Distributions of Lanostene-Derived Triterpenoids and Glucan Content in the Fruiting Bodies of the Australian Ganoderma Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Ganoderma Cultivation
2.3. Morphological Characterisation
2.4. Sample Preparation and Extraction
2.5. Antioxidant Activity
2.5.1. ABTS Radical-Scavenging Activity
2.5.2. DPPH Free Radical-Scavenging Activity
2.5.3. Ferric Reducing Antioxidant Ability (FRAP)
2.6. Glucan Quantification
2.7. Identification of LDTs Through UHPLC-MS/MS
3. Results and Discussion
3.1. Morphological Characterisation of Ganoderma Fruiting Bodies
3.2. Antioxidant Activity of Ganoderma Methanolic Extracts
3.3. Glucan Content in Ganoderma Fruiting Bodies
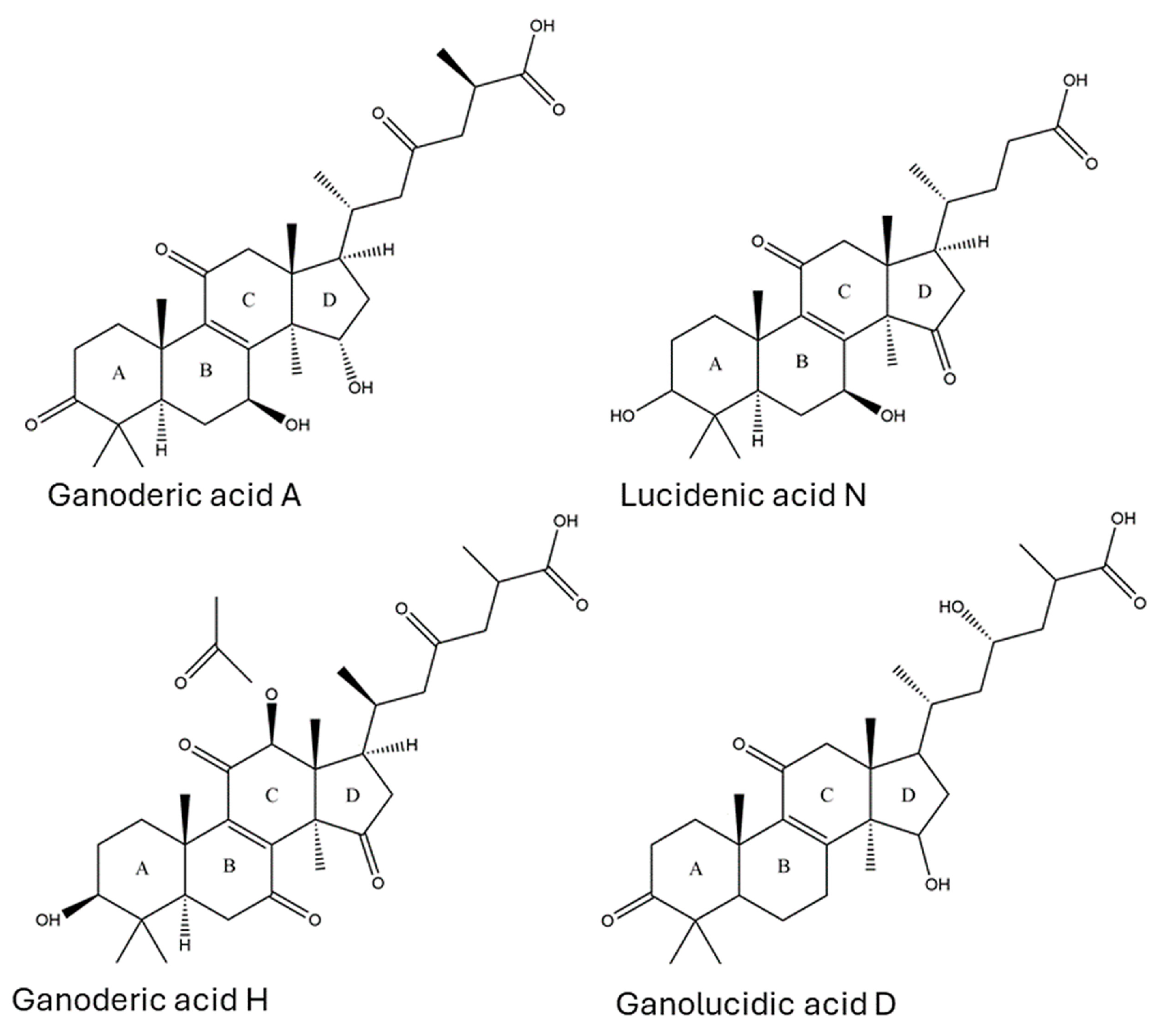
3.4. Identification of LDTs in Australian Ganoderma Fruiting Bodies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El Sheikha, A.F. Nutritional profile and health benefits of Ganoderma lucidum “Lingzhi, Reishi, or Mannentake” as functional foods: Current scenario and future perspectives. Foods 2022, 11, 1030. [Google Scholar] [CrossRef] [PubMed]
- Moncalvo, J.M.; Ryvarden, L. A Nomenclatural Study of the Ganodermataceae Donk; Fungiflora: Oslo, Norway, 1997. [Google Scholar]
- Moncalvo, J.M.; Wang, H.F.; Hseu, R.S. Gene phylogeny of the Ganoderma lucidum complex based on ribosomal DNA sequences. Comparison with traditional taxonomic characters. Mycol. Res. 1995, 99, 1489–1499. [Google Scholar] [CrossRef]
- Islam, M.; Sarker, N.C.; Biswas, S.K.; Amin, R. Effect of light intensity and its management practices on yield of Ganoderma lucidum. Bangladesh J. Mushroom 2011, 5, 17–22. [Google Scholar]
- Gryzenhout, M.; Ghosh, S.; Tchotet Tchoumi, J.M.; Vermeulen, M.; Kinge, T.R. Ganoderma: Diversity, Ecological Significances, and Potential Applications in Industry and Allied Sectors. In Industrially Important Fungi for Sustainable Development; Springer: Berlin/Heidelberg, Germany, 2021; pp. 295–334. [Google Scholar]
- Zhou, L.W.; Cao, Y.; Wu, S.H.; Vlasak, J.; Li, D.W.; Li, M.J.; Dai, Y.C. Global diversity of the Ganoderma lucidum complex (Ganodermataceae, Polyporales) inferred from morphology and multilocus phylogeny. Phytochemistry 2015, 114, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Andrejč, D.C.; Knez, Ž.; Marevci, M.K. Antioxidant, antibacterial, antitumor, antifungal, antiviral, anti-inflammatory, and nevro-protective activity of Ganoderma lucidum: An overview. Front. Pharmacol. 2022, 13, 934982. [Google Scholar] [CrossRef]
- Jiang, G.; Lei, A.; Chen, Y.; Yu, Q.; Xie, J.; Yang, Y.; Yuan, T.; Su, D. The protective effects of the Ganoderma atrum polysaccharide against acrylamide-induced inflammation and oxidative damage in rats. Food Funct. 2021, 12, 397–407. [Google Scholar] [CrossRef]
- Pan, Y.; Yuan, S.; Teng, Y.; Zhang, Z.; He, Y.; Zhang, Y.; Liang, H.; Wu, X.; Li, J.; Yang, H. Antioxidation of a proteoglycan from Ganoderma lucidum protects pancreatic β-cells against oxidative stress-induced apoptosis in vitro and in vivo. Int. J. Biol. Macromol. 2022, 200, 470–486. [Google Scholar] [CrossRef]
