Overview of the Current Challenges in Pulmonary Coccidioidomycosis
Abstract
1. Introduction
2. Biology and Ecology
Current Challenges
- Inability to detect rodents infected with the disease to control the spread of coccidioidomycosis;
- Incomplete understanding of the environmental reservoir of Coccidioides in soil.
3. Epidemiology
4. Pathophysiology
Current Challenges
- Difficulty in detecting arthroconidia in the air to predict outbreaks and create a preventive measure [24];
- High-risk occupations in the endemic areas have developed some preventive measures such as watering construction sites, paving roads, or planting grass, but none of these measures are supported by data [19];
- There are no robust tools to track the source of the occupational outbreaks;
- Limited guidance about high-risk postexposure prophylaxis.
5. Immunology of Coccidioidomycosis
Current Challenges
- Patients who develop severe or disseminated disease without clear immunological defects are not being investigated properly in a daily clinical setting;
- There is an essential need to better understand the immune response to improve the identification of high-risk patients;
- There is unclear guidance for patients who live in endemic areas and take immunosuppressive drugs as to whether they should take chronic antifungal medication.
6. Clinical Manifestation of Coccidioidomycosis
6.1. Pulmonary Coccidioidomycosis Manifestations
6.2. Asymptomatic or Mild Respiratory Tract Infection
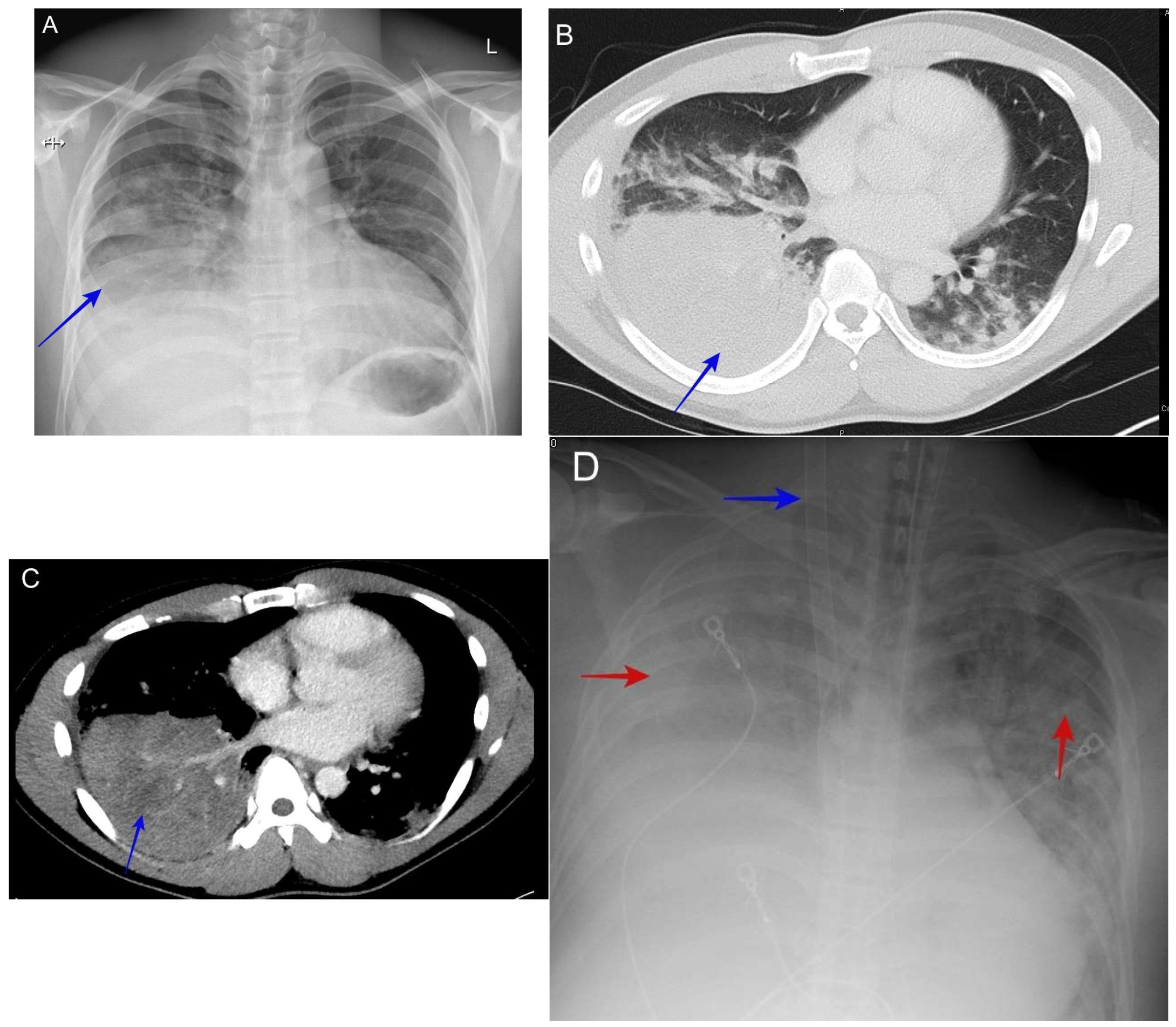
6.3. Acute Pneumonia Resembling Community-Acquired Pneumonia
6.4. Pleuritic Chest Pain and Effusion
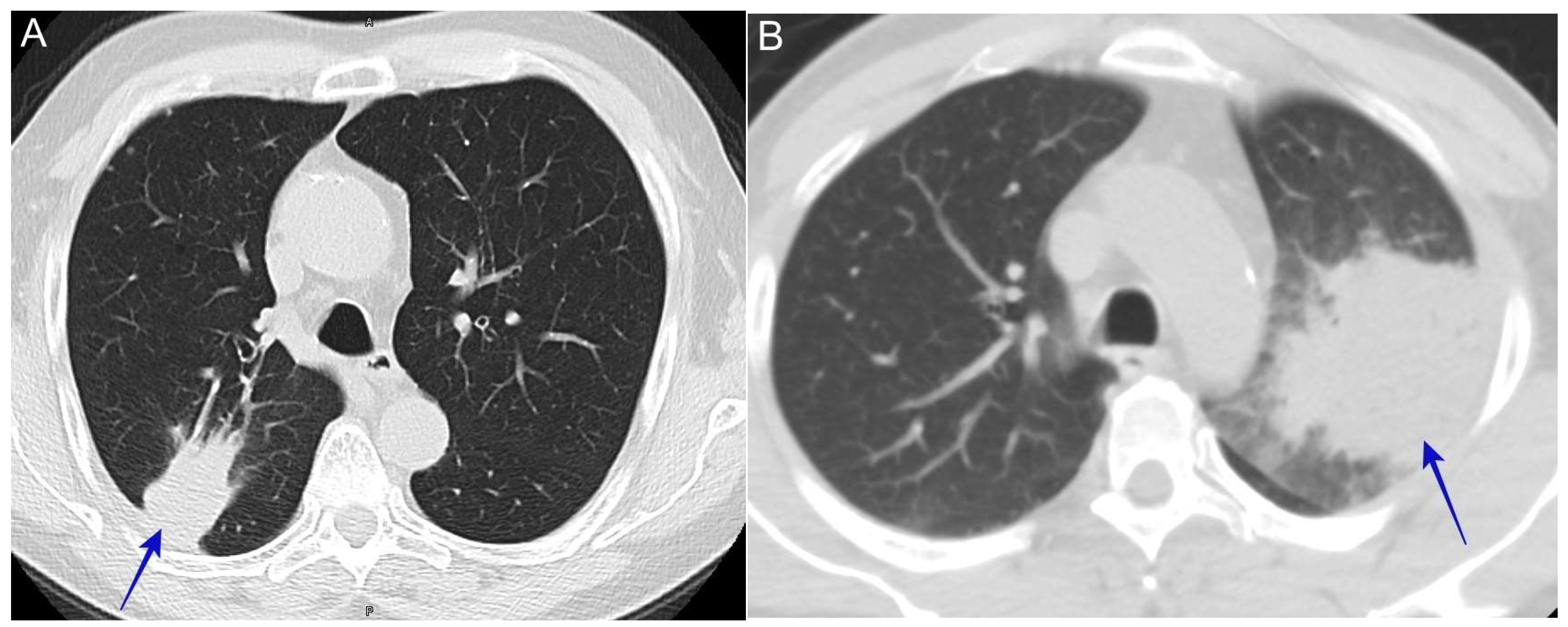
6.5. Lung Mass
6.6. Pulmonary Cavitary Disease
6.7. Severe Pneumonia with Diffuse Reticulonodular Opacities (Miliary Nodular Pattern)
6.8. Severe Pneumonia with Acute Respiratory Distress Syndrome (ARDS)
6.9. Complicated Pyopneumothorax or Hydropneumothorax
6.10. Lung Nodule
6.11. Post-Coccidioidomycosis Syndrome (Fatigue)
6.12. Current Challenges
- Multiple different manifestations make diagnosis difficult;
- Differentiating bacterial pneumonia from coccidioidomycosis;
- Differentiated lung nodules due to Coccidioides vs. malignancy.
6.13. Extrapulmonary Manifestation
6.14. Diagnostic Tools
6.14.1. Serology
