.; Kvam, A.J.; Gates-Hollingsworth, M.; Green, H.R.; Soukup, E.; Limper, A.H.; Kozel, T.R. Conservation of Mannan Synthesis in Fungi of the Zygomycota and Ascomycota Reveals a Broad Diagnostic Target. mSphere 2018, 3, e00094-18. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.E.; Thornton, C.R. Development of a Monoclonal Antibody and a Serodiagnostic Lateral-Flow Device Specific to Rhizopus arrhizus (Syn. R. oryzae), the Principal Global Agent of Mucormycosis in Humans. J. Fungi 2022, 8, 756. [Google Scholar] [CrossRef] [PubMed]
- Jensen, H.E. Histopathology in the Diagnosis of Invasive Fungal Diseases. Curr. Fungal Infect. Rep. 2021, 15, 23–31. [Google Scholar] [CrossRef]
- Sadamoto, S.; Mitsui, Y.; Nihonyanagi, Y.; Amemiya, K.; Shinozaki, M.; Murayama, S.Y.; Abe, M.; Umeyama, T.; Tochigi, N.; Miyazaki, Y.; et al. Comparison Approach for Identifying Missed Invasive Fungal Infections in Formalin-Fixed, Paraffin-Embedded Autopsy Specimens. J. Fungi 2022, 8, 337. [Google Scholar] [CrossRef]
- Rickerts, V.; Just-Nübling, G.; Konrad, F.; Kern, J.; Lambrecht, E.; Böhme, A.; Jacobi, V.; Bialek, R. Diagnosis of invasive aspergillosis and mucormycosis in immunocompromised patients by seminested PCR assay of tissue samples. Eur. J. Clin. Microbiol. Infect. Dis. 2006, 25, 8–13. [Google Scholar] [CrossRef]
- Lau, A.; Chen, S.; Sorrell, T.; Carter, D.; Malik, R.; Martin, P.; Halliday, C. Development and Clinical Application of a Panfungal PCR Assay To Detect and Identify Fungal DNA in Tissue Specimens. J. Clin. Microbiol. 2007, 45, 380–385. [Google Scholar] [CrossRef]
- Dannaoui, E. Recent Developments in the Diagnosis of Mucormycosis. J. Fungi 2022, 8, 457. [Google Scholar] [CrossRef]
- Lackner, N.; Posch, W.; Lass-Flörl, C. Microbiological and molecular diagnosis of mucormycosis: From old to new. Microorganisms 2021, 9, 1518. [Google Scholar] [CrossRef]
- Valero, C.; de la Cruz-Villar, L.; Zaragoza, Ó.; Buitrago, M.J. New Panfungal Real-Time PCR Assay for Diagnosis of Invasive Fungal Infections. J. Clin. Microbiol. 2016, 54, 2910–2918. [Google Scholar] [CrossRef]
- Gade, L.; Hurst, S.; Balajee, S.A.; Lockhart, S.R.; Litvintseva, A.P. Detection of mucormycetes and other pathogenic fungi in formalin fixed paraffin embedded and fresh tissues using the extended region of 28S rDNA. Med. Mycol. 2017, 55, 385–395. [Google Scholar] [CrossRef]
- Camp, I.; Manhart, G.; Schabereiter-Gurtner, C.; Spettel, K.; Selitsch, B.; Willinger, B. Clinical evaluation of an in-house panfungal real-time PCR assay for the detection of fungal pathogens. Infection 2020, 48, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, S.; Kumari, P.; Gupta, D. Utility of Panfungal PCR in the diagnosis of invasive fungal infections in febrile neutropenia. J. Family Med. Prim. Care 2021, 10, 2533–2540. [Google Scholar] [CrossRef] [PubMed]
- Baldin, C.; Soliman, S.S.M.; Jeon, H.H.; Alkhazraji, S.; Gebremariam, T.; Gu, Y.; Bruno, V.M.; Cornely, O.A.; Leather, H.L.; Sugrue, M.W.; et al. PCR-Based Approach Targeting Mucorales-Specific Gene Family for Diagnosis of Mucormycosis. J. Clin. Microbiol. 2018, 56, e00746-18. [Google Scholar] [CrossRef] [PubMed]
- Kwon-Chung, K.J.; Bennett, J.E. Medical mycology. Med. Mycol. 1992, 34, 504. [Google Scholar] [CrossRef]
- Zeller, I.; Schabereiter-Gurtner, C.; Mihalits, V.; Selitsch, B.; Barousch, W.; Hirschl, A.M.; Makristathis, A.; Willinger, B. Detection of fungal pathogens by a new broad range real-time PCR assay targeting the fungal ITS2 region. J. Med. Microbiol. 2017, 66, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Hammond, S.P.; Bialek, R.; Milner, D.A.; Petschnigg, E.M.; Baden, L.R.; Marty, F.M. Molecular methods to improve diagnosis and identification of mucormycosis. J. Clin. Microbiol. 2011, 49, 2151–2153. [Google Scholar] [CrossRef]