- Baby, S.; Johnson, A.J.; Govindan, B. Secondary metabolites from Ganoderma. Phytochemistry 2015, 114, 66–101. [Google Scholar] [CrossRef]
- Radwan, F.F.; Hossain, A.; God, J.M.; Leaphart, N.; Elvington, M.; Nagarkatti, M.; Tomlinson, S.; Haque, A. Reduction of myeloid-derived suppressor cells and lymphoma growth by a natural triterpenoid. J. Cell Biochem. 2015, 116, 102–114. [Google Scholar] [CrossRef]
- Jiang, J.; Grieb, B.; Thyagarajan, A.; Sliva, D. Ganoderic acids suppress growth and invasive behavior of breast cancer cells by modulating AP-1 and NF-kappaB signaling. Int. J. Mol. Med. 2008, 21, 577–584. [Google Scholar]
- Lin, W.; Gu, L.; Zhu, L.Y.; Zhou, S.; Lian, D.; Xu, Y.; Zheng, L.; Liu, X.; Li, L. Extract of Ganoderma sinensis spores induces cell cycle arrest of hepatoma cell via endoplasmic reticulum stress. Pharm. Biol. 2021, 59, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Gill, B.S.; Kumar, S.; Navgeet. Evaluating anti-oxidant potential of ganoderic acid A in STAT 3 pathway in prostate cancer. Mol. Biol. Rep. 2016, 43, 1411–1422. [Google Scholar] [CrossRef] [PubMed]
- Galappaththi, M.C.; Patabendige, N.M.; Premarathne, B.M.; Hapuarachchi, K.K.; Tibpromma, S.; Dai, D.-Q.; Suwannarach, N.; Rapior, S.; Karunarathna, S.C. A review of Ganoderma triterpenoids and their bioactivities. Biomolecules 2022, 13, 24. [Google Scholar] [CrossRef]
- Han, X.; Luo, R.; Ye, N.; Hu, Y.; Fu, C.; Gao, R.; Fu, S.; Gao, F. Research progress on natural beta-glucan in intestinal diseases. Int. J. Biol. Macromol. 2022, 219, 1244–1260. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.W.; Kim, B.M.; Kim, Y.C.; Lee, S.H.; Chung, S.K. Improvement in β-glucan extraction from Ganoderma lucidum with high-pressure steaming and enzymatic pre-treatment. Appl. Biol. Chem. 2018, 61, 235–242. [Google Scholar] [CrossRef]
- Zhao, S.Z.; Lei, M.; Xu, H.; He, H.L.; Suvorov, A.; Wang, J.H.; Qiu, J.Y.; Zhou, Q.X.; Yang, J.Y.; Chen, L.L. The normal cell proliferation and wound healing effect of polysaccharides from. Food Sci. Hum. Wellness 2021, 10, 508–513. [Google Scholar] [CrossRef]
- Seweryn, E.; Ziała, A.; Gamian, A. Health-promoting of polysaccharides extracted from Ganoderma lucidum. Nutrients 2021, 13, 2725. [Google Scholar] [CrossRef]
- Teelixir. Reishi Mushroom. Available online: https://teelixir.com.au/collections/medicinal-mushrooms/products/reishi-mushroom?variant=44436431700243 (accessed on 7 April 2023).
- Ha, D.T.; Loan, L.T.; Hung, T.M.; Han, L.V.N.; Khoi, N.M.; Dung, L.V.; Min, B.S.; Nguyen, N.P.D. An improved HPLC-DAD method for quantitative comparisons of triterpenes in Ganoderma lucidum and its five related species originating from Vietnam. Molecules 2015, 20, 1059–1077. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Du, Z.; Dong, C.H.; Wang, K.; Yao, Y.J. Classification, Biological Characteristics and Cultivations of Ganoderma. Adv. Exp. Med. Biol. 2019, 1181, 15–58. [Google Scholar] [CrossRef]