6.14.2. Enzyme Immunoassay (EIA)
6.14.3. Immunodiffusion-Based (ID)
6.14.4. Tube Precipitin-Type (TP) Antibodies
6.14.5. Complement Fixation (CF)
6.14.6. Lateral Flow Assay (LFA)
6.14.7. Antigen Detection
6.14.8. Cultures
6.15. Molecular Methods
6.15.1. Polymerase Chain Reaction (PCR)
6.15.2. Metagenomic Next-Generation Sequencing (NGS)
6.15.3. Pathology
6.16. Current Challenges
- Different Coccidioides tests with their own sensitivity and specificity makes it challenging for physicians to order and interpret the testing;
- Lack of testing for coccidioidomycosis in outpatient care settings within a region endemic for coccidioidomycosis.
6.17. Making Accurate Assessments
6.18. Suspecting the Diagnosis
6.19. Ordering the Correct Testing
6.20. Management of Coccidioidomycosis
6.21. Identifying High-Risk Patients for Initial Therapies
6.22. Medical Management of Acute Coccidioidomycosis
6.22.1. Level of Care
6.22.2. Outpatient Therapy (Mild or Moderate Disease)
6.23. Inpatient Therapy (Severe Disease)
6.24. Critical Care Management (Serious or Critical Disease)
6.25. Current Challenges
- Clinicians lack evidence-based therapy options for patients who do not have high risk factors for complications;
- Outpatient therapy and monitoring are challenging given the paucity of specialized clinics for coccidioidomycosis;
- Data of the newer generation of triazole therapies are limited;
- There is no available therapy to boost the immune system against coccidioidomycosis in critically ill patients who develop disseminated diseases;
- Side effects and toxicity during the ICU are much more common compared to outpatient or regular inpatient therapies.
6.26. Surgical Management of Pulmonary Coccidioidomycosis
6.27. Acute Indications for Surgical Intervention
6.28. Current Challenges
- Surgical intervention for pulmonary coccidioidomycosis is usually unclear to many physicians and surgeons and a lot of times the surgery is offered too early or too late;
- Lifelong antifungal therapy for chronic pulmonary coccidioidomycosis can have an alternative option, which is surgical intervention, but some of these patients have uncontrolled diabetes, which should be managed well (mainly because of the lack of available primary care) prior to surgical intervention.