- Baldin, C.; Ibrahim, A.S. Molecular mechanisms of mucormycosis—The bitter and the sweet. PLoS Pathog. 2017, 13, e1006408. [Google Scholar] [CrossRef]
- Legrand, M.; Gits-Muselli, M.; Boutin, L.; Garcia-Hermoso, D.; Maurel, V.; Soussi, S.; Benyamina, M.; Ferry, A.; Chaussard, M.; Hamane, S.; et al. Detection of Circulating Mucorales DNA in Critically Ill Burn Patients: Preliminary Report of a Screening Strategy for Early Diagnosis and Treatment. Clin. Infect. Dis. 2016, 63, 1312–1317. [Google Scholar] [CrossRef]
- Caillot, D.; Valot, S.; Lafon, I.; Basmaciyan, L.; Chretien, M.L.; Sautour, M.; Millon, L.; Legouge, C.; Payssot, A.; Dalle, F. Is It Time to Include CT “Reverse Halo Sign” and qPCR Targeting Mucorales in Serum to EORTC-MSG Criteria for the Diagnosis of Pulmonary Mucormycosis in Leukemia Patients? Open Forum Infect. Dis. 2016, 3, ofw190. [Google Scholar] [CrossRef]
- Millon, L.; Larosa, F.; Lepiller, Q.; Legrand, F.; Rocchi, S.; Daguindau, E.; Scherer, E.; Bellanger, A.-P.; Leroy, J.; Grenouillet, F. Quantitative Polymerase Chain Reaction Detection of Circulating DNA in Serum for Early Diagnosis of Mucormycosis in Immunocompromised Patients. Clin. Infect. Dis. 2013, 56, e95–e101. [Google Scholar] [CrossRef]
- Millon, L.; Herbrecht, R.; Grenouillet, F.; Morio, F.; Alanio, A.; Letscher-Bru, V.; Cassaing, S.; Chouaki, T.; Kauffmann-Lacroix, C.; Poirier, P.; et al. Early diagnosis and monitoring of mucormycosis by detection of circulating DNA in serum: Retrospective analysis of 44 cases collected through the French Surveillance Network of Invasive Fungal Infections (RESSIF). Clin. Microbiol. Infect. 2016, 22, e1–e810. [Google Scholar] [CrossRef] [PubMed]
- Coste, A.; Conrad, A.; Porcher, R.; Poirée, S.; Peterlin, P.; Defrance, C.; Letscher-Bru, V.; Morio, F.; Gastinne, T.; Bougnoux, M.-E.; et al. Improving Diagnosis of Pulmonary Mucormycosis: Leads From a Contemporary National Study of 114 Cases. Chest 2023, 164, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Rompally, S.; Choudary, H.; Rudramurthy, S.M.; Kaur, H.; Bansal, D.; Malhotra, P.; Narayana Yaddanapudi, L.; Chakrabarti, A. P407 Standardization of PCR for the diagnosis of invasive aspergillosis and invasive mucormycosis from blood samples. Med. Mycol. 2022, 60, myac072P407. [Google Scholar] [CrossRef]
- Millon, L.; Caillot, D.; Berceanu, A.; Bretagne, S.; Lanternier, F.; Morio, F.; Letscher-Bru, V.; Dalle, F.; Denis, B.; Alanio, A.; et al. Evaluation of Serum Mucorales Polymerase Chain Reaction (PCR) for the Diagnosis of Mucormycoses: The MODIMUCOR Prospective Trial. Clin. Infect. Dis. 2022, 75, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Lengerova, M.; Racil, Z.; Hrncirova, K.; Kocmanova, I.; Volfova, P.; Ricna, D.; Bejdak, P.; Moulis, M.; Pavlovsky, Z.; Weinbergerova, B.; et al. Rapid Detection and Identification of Mucormycetes in Bronchoalveolar Lavage Samples from Immunocompromised Patients with Pulmonary Infiltrates by Use of High-Resolution Melt Analysis. J. Clin. Microbiol. 2014, 52, 2824–2828. [Google Scholar] [CrossRef]
- Scherer, E.; Iriart, X.; Bellanger, A.P.; Dupont, D.; Guitard, J.; Gabriel, F.; Cassaing, S.; Charpentier, E.; Guenounou, S.; Cornet, M.; et al. Quantitative PCR (qPCR) Detection of Mucorales DNA in Bronchoalveolar Lavage Fluid To Diagnose Pulmonary Mucormycosis. J. Clin. Microbiol. 2018, 56, e00289-18. [Google Scholar] [CrossRef]