- Smith, B.J.; Sivasithamparam, K. Morphological studies of Ganoderma (Ganodermataceae) from the Australasian and Pacific regions. Aust. Syst. Bot. 2003, 16, 487–503. [Google Scholar] [CrossRef]
- Atkinson, G.F. Observations on Polyporus lucidus leys. and some of its Allies from Europe and North America. Bot. Gaz. 1908, 46, 321–338. [Google Scholar] [CrossRef]
- Sudheer, S.; Taha, Z.; Manickam, S.; Ali, A.; Cheng, P.G. Development of antler-type fruiting bodies of Ganoderma lucidum and determination of its biochemical properties. Fungal Biol. 2018, 122, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Bhosle, S.; Ranadive, K.; Bapat, G.; Garad, S.; Deshpande, G.; Vaidya, J. Taxonomy and diversity of Ganoderma from the Western parts of Maharashtra (India). Mycosphere 2010, 1, 249–262. [Google Scholar]
- Ahmad, M.F.; Wahab, S.; Ahmad, F.A.; Ashraf, S.A.; Abullais, S.S.; Saad, H.H. Ganoderma lucidum: A potential pleiotropic approach of ganoderic acids in health reinforcement and factors influencing their production. Fungal Biol. Rev. 2022, 39, 100–125. [Google Scholar] [CrossRef]
- Vaithanomsat, P.; Boonlum, N.; Chaiyana, W.; Tima, S.; Anuchapreeda, S.; Trakunjae, C.; Apiwatanapiwat, W.; Janchai, P.; Boondaeng, A.; Nimitkeatkai, H.; et al. Mushroom beta-Glucan Recovered from Antler-Type Fruiting Body of Ganoderma lucidum by Enzymatic Process and Its Potential Biological Activities for Cosmeceutical Applications. Polymers 2022, 14, 4202. [Google Scholar] [CrossRef]
- Tehranian, M.J.; Jouki, M.; Shakouri, M.J.; Jafari, S. Functional properties of Ganoderma lucidum extract: Antimicrobial and antioxidant activities. Food Sci. Technol. 2023, 43, e21423. [Google Scholar] [CrossRef]
- Raseta, M.; Popovic, M.; Beara, I.; Sibul, F.; Zengin, G.; Krstic, S.; Karaman, M. Anti-Inflammatory, Antioxidant and Enzyme Inhibition Activities in Correlation with Mycochemical Profile of Selected Indigenous Ganoderma spp. from Balkan Region (Serbia). Chem. Biodivers. 2021, 18, e2000828. [Google Scholar] [CrossRef]
- Yalcin, O.U.; Sarikurkcu, C.; Cengiz, M.; Gungor, H.; Cavar Zeljkovic, S. Ganoderma carnosum and Ganoderma pfeifferi: Metal concentration, phenolic content, and biological activity. Mycologia 2020, 112, 1–8. [Google Scholar] [CrossRef]
- Dong, Q.; He, D.; Ni, X.; Zhou, H.; Yang, H. Comparative study on phenolic compounds, triterpenoids, and antioxidant activity of Ganoderma lucidum affected by different drying methods. J. Food Meas. Charact. 2019, 13, 3198–3205. [Google Scholar] [CrossRef]
- Sułkowska-Ziaja, K.; Balik, M.; Szczepkowski, A.; Trepa, M.; Zengin, G.; Kała, K.; Muszyńska, B. A review of chemical composition and bioactivity studies of the most promising species of Ganoderma spp. Diversity 2023, 15, 882. [Google Scholar] [CrossRef]