6.29. Post-Coccidioidomycosis Sequelae Management
6.29.1. Monitoring the Disease
6.29.2. Management of Incidental Lung Nodules
6.29.3. Post-Coccidioidomycosis Fatigue Management
6.29.4. Post-Coccidioidomycosis Psychological Impact
6.30. Current Challenges
- Commonly used lung nodule calculators for lung cancer overestimate the risk of lung cancer in areas where coccidioidomycosis is endemic [52]. Therefore, new risk stratification tools for use in these endemic areas are required;
- The natural history of lung nodules due to coccidioidomycosis is unclear and there is no clear guidance about how long it should be monitored radiologically;
- There are generally no clear guidelines for follow-up after acute infection or evidence-based approaches for managing post-coccidioidomycosis fatigue or chronic pulmonary coccidioidomycosis.
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benedict, K.; McCotter, O.Z.; Brady, S.; Komatsu, K.; Sondermeyer-Cooksey, G.L.; Nguyen, A.; Jain, S.; Vugia, D.J.; Jackson, B.R. Surveillance for coccidioidomycosis—United States, 2011–2017. MMWR 2019, 68, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Control and Prevention. Valley Fever Map. Available online: https://www.cdc.gov/fungal/pdf/more-information-about-fungal-maps-508.pdf (accessed on 11 October 2024).
- Gorris, M.E.; Treseder, K.K.; Zender, C.S.; Randerson, J.T. Expansion of Coccidioidomycosis Endemic Regions in the United States in Response to Climate Change. Geohealth 2019, 3, 308–327. [Google Scholar] [CrossRef] [PubMed]
- Deresinski, S.; Mirels, L.F. Coccidioidomycosis: What a long strange trip it’s been. Med. Mycol. 2019, 57, S3–S15. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, T.N.; Stevens, D.A.; Hung, C.-Y.; Beyhan, S.; Taylor, J.W.; Shubitz, L.F.; Duttke, S.H.; Heidari, A.; Johnson, R.H.; Deresinski, S.C.; et al. Coccidioides Species: A Review of Basic Research: 2022. J. Fungi. 2022, 8, 859. [Google Scholar] [CrossRef]
- Sharpton, T.J.; Stajich, J.E.; Rounsley, S.D.; Gardner, M.J.; Wortman, J.R.; Jordar, V.S.; Maiti, R.; Kodira, C.D.; Neafsey, D.E.; Zeng, Q.; et al. Comparative genomic analyses of the human fungal pathogens Coccidioides and their relatives. Genome Res. 2009, 19, 1722–1731. [Google Scholar] [CrossRef]
- Koufopanou, V.; Burt, A.; Taylor, J.W. Concordance of gene genealogies reveals reproductive isolation in the pathogenic fungus Coccidioides immitis. Proc. Natl. Acad. Sci. USA 1997, 94, 5478–5482. [Google Scholar] [CrossRef]
- Fisher, M.C.; Koenig, G.L.; White, T.J.; Taylor, J.W. Molecular and phenotypic description of Coccidioides posadasii sp. nov., previously recognized as the non-California population of Coccidioides immitis. Mycologia 2002, 94, 73–84. [Google Scholar] [CrossRef]
- Lewis, E.R.G.; Bowers, J.R.; Barker, B.M. Dust devil: The life and times of the fungus that causes valley Fever. PLoS Pathog. 2015, 11, 5. [Google Scholar] [CrossRef]
- Taylor, J.W.; Barker, B.M. The endozoan, small-mammal reservoir hypothesis and the life cycle of Coccidioides species. Med. Mycol. 2019, 57, S16–S20. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Increase in reported coccidioidomycosis—United States, 1998–2011. MMWR Morb. Mortal Wkly. Rep. 2013, 62, 217–221. [Google Scholar]
- Available online: https://www.cdc.gov/valley-fever/php/statistics/index.html (accessed on 11 October 2024).
- Gail, L.; Sondermeyer, C.; Alyssa, N.; Duc, V.; Seema, J. Regional Analysis of Coccidioidomycosis Incidence—California, 2000–2018. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1817–1821. [Google Scholar]
- Drutz, D.J.; Huppert, M.; Sun, S.H.; McGuire, W.L. Human sex hormones stimulate the growth and maturation of Coccidioides immitis. Infect Immun. 1981, 32, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Pappagianis, D.; Lindsay, S.; Beall, S.; Williams, P. Ethnic background and the clinical course of coccidioidomycosis. Am. Rev. Respir. Dis. 1979, 120, 959–961. [Google Scholar] [PubMed]
- Center for disease control and prevention. In Coccidioidomycosis/Valley Fever CDC Yellow Book 2024; Oxford University Press: Oxford, UK, 2024.