- Deurenberg, R.H.; Bathoorn, E.; Chlebowicz, M.A.; Couto, N.; Ferdous, M.; García-Cobos, S.; Kooistra-Smid, A.M.D.; Raangs, E.C.; Rosema, S.; Veloo, A.C.M.; et al. Application of next generation sequencing in clinical microbiology and infection prevention. J. Biotechnol. 2017, 243, 16–24. [Google Scholar] [CrossRef]
- Miao, Q.; Ma, Y.; Wang, Q.; Pan, J.; Zhang, Y.; Jin, W.; Yao, Y.; Su, Y.; Huang, Y.; Wang, M.; et al. Microbiological Diagnostic Performance of Metagenomic Next-generation Sequencing When Applied to Clinical Practice. Clin. Infect. Dis. 2018, 67, S231–S240. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, X.; Liu, Y.; Wang, H.; Zhao, R.; Lv, X.; Wei, X.; Zhou, K.S. Nasal and cutaneous mucormycosis in two patients with lymphoma after chemotherapy and target therapy: Early detection by metagenomic next-generation sequencing. Front. Cell Infect. Microbiol. 2022, 12, 12–960766. [Google Scholar] [CrossRef]
- Liu, M.; Bruni, G.O.; Taylor, C.M.; Zhang, Z.; Wang, P. Comparative genome-wide analysis of extracellular small RNAs from the mucormycosis pathogen Rhizopus delemar. Sci. Rep. 2018, 8, 5243. [Google Scholar] [CrossRef]
- Chen, X.; Ding, S.; Lei, C.; Qin, J.; Guo, T.; Yang, D.; Yang, M.; Qing, J.; He, W.; Song, M.; et al. Blood and Bronchoalveolar Lavage Fluid Metagenomic Next-Generation Sequencing in Pneumonia. Can. J. Infect. Dis. Med. Microbiol. 2020, 2020, 6839103. [Google Scholar] [CrossRef] [PubMed]
- Weiss, Z.F.; Leon, A.; Koo, S. The evolving landscape of fungal diagnostics, current and emerging microbiological approaches. J. Fungi 2021, 7, 127. [Google Scholar] [CrossRef] [PubMed]
- Blauwkamp, T.A.; Thair, S.; Rosen, M.J.; Blair, L.; Lindner, M.S.; Vilfan, I.D.; Kawli, T.; Christians, F.C.; Venkatasubrahmanyam, S.; Wall, G.D.; et al. Analytical and clinical validation of a microbial cell-free DNA sequencing test for infectious disease. Nat. Microbiol. 2019, 4, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Keller, N.P.; Turner, G.; Bennett, J.W. Fungal secondary metabolism—From biochemistry to genomics. Nat. Rev. Microbiol. 2005, 3, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Acharige, M.J.T.; Koshy, S.; Ismail, N.; Aloum, O.; Jazaerly, M.; Astudillo, C.L.; Koo, S. Breath-based diagnosis of fungal infections. J. Breath Res. 2018, 12, 027108. [Google Scholar] [CrossRef]
- Koshy, S.; Ismail, N.; Astudillo, C.L.; Haeger, C.M.; Aloum, O.; Acharige, M.T.; Farmakiotis, D.; Baden, L.R.; Marty, F.M.; Kontoyiannis, D.P.; et al. Breath-Based Diagnosis of Invasive Mucormycosis (IM). Open Forum Infect. Dis. 2017, 4, S53–S54. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safiia, J.; Díaz, M.A.; Alshaker, H.; Atallah, C.J.; Sakr, P.; Moshovitis, D.G.; Nawlo, A.; Franceschi, A.E.; Liakos, A.; Koo, S. Recent Advances in Diagnostic Approaches for Mucormycosis. J. Fungi 2024, 10, 727. https://doi.org/10.3390/jof10100727
Safiia J, Díaz MA, Alshaker H, Atallah CJ, Sakr P, Moshovitis DG, Nawlo A, Franceschi AE, Liakos A, Koo S. Recent Advances in Diagnostic Approaches for Mucormycosis. Journal of Fungi. 2024; 10(10):727. https://doi.org/10.3390/jof10100727
Chicago/Turabian StyleSafiia, Jawad, Marco Aurelio Díaz, Hassan Alshaker, Christine J. Atallah, Paul Sakr, Dimitrios G. Moshovitis, Ahmad Nawlo, Andres E. Franceschi, Alexis Liakos, and Sophia Koo. 2024. "Recent Advances in Diagnostic Approaches for Mucormycosis" Journal of Fungi 10, no. 10: 727. https://doi.org/10.3390/jof10100727
APA StyleSafiia, J., Díaz, M. A., Alshaker, H., Atallah, C. J., Sakr, P., Moshovitis, D. G., Nawlo, A., Franceschi, A. E., Liakos, A., & Koo, S. (2024). Recent Advances in Diagnostic Approaches for Mucormycosis. Journal of Fungi, 10(10), 727. https://doi.org/10.3390/jof10100727