- Atila, F.; Ogutcu, H.; Bilginoglu, E.; Kazankaya, A.; Kumar, P.; Fayssal, S.A. Effect of phenolic-rich forest and Agri-food wastes on yield, antioxidant, and antimicrobial activities of Ganoderma lucidum. Biomass Convers. Biorefinery 2023, 14, 1–11. [Google Scholar] [CrossRef]
- Oludemi, T.; Barros, L.; Prieto, M.A.; Heleno, S.A.; Barreiro, M.F.; Ferreira, I. Extraction of triterpenoids and phenolic compounds from Ganoderma lucidum: Optimization study using the response surface methodology. Food Funct. 2018, 9, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, I. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.-J.; Lee, J.-H. Comparison of antioxidant activities expressed as equivalents of standard antioxidant. Food Sci. Technol. 2023, 43, e121522. [Google Scholar] [CrossRef]
- Zengin, G.; Sarikurkcu, C.; Gunes, E.; Uysal, A.; Ceylan, R.; Uysal, S.; Gungor, H.; Aktumsek, A. Two Ganoderma species: Profiling of phenolic compounds by HPLC-DAD, antioxidant, antimicrobial and inhibitory activities on key enzymes linked to diabetes mellitus, Alzheimer’s disease and skin disorders. Food Funct. 2015, 6, 2794–2802. [Google Scholar] [CrossRef]
- Sulkowska-Ziaja, K.; Zengin, G.; Gunia-Krzyzak, A.; Popiol, J.; Szewczyk, A.; Jaszek, M.; Rogalski, J.; Muszynska, B. Bioactivity and Mycochemical Profile of Extracts from Mycelial Cultures of Ganoderma spp. Molecules 2022, 27, 275. [Google Scholar] [CrossRef]
- Sipping, M.T.K.; Mediesse, F.K.; Sombes, A.Y.N.; Mfopa, A.; Boudjeko, T. Antioxidant and anti-inflammatory activities of Ganoderma resinaceum (Boud) fruiting bodies extracts. J. Herbmed. Pharmacol. 2022, 11, 348–359. [Google Scholar] [CrossRef]
- McCleary, B.V.; Draga, A. Measurement of beta-Glucan in Mushrooms and Mycelial Products. J. AOAC Int. 2016, 99, 364–373. [Google Scholar] [CrossRef]
- Kiss, A.; Grunvald, P.; Ladanyi, M.; Papp, V.; Papp, I.; Nemedi, E.; Mirmazloum, I. Heat Treatment of Reishi Medicinal Mushroom (Ganoderma lingzhi) Basidiocarp Enhanced Its beta-glucan Solubility, Antioxidant Capacity and Lactogenic Properties. Foods 2021, 10, 2015. [Google Scholar] [CrossRef]
- Sari, M.; Prange, A.; Lelley, J.I.; Hambitzer, R. Screening of beta-glucan contents in commercially cultivated and wild growing mushrooms. Food Chem. 2017, 216, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Kozarski, M.; Klaus, A.; Niksic, M.; Jakovljevic, D.; Helsper, J.P.F.G.; Van Griensven, L.J.L.D. Antioxidative and immunomodulating activities of polysaccharide extracts of the medicinal mushrooms Agaricus bisporus, Agaricus brasiliensis, Ganoderma lucidum and Phellinus linteus. Food Chem. 2011, 129, 1667–1675. [Google Scholar] [CrossRef]
- Li, Z.; Shi, Y.; Zhang, X.; Xu, J.; Wang, H.; Zhao, L.; Wang, Y. Screening Immunoactive Compounds of Ganoderma lucidum Spores by Mass Spectrometry Molecular Networking Combined With in vivo Zebrafish Assays. Front. Pharmacol. 2020, 11, 287. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Liang, W.; Chen, W.; Li, S.; Cui, Y.; Qi, Q.; Zhang, L. Screening and Analysis of the Marker Components in Ganoderma lucidum by HPLC and HPLC-MS(n) with the Aid of Chemometrics. Molecules 2017, 22, 584. [Google Scholar] [CrossRef]