- Laniado-Laborin, R. Expanding understanding of epidemiology of coccidioidomycosis in the Western hemisphere. Ann. N. Y. Acad. Sci. 2007, 1111, 19–34. [Google Scholar] [CrossRef]
- A de Perio, M.; Materna, B.L.; Cooksey, G.L.S.; Vugia, D.J.; Su, C.-P.; E Luckhaupt, S.; McNary, J.; A Wilken, J. Occupational coccidioidomycosis surveillance and recent outbreaks in California. Med. Mycol. 2019, 57 (Suppl. S1), S41–S45. [Google Scholar] [CrossRef]
- Brown, J.; Benedict, K.; Park, B.J.; Thompson, G.R., 3rd. Coccidioidomycosis: Epidemiology. Clin. Epidemiol. 2013, 5, 185–197. [Google Scholar]
- Flynn, N.M.; Hoeprich, P.D.; Kawachi, M.M.; Lee, K.K.; Lawrence, R.M.; Goldstein, E.; Jordan, G.W.; Kundargi, R.S.; Wong, G.A. An unusual outbreak of windborne coccidioidomycosis. N. Engl. J. Med. 1979, 301, 358–361. [Google Scholar] [CrossRef]
- Nicas, M.; Hubbard, A. A risk analysis for airborne pathogens with low infectious doses: Application to respirator selection against Coccidioides immitis spores. Risk Anal. 2002, 22, 1153–1163. [Google Scholar] [CrossRef]
- Peterson, C.; Chu, V.; Lovelace, J.; Almekdash, M.H.; Lacy, M. Coccidioidomycosis Cases at a Regional Referral Center, West Texas, USA, 2013–2019. Emerg. Infect Dis. 2022, 28, 848–851. [Google Scholar] [CrossRef]
- Muñoz-Hernández, B.; Palma-Cortés, G.; Cabello-Gutiérrez, C.; Martínez-Rivera, M.A. Parasitic polymorphism of Coccidioides spp. BMC Infect. Dis. 2014, 14, 213. [Google Scholar] [CrossRef]
- Gade, L.; McCotter, O.Z.; Bowers, J.R.; Waddell, V.; Brady, S.; Carvajal, J.A.; Sunenshine, R.; Komatsu, K.K.; Engelthaler, D.M.; Chiller, T.; et al. The detection of Coccidioides from ambient air in Phoenix, Arizona: Evidence of uneven distribution and seasonality. Med. Mycol. 2020, 58, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.-Y.; Hsu, A.P.; Holland, S.M.; Fierer, J. A review of innate and adaptive immunity to coccidioidomycosis. Med. Mycol. 2019, 57 (Suppl. S1), S85–S92. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, S.W.; Bonham, K.S.; Zanoni, I.; Kagan, J.C. Innate immune pattern recognition: A cell biological perspective. Rev. Annu. Rev. Immunol. 2015, 33, 257–290. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Wüthrich, M.; Deepe, G.; Klein, B. Adaptive immunity to fungi. Rev. Cold Spring Harb. Perspect. Med. 2014, 5, a019612. [Google Scholar] [CrossRef]
- Erwig, L.P.; Gow, N.A.R. Interactions of fungal pathogens with phagocytes. Nat. Rev. Microbiol. 2016, 14, 163–176. [Google Scholar] [CrossRef]
- Speakman, E.A.; Dambuza, I.M.; Salazar, F.; Brown, G.D. T Cell Antifungal Immunity and the Role of C-Type Lectin Receptors. Trends Immunol. 2020, 41, 61–76. [Google Scholar] [CrossRef]
- Fernández-García, O.A.; Cuellar-Rodríguez, J.M. Immunology of Fungal Infections. Infect. Dis. Clin. N. Am. 2021, 35, 373–388. [Google Scholar] [CrossRef]
- Donovan, F.M.; A Ramadan, F.; Lim, J.R.; E Buchfuhrer, J.; Khan, R.N.; DeQuillfeldt, N.P.; Davis, N.M.; Kaveti, A.; De Shadarevian, M.; Bedrick, E.J.; et al. Contribution of Biologic Response Modifiers to the Risk of Coccidioidomycosis Severity. Open Forum Infect. Dis. 2022, 9, ofac032. [Google Scholar] [CrossRef]
- Bergstrom, L.; Yocum, D.E.; Ampel, N.M.; Villanueva, I.; Lisse, J.; Gluck, O.; Tesser, J.; Posever, J.; Miller, M.; Araujo, J.; et al. Increased risk of coccidioidomycosis in patients treated with tumor necrosis factor alpha antagonists. Arthritis Rheum. 2004, 50, 1959–1966. [Google Scholar] [CrossRef]
- Li, X.; Lau, S.K.; Woo, P.C. Fungal infection risks associated with the use of cytokine antagonists and immune checkpoint inhibitors. Exp. Biol. Med. 2020, 245, 1104–1114. [Google Scholar] [CrossRef]
- Bays, D.J.; Thompson, G.R.; Reef, S.; Snyder, L.; Freifeld, A.J.; Huppert, M.; Salkin, D.; Wilson, M.D.; Galgiani, J.N. Natural History of Disseminated Coccidioidomycosis: Examination of the Veterans Affairs–Armed Forces Database. Clin. Infect. Dis. 2020, 73, e3814–e3819. [Google Scholar] [CrossRef] [PubMed]
- Bays, D.J.; Thompson, G.R. Coccidioidomycosis. Infect. Dis. Clin. N. Am. 2021, 35, 453–469. [Google Scholar] [CrossRef] [PubMed]
- Rosenstein, N.E.; Emery, K.W.; Werner, S.B.; Kao, A.; Johnson, R.; Rogers, D.; Vugia, D.; Reingold, A.; Talbot, R.; Plikaytis, B.D.; et al. Risk factors for severe pulmonary and disseminated coccidioidomycosis: Kern County, California, 1995–1996. Clin. Infect. Dis. 2001, 32, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Drutz, D.J.; Catanzaro, A. Coccidioidomycosis. Part. I and part II. Am. Rev. Respir. Dis. 1978, 117, 559–585. [Google Scholar]
- Smith, C.E.; Beard, R.R. Varieties of coccidioidal infection in relation to the epidemiology and control of the diseases. Am. J. Public. Health Nations Health 1946, 36, 1394–1402. [Google Scholar] [CrossRef]
- Galgiani, J.N. Coccidioidomycosis. West. J. Med. 1993, 159, 153–171. [Google Scholar]
- Stevens, D.A. Coccidioidomycosis. N. Engl. J. Med. 1995, 332, 1077–1082. [Google Scholar] [CrossRef]
- Valdivia, L.; Nix, D.; Wright, M.; Lindberg, E.; Fagan, T.; Lieberman, D.; Stoffer, T.; Ampel, N.M.; Galgiani, J.N. Coccidioidomycosis as a common cause of community-acquired pneumonia. Infect Dis. 2006, 12, 958–962. [Google Scholar] [CrossRef]
- Galgiani, J.N.; Kauffman, C.A. Coccidioidomycosis and Histoplasmosis in Immunocompetent Persons. N. Engl. J. Med. 2024, 390, 536–547. [Google Scholar] [CrossRef]
- Merchant, M.; Romero, A.O.; Libke, R.D.; Joseph, J. Pleural effusion in hospitalized patients with Coccidioidomycosis. Respir. Med. 2008, 102, 537–540. [Google Scholar] [CrossRef]
- Guimarães, M.D.; Marchiori, E.; Meirelles, G.d.S.P.; Hochhegger, B.; Santana, P.R.P.; Gross, J.L.; Bitencourt, A.G.V.; Boonsirikamchai, P.; Godoy, M.C.B. Fungal infection mimicking pulmonary malignancy: Clinical and radiological characteristics. Lung 2013, 191, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.E.; Beard, R.R.; Saito, M.T. Pathogenesis of coccidioidomycosis with special reference to pulmonary cavitation. Ann. Intern. Med. 1948, 29, 623–655. [Google Scholar] [PubMed]
- Kooner, L.; Munoz, A.; Garcia, A.; Kaur, A.; Sharma, R.; Bustamante, V.; Narang, V.; Thompson, G.R., III; Kuran, R.; Berjis, A.; et al. Coccidioidal Pulmonary Cavitation: A New Age. J. Fungi. 2023, 9, 561. [Google Scholar] [CrossRef] [PubMed]
- Shemuel, J.; Bays, D.J.; Thompson, G.R.; Reef, S.; Snyder, L.; Freifeld, A.J.; Huppert, M.; Salkin, D.; Wilson, M.D.; Galgiani, J.N. Natural history of pulmonary coccidioidomycosis: Further examination of the VA-Armed Forces Database. Med. Mycol. 2022, 60, myac054. [Google Scholar] [CrossRef]
- Monroy-Nieto, J.; Gade, L.; Benedict, K.; Etienne, K.A.; Litvintseva, A.P.; Bowers, J.R.; Engelthaler, D.M.; Chow, N.A. Genomic Epidemiology Linking Nonendemic Coccidioidomycosis to Travel. Emerg. Infect. Dis. 2023, 29, 110–117. [Google Scholar] [CrossRef]
- Singh, V.R.; Smith, D.K.; Lawerence, J.; Kelly, P.C.; Thomas, A.R.; Spitz, B.; Sarosi, G.A. Coccidioidomycosis in patients infected with human immunodeficiency virus: Review of 91 cases at a single institution. Clin. Infect. Dis. 1996, 23, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Studemeister, A.; Studemeister, L.; Brun, F. The role of extracorporeal membrane oxygenation in severe pulmonary coccidioidomycosis. Heart Lung 2018, 47, 261–263. [Google Scholar] [CrossRef]
- Fayed, M.; Vempilly, J. Rare Initial Presentation of Pulmonary Coccidioidomycosis. Chest Infect. 2015, 148, 91A. [Google Scholar] [CrossRef]
- Peterson, M.W.; Jain, R.; Hildebrandt, K.; Carson, W.K.; Fayed, M.A. Differentiating Lung Nodules Due to Coccidioides from Those Due to Lung Cancer Based on Radiographic Appearance. J. Fungi 2023, 9, 641. [Google Scholar] [CrossRef]
- Schweigert, M.; Dubecz, A.; Beron, M.; Ofner, D.; Stein, H.J. Pulmonary infections imitating lung cancer: Clinical presentation and therapeutical approach. Ir. J. Med. Sci. 2013, 182, 73–80. [Google Scholar] [CrossRef]
- Garrett, A.L.; Chang, Y.-H.H.; Ganley, K.; Blair, J.E. Uphill both ways: Fatigue and quality of life in valley fever. Med. Mycol. 2016, 54, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Galgiani, J.N.; Ampel, N.M.; Blair, J.E.; Catanzaro, A.; Geertsma, F.; Hoover, S.E.; Johnson, R.H.; Kusne, S.; Lisse, J.; MacDonald, J.D.; et al. 2016 Infectious Diseases Society of America (IDSA) Clinical Practice Guideline for the Treatment of Coccidioidomycosis. Clin. Infect Dis. 2016, 63, e112–e146. [Google Scholar] [CrossRef] [PubMed]
- Seitz, A.E.; Prevots, D.R.; Holland, S.M. Hospitalizations associated with disseminated coccidioidomycosis, Arizona and California, USA. Emerg. Infect. Dis. 2012, 18, 1476–1479. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.P.; Morris, M.F.; Panse, P.M.; Ko, M.G.; Files, J.A.; Ruddy, B.E.; Blair, J.E. Does the presence of mediastinal adenopathy confer a risk for disseminated infection in immunocompetent persons with pulmonary coccidioidomycosis. Mycoses 2013, 56, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Ruan, Q.; Jin, J.; Zheng, J.; Shao, L.; Li, N.; Zhu, L.; Zhang, W.; Hu, Y.; Chen, M. Disseminated coccidioidomycosis in immunocompetent patients in non-endemic areas: A case series and literature review. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 925–939. [Google Scholar] [CrossRef]