- Biswal, R.P.; Dandamudi, R.B.; Patnana, D.P.; Pandey, M.; Vutukuri, V.R.K. Metabolic fingerprinting of Ganoderma spp. using UHPLC-ESI-QTOF-MS and its chemometric analysis. Phytochemistry 2022, 199, 113169. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Han, W.; Liu, Y.; Feng, J.; Tang, C.; Feng, N.; Tang, Q. One single standard substance for the simultaneous determination of 17 triterpenes in Ganoderma lingzhi and its related species using high-performance liquid chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1068–1069, 49–55. [Google Scholar] [CrossRef]
- Yang, M.; Wang, X.; Guan, S.; Xia, J.; Sun, J.; Guo, H.; Guo, D.A. Analysis of triterpenoids in Ganoderma lucidum using liquid chromatography coupled with electrospray ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 2007, 18, 927–939. [Google Scholar] [CrossRef]
- Wu, T.S.; Shi, L.S.; Kuo, S.C. Cytotoxicity of Ganoderma lucidum triterpenes. J. Nat. Prod. 2001, 64, 1121–1122. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Nishimura, N.; Bando, S.; Arihara, S.; Matsumura, E.; Katayama, S. New lanostanoids, elfvingic acids A-H, from the fruit body of. J. Nat. Prod. 2002, 65, 548–552. [Google Scholar] [CrossRef]
- Richter, C.; Wittstein, K.; Kirk, P.M.; Stadler, M. An assessment of the taxonomy and chemotaxonomy of Ganoderma. Fungal Divers. 2015, 71, 1–15. [Google Scholar] [CrossRef]
- Chairul, S.M.; Hayashi, Y. Lanostanoid triterpenes from Ganoderma applanatum. Phytochemistry 1994, 35, 1305–1308. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, A.; Zhang, F.; Linhardt, R.J.; Zhu, Z.; Yang, Y.; Zhang, T.; Lin, Z.; Zhang, S.; Zhao, H.; et al. Ganoderenic acid D-loaded functionalized graphene oxide-based carrier for active targeting therapy of cervical carcinoma. Biomed. Pharmacother. 2023, 164, 114947. [Google Scholar] [CrossRef] [PubMed]
- Yue, Q.X.; Cao, Z.W.; Guan, S.H.; Liu, X.H.; Tao, L.; Wu, W.Y.; Li, Y.X.; Yang, P.Y.; Liu, X.; Guo, D.A. Proteomics characterization of the cytotoxicity mechanism of ganoderic acid D and computer-automated estimation of the possible drug target network. Mol. Cell Proteom. 2008, 7, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Gill, B.S.; Sharma, P.; Kumar, R.; Kumar, S. Misconstrued versatility of Ganoderma lucidum: A key player in multi-targeted cellular signaling. Tumour. Biol. 2016, 37, 2789–2804. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Tian, D.; Liu, Y.; Li, H.; Zhu, J.; Li, M.; Xin, M.; Xia, J. Review of the molecular mechanisms of Ganoderma lucidum triterpenoids: Ganoderic acids A, C2, D, F, DM, X and Y. Eur. J. Med. Chem. 2019, 174, 130–141. [Google Scholar] [CrossRef]