- Vincent, T.; Galgiani, J.N.; Huppert, M.; Salkin, D. The natural history of coccidioidal meningitis: VA-Armed Forces cooperative studies, 1955–1958. Clin. Infect Dis. 1993, 16, 247–254. [Google Scholar] [CrossRef]
- McHardy, I.H.; Barker, B.; Thompson, G.R. Review of Clinical and Laboratory Diagnostics for Coccidioidomycosis. J. Clin. Microbiol. 2023, 61, e0158122. [Google Scholar] [CrossRef]
- Martins, T.B.; Jaskowski, T.D.; Mouritsen, C.L.; Hill, H.R. Comparison of commercially available enzyme immunoassay with traditional serological tests for detection of antibodies to Coccidioides immitis. J. Clin. Microbiol. 1995, 33, 940–943. [Google Scholar] [CrossRef]
- Pappagianis, D.; Zimmer, B.L. Serology of coccidioidomycosis. Clin. Microbiol. Rev. 1990, 3, 247–268. [Google Scholar] [CrossRef]
- Kuberski, T.; Herrig, J.; Pappagianis, D. False-positive IgM serology in coccidioidomycosis. J. Clin. Microbiol. 2010, 48, 2047–2049. [Google Scholar] [CrossRef]
- Smith, C.E.; Saito, M.T. Serologic reactions in coccidioidomycosis. Chronic Dis. 1957, 5, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Donovan, F.M.; Ramadan, F.A.; Khan, S.A.; Bhaskara, A.; Lainhart, W.D.; Narang, A.T.; Mosier, J.M.; Ellingson, K.D.; Bedrick, E.J.; Saubolle, M.A.; et al. Comparison of a novel rapid lateral flow assay to enzyme immunoassay results for early diagnosis of coccidioidomycosis. Clin. Infect Dis. 2021, 73, e2746–e2753. [Google Scholar] [CrossRef] [PubMed]
- Durkin, M.; Connolly, P.; Kuberski, T.; Myers, R.; Kubak, B.M.; Bruckner, D.; Pegues, D.; Wheat, L.J. Diagnosis of coccidioidomycosis with use of the Coccidioides antigen enzyme immunoassay. Clin. Infect. Dis. 2008, 47, e69–e73. [Google Scholar] [CrossRef] [PubMed]
- Kassis, C.; Zaidi, S.; Kuberski, T.; Moran, A.; Gonzalez, O.; Hussain, S.; Hartmann-Manrique, C.; Al-Jashaami, L.; Chebbo, A.; Myers, R.A.; et al. Role of Coccidioides Antigen Testing in the Cerebrospinal Fluid for the Diagnosis of Coccidioidal Meningitis. Clin. Infect. Dis. 2015, 61, 1521–1526. [Google Scholar] [CrossRef]
- Vucicevic, D.; Blair, J.E.; Binnicker, M.J.; McCullough, A.E.; Kusne, S.; Vikram, H.R.; Parish, J.M.; Wengenack, N.L. The utility of Coccidioides polymerase chain reaction testing in the clinical setting. Mycopathologia 2010, 170, 345–351. [Google Scholar] [CrossRef]
- Mead, H.L.; Van Dyke, M.C.C.; Barker, B.M. Proper Care and Feeding of Coccidioides: A Laboratorian’s Guide to Cultivating the Dimorphic Stages of C. immitis and C. posadasii. Curr. Protoc. Microbiol. 2020, 58, e113. [Google Scholar]
- Saubolle, M.A.; Wojack, B.R.; Wertheimer, A.M.; Fuayagem, A.Z.; Young, S.; Koeneman, B.A. Multicenter Clinical Validation of a Cartridge-Based Real-Time PCR System for Detection of Coccidioides spp. in Lower Respiratory Specimens. J. Clin. Microbiol. 2018, 56, 2. [Google Scholar] [CrossRef]
- Mitchell, M.; Dizon, D.; Libke, R.; Peterson, M.; Slater, D.; Dhillon, A. Development of a real-time PCR Assay for identification of Coccidioides immitis by use of the BD Max system. J. Clin. Microbiol. 2015, 53, 926–929. [Google Scholar] [CrossRef]
- Dizon, D.; Mitchell, M.; Dizon, B.; Libke, R.; Peterson, M.W. The utility of real-time polymerase chain reaction in detecting Coccidioides immitis among clinical specimens in the Central California San. Joaquin Valley. Med. Mycol. 2019, 57, 688–693. [Google Scholar] [CrossRef]
- Daher, M.; Iordanov, R.; Al Mohajer, M.; Sohail, M.R.; Staggers, K.A.; Hamdi, A.M. Clinical utility of metagenomic next-generation sequencing in fever of undetermined origin. Ther. Adv. Infect. Dis. 2024, 11, 20499361241244969. [Google Scholar] [CrossRef]
- Mao, Y.; Li, X.; Lou, H.; Shang, X.; Mai, Y.; Yang, L.; Peng, F.; Fu, X. Detection of Coccidioides posadasii in a patient with meningitis using metagenomic next-generation sequencing: A case report. BMC Infect. Dis. 2021, 21, 968. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.Z.; Mathisen, G. Diagnosis of severe pulmonary coccidioidomycosis via plasma cell-free DNA. Chest Infect. 2023, 164, a952–a953. [Google Scholar] [CrossRef]
- Shah, R.A.; Vempilly, J.J.; Husnain, S.M.N.U.; Hegde, P. Combined Endosonography Reduces Time to Diagnose Pulmonary Coccidioidomycosis. J. Bronchol. Interv. Pulmonol. 2018, 25, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Galgiani, J.N.; Thompson, G.R., III; Board of Valley Fever Alliance of Arizona Clinicians. Valley Fever (Coccidioidomycosis) Tutorial for Primary Care Professionals; Valley Fever Center for Excellence, The University of Arizona: Tucson, AZ, USA, 2016. Available online: http://www.vfce.arizona.edu/sites/vfce/files/tutorial_for_primary_care_professionals.pdf (accessed on 11 October 2024).