- Yu, Z.R.; Jia, W.H.; Liu, C.; Wang, H.Q.; Yang, H.G.; He, G.R.; Chen, R.Y.; Du, G.H. Ganoderic acid A protects neural cells against NO stress injury in vitro via stimulating beta adrenergic receptors. Acta Pharmacol. Sin. 2020, 41, 516–522. [Google Scholar] [CrossRef]
- Bashir, M.A.; Shao, C.-S.; Abdalla, M.; Lin, X.; Li, L.; Wu, Y.; Huang, Q. Ganoderic acid A targets IL-1R1 and disrupts IL-1β binding in human cancer cells. J. Mol. Struct. 2023, 1301, 137431. [Google Scholar] [CrossRef]
- Tung, N.T.; Cuong, T.D.; Hung, T.M.; Lee, J.H.; Woo, M.H.; Choi, J.S.; Kim, J.; Ryu, S.H.; Min, B.S. Inhibitory effect on NO production of triterpenes from the fruiting bodies of Ganoderma lucidum. Bioorg. Med. Chem. Lett. 2013, 23, 1428–1432. [Google Scholar] [CrossRef]
- Farrell, A.J.; Blake, D.R.; Palmer, R.M.; Moncada, S. Increased concentrations of nitrite in synovial fluid and serum samples suggest increased nitric oxide synthesis in rheumatic diseases. Ann. Rheum. Dis. 1992, 51, 1219–1222. [Google Scholar] [CrossRef]




| Sample ID | Total Glucan (mg/g DW) | α-Glucan (%) | β-Glucan (%) | |
|---|---|---|---|---|
| G. lucidum extract | 619.2 a | 97.4 | 2.6 | |
| Group 1 | G1 | 237.3 gi | 0.8 | 99.2 |
| G2 | 300.2 ef | 0.7 | 99.3 | |
| G3 | 293.0 eg | 0.7 | 99.3 | |
| G4 | 240.4 gi | 0.8 | 99.2 | |
| G5 | 272.6 fg | 0.7 | 99.3 | |
| G6 | 198.2 i | 1.5 | 98.5 | |
| Group 2 | G8 | 215.0 hi | 0.5 | 99.5 |
| G9 | 301.4 ef | 3.3 | 96.7 | |
| G10 | 270.4 fg | 5.2 | 94.8 | |
| Group 3 | G11 | 285.8 eg | 0.7 | 99.3 |
| G12 | 359.6 cd | 0.8 | 99.2 | |
| G13 | 294.9 ef | 1.0 | 99.0 | |
| G14 | 321.2 de | 0.6 | 99.4 | |
| G15 | 385.3 bc | 0.5 | 99.5 | |
| G16 | 368.0 cd | 0.3 | 99.7 | |
| G17 | 301.1 eg | 0.7 | 99.3 | |
| G18 | 443.5 b | 0.7 | 99.3 | |
| G19 | 299.9 ef | 0.7 | 99.3 | |
| Group 4 | G21 | 214.0 fgh | 1.4 | 98.6 |
| G22 | 210.6 hi | 1.5 | 98.5 | |
| G23 | 270.9 eg | 0.8 | 99.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Oliveira Campos, A.; Harrison, M.D.; Marshall, D.L.; Strong, P.J. Distributions of Lanostene-Derived Triterpenoids and Glucan Content in the Fruiting Bodies of the Australian Ganoderma Species. J. Fungi 2024, 10, 723. https://doi.org/10.3390/jof10100723
De Oliveira Campos A, Harrison MD, Marshall DL, Strong PJ. Distributions of Lanostene-Derived Triterpenoids and Glucan Content in the Fruiting Bodies of the Australian Ganoderma Species. Journal of Fungi. 2024; 10(10):723. https://doi.org/10.3390/jof10100723
Chicago/Turabian StyleDe Oliveira Campos, Aline, Mark D. Harrison, David L. Marshall, and Peter James Strong. 2024. "Distributions of Lanostene-Derived Triterpenoids and Glucan Content in the Fruiting Bodies of the Australian Ganoderma Species" Journal of Fungi 10, no. 10: 723. https://doi.org/10.3390/jof10100723
APA StyleDe Oliveira Campos, A., Harrison, M. D., Marshall, D. L., & Strong, P. J. (2024). Distributions of Lanostene-Derived Triterpenoids and Glucan Content in the Fruiting Bodies of the Australian Ganoderma Species. Journal of Fungi, 10(10), 723. https://doi.org/10.3390/jof10100723