- Werner, S.B.; Pappagianis, D.; Heindl, I.; Mickel, A. An epidemic of coccidioidomycosis among archeology students in northern California. N. Engl. J. Med. 1972, 286, 507–512. [Google Scholar] [CrossRef]
- Jude, C.M.; Nayak, N.B.; Patel, M.K.; Deshmukh, M.; Batra, P. Pulmonary coccidioidomycosis: Pictorial review of chest radiographic and CT findings. Radiographics 2014, 34, 912–925. [Google Scholar] [CrossRef]
- Capone, D.; Marchiori, E.; Wanke, B.; E Dantas, K.; Cavalcanti, M.A.S.; Filho, A.D.; Escuissato, D.L.; Warszawiak, D. Acute pulmonary coccidioidomycosis: CT findings from 15 patients. Br. J. Radiol. 2008, 81, 721–724. [Google Scholar] [CrossRef]
- Available online: https://www.cdc.gov/valley-fever/hcp/testing-algorithm/?CDC_AAref_Val=https://www.cdc.gov/fungal/diseases/coccidioidomycosis/diagnosticalgorithms/ (accessed on 11 October 2024).
- Ruddy, B.E.; Mayer, A.P.; Ko, M.G.; Labonte, H.R.; Borovansky, J.A.; Boroff, E.S.; Blair, J.E. Coccidioidomycosis in African Americans. Mayo. Clin. Proc. 2011, 86, 63–69. [Google Scholar] [CrossRef]
- Ampel, N.M.; Ryan, K.J.; Carry, P.J.; A Wieden, M.; Schifman, R.B. Fungemia due to Coccidioides immitis. An analysis of 16 episodes in 15 patients and a review of the literature. Medicine 1986, 65, 312–321. [Google Scholar] [CrossRef]
- Deresinski, S.C.; Stevens, D.A. Coccidioidomycosis in compromised hosts. Experience at Stanford University Hospital. Medicine 1975, 54, 377–395. [Google Scholar] [CrossRef]
- Blair, J.E.; Ampel, N.M.; Hoover, S.E. Coccidioidomycosis in selected immunosuppressed hosts. Med. Mycol. 2019, 57 (Suppl. S1), S56–S63. [Google Scholar] [CrossRef]
- Bercovitch, R.S.; Catanzaro, A.; Schwartz, B.S.; Pappagianis, D.; Watts, D.; Ampel, N.M. Coccidioidomycosis during pregnancy: A review and recommendations for management. Clin. Infect. Dis. 2011, 53, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Santelli, A.C.; Blair, J.E.; Roust, L.R. Coccidioidomycosis in patients with diabetes mellitus. Am. J. Med. 2006, 119, 964–969. [Google Scholar] [CrossRef] [PubMed]
- Liapikou, A.; Ferrer, M.; Polverino, E.; Balasso, V.; Esperatti, M.; Piñer, R.; Mensa, J.; Luque, N.; Ewig, S.; Menendez, R.; et al. Severe community-acquired pneumonia: Validation of the Infectious Diseases Society of America/American Thoracic Society guidelines to predict an intensive care unit admission. Clin. Infect. Dis. 2009, 48, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Phua, J.; See, K.C.; Chan, Y.H.; Widjaja, L.S.; Aung, N.W.; Ngerng, W.J.; Lim, T.K. Validation and clinical implications of the IDSA/ATS minor criteria for severe community-acquired pneumonia. Thorax 2009, 64, 598–603. [Google Scholar] [CrossRef]
- Chalmers, J.D.; Taylor, J.K.; Mandal, P.; Choudhury, G.; Singanayagam, A.; Akram, A.R.; Hill, A.T. Validation of the Infectious Diseases Society of America/American Thoracic Society minor criteria for intensive care unit admission in community-acquired pneumonia patients without major criteria or contraindications to intensive care unit care. Clin. Infect. Dis. 2011, 53, 503–511. [Google Scholar] [CrossRef]
- Janis, E.B.; Neil, M.A.; Carol, A.K.; Milana, B. Primary Pulmonary Coccidioidal Infection. Available online: www.uptodate.com (accessed on 9 August 2024).
- Heidari, A.; Sharma, R.; Shakir, Q.; Shah, M.; Clement, J.; Donnelley, M.A.; Reynolds, T.; Trigg, K.; Jolliff, J.; Kuran, R.; et al. Isavuconazole in the Treatment of Chronic Forms of Coccidioidomycosis. Clin. Infect. Dis. 2023, 76, 2196–2199. [Google Scholar] [CrossRef]
- Timothy TKuberski Ronald JServi Phillip, J. Rubin. Successful Treatment of a Critically Ill Patient with Disseminated Coccidioidomycosis, Using Adjunctive Interferon. Clin. Infect. Dis. 2004, 38, 910–912. [Google Scholar]
- James, R.M.L.; Ashley, S.; Rebecca, W.; Rachel, V.T.; Emily, R.H.; Candidate, T.Z.; Mohanad, A.O. Clinical Outcomes of Coccidioidomycosis in Patients Requiring Intensive Care Unit Hospitalization. Open Forum Infect. Dis. 2023, 10 (Suppl. S2), ofad500.868. [Google Scholar]
- Khalafi, S.; Brockman, M.J.; Dihowm, F. Coccidioides-Induced Pyopneumothorax in an Immunocompetent Patient. Cureus 2023, 15, e39782. [Google Scholar] [CrossRef]
- Narang, V.K.; Dao, K.; Jaratanian, S.; D’assumpcao, C.; Kuran, R.; Munoz, A.; Heidari, A. Pulmonary Giant Cavitary Coccidioides with Fungal Ball and Hemoptysis. J. Investig. Med. High. Impact Case Rep. 2022, 10, 23247096221084852. [Google Scholar] [CrossRef]
- Jaroszewski, D.E.; Halabi, W.J.; Blair, J.E.; Coakley, B.J.; Wong, R.K.; Parish, J.M.; Vaszar, L.T.; Kusne, S.; Vikram, H.R.; DeValeria, P.A.; et al. Surgery for pulmonary coccidioidomycosis: A 10-year experience. Ann. Thorac. Surg. 2009, 88, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Pappagianis, D. Serologic studies in coccidioidomycosis. Semin. Respir. Infect. 2001, 16, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Saubolle, M.A. Laboratory aspects in the diagnosis of coccidioidomycosis. Review Ann. N. Y. Acad. Sci. 2007, 1111, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Al-Ameri, A.; Malhotra, P.; Thygesen, H.; Plant, P.K.; Vaidyanathan, S.; Karthik, S.; Scarsbrook, A.; Callister, M.E. Risk of malignancy in pulmonary nodules: A validation study of four prediction models. Lung Cancer 2015, 89, 27–30. [Google Scholar] [CrossRef]
- Reyes, N.; Onadeko, O.O.; Luraschi-Monjagatta, M.D.C.; Knox, K.S.; Rennels, M.A.; Walsh, T.K.; Ampel, N.M. Positron emission tomography in the evaluation of pulmonary nodules among patients living in a coccidioidal endemic region. Lung 2014, 192, 589–593. [Google Scholar] [CrossRef]
- Ganley, K.J.; Bosch, P.R.; Blair, J.E.; Rischard, F.; Galgiani, J.N. Oxygen Consumption Deficits in Patients with Residual Fatigue After Primary Coccidioidomycosis. Open Forum Infect. Dis. 2017, 4, ofx136. [Google Scholar] [CrossRef]
- Jie, P.; Fariba, M.D.; Kate, E.; Gondy, L.; Jeff, S.; Edward, B.; John, N.G. Clinician Practice Patterns That Result in the Diagnosis of Coccidioidomycosis Before or During Hospitalization. Clin. Infect. Dis. 2021, 73, e1587–e1593. [Google Scholar]
- Gaab, E.M.; Naeem, F. Pediatric Coccidioidomycosis Patients: Perceptions, Quality of Life and Psychosocial Factors. Healthcare 2015, 3, 775–795. [Google Scholar] [CrossRef]














| Test | Availability | Detection |
|---|---|---|
| Enzyme-linked immunoassays (EIA) | Available locally in many centers in endemic areas. Available by commercial laboratories. | Anticoccidioidal antibodies IgM and IgG. |
| Immunodiffusion | University of California Davis lab (UC Davis). Available by commercial laboratories. | Immunodiffusion (ID) for detection of coccidioidal IgM (“precipitin”) (sometimes termed as IDTP or antibody to TP antigen). Immunodiffusion (ID) for detection of coccidioidal IgG (sometimes termed as IDCF or antibody to F antigen). |
| Tube precipitin (TP)-type antibodies | Largely replaced by the current immunodiffusion. | Anticoccidioidal antibodies IgM and IgG. |
| Lateral flow assay (LFA) | It is available commercially via IMMY. | Detects Coccidioides-specific IgM and IgG. It is a rapid test and results can be available within 1 h. It carries a poor sensitivity but high specificity. |
| Complement-fixation (CF) | Available by commercial laboratories, some California county labs, and the University of California Davis lab (UC Davis). | Measures the binding of the complement by the IgG antibody as determined by the inhibition of lysis of foreign red blood cells (the greater the dilution then the more likely the patient has extensive infection). Detects antibodies in cerebrospinal fluid. |
| Cultures | Available in most centers locally and commercially. It must be carried out at biosafety level 3 (BSL-3). | Cultures can be obtained from sputum, endotracheal aspirate, bronchoalveolar lavage, body fluids, and tissue. |
| Polymerase chain reaction (PCR) | Available locally in some medical centers and commercially via Genestate. | PCRs can be obtained from sputum, endotracheal aspirate, bronchoalveolar lavage, body fluids, and tissue. |
| Metagenomic next-generation sequencing (NGS) | Available locally in some medical centers and commercially: for example, Karius. | It analyzes cell-free genetic material from a given sample. It can be carried out in a body sample or serum. Sensitivity is currently unclear. |
| Antigen coccidioidal detection via EIA | Available commercially via ARUP and MiraVista. | Antigen in body fluids such as cerebrospinal fluid, serum, and urine. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fayed, M.A.; Evans, T.M.; Almasri, E.; Bilello, K.L.; Libke, R.; Peterson, M.W. Overview of the Current Challenges in Pulmonary Coccidioidomycosis. J. Fungi 2024, 10, 724. https://doi.org/10.3390/jof10100724
Fayed MA, Evans TM, Almasri E, Bilello KL, Libke R, Peterson MW. Overview of the Current Challenges in Pulmonary Coccidioidomycosis. Journal of Fungi. 2024; 10(10):724. https://doi.org/10.3390/jof10100724
Chicago/Turabian StyleFayed, Mohamed A., Timothy M. Evans, Eyad Almasri, Kathryn L. Bilello, Robert Libke, and Michael W. Peterson. 2024. "Overview of the Current Challenges in Pulmonary Coccidioidomycosis" Journal of Fungi 10, no. 10: 724. https://doi.org/10.3390/jof10100724
APA StyleFayed, M. A., Evans, T. M., Almasri, E., Bilello, K. L., Libke, R., & Peterson, M. W. (2024). Overview of the Current Challenges in Pulmonary Coccidioidomycosis. Journal of Fungi, 10(10), 724. https://doi.org/10.3390/jof10100724







