Exploring Plant Growth-Promoting Traits of Endophytic Fungi Isolated from Ligusticum chuanxiong Hort and Their Interaction in Plant Growth and Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Endophytic Fungi and Plant Materials
2.2. Evaluation of PGP Traits of Endophytic Fungi
2.2.1. Analysis of Indole-3-Acetic Acid (IAA)
2.2.2. Phosphate Solubilization
2.2.3. Nitrogen Fixation
2.2.4. Siderophores Production
2.2.5. Potassium Solubilization
2.2.6. Ammonia Production
2.3. Cocultivation of Endophytic Fungal Strains with Tobacco
2.4. In Vitro Germination and Seedling Vigor Test
2.5. Pot Experiment
2.6. Determination of Fungus Colonization in Tobacco Roots
2.7. Analysis of Chlorophyll Content and Photosynthesis Parameters
2.8. Analysis of Soluble Protein and Soluble Sugar Content
2.9. Quantification of Antioxidant Enzyme Activity and Oxidative Stress Markers
2.10. Detection of Expression Levels of Growth-Related Genes by Real-Time PCR (RT-qPCR)
2.11. Statistical Analyses
3. Results
3.1. In Vitro Assessment for PGP Traits of Endophytic Fungi
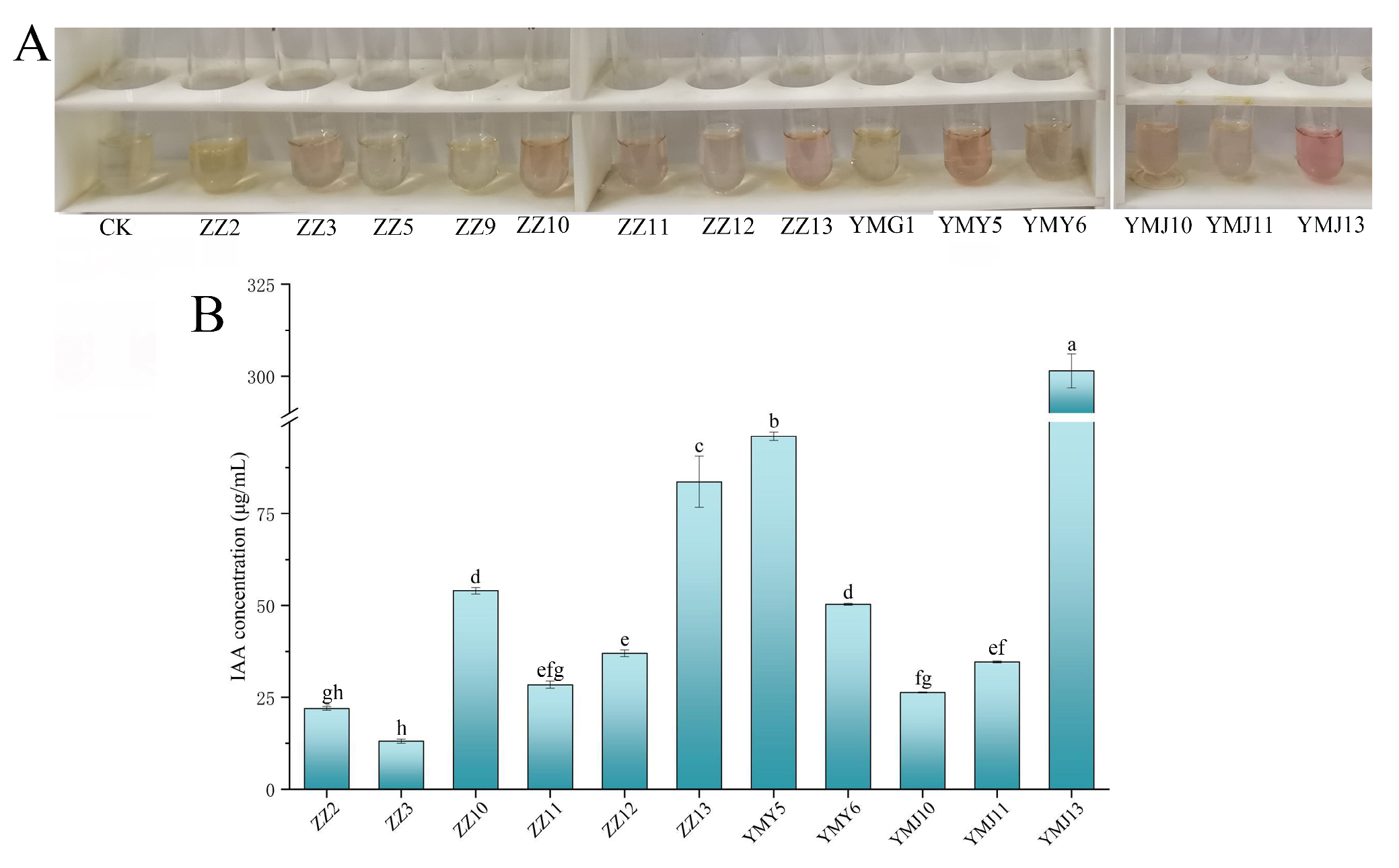
3.1.1. Production of Indole-3-Acidic Acid (IAA)
3.1.2. Qualitative and Quantitative Analysis of Phosphate Solubilization
3.1.3. Siderophores and Nitrogen Fixation
3.1.4. Potassium Solubilization and Ammonia Production
3.2. Cocultivation of Endophytic Fungal and Tobacco Seedlings
3.3. Effects of Endophytic Fungi on Tobacco Seed Viability
3.4. The Effect of Endophytic Fungi on Tobacco Growth in Pot Experiments
3.5. Effects of Endophytic Fungi on Chlorophyll Content and Photosynthesis Parameters of Tobacco
3.6. Effects of Endophytic Fungi on the Content of Soluble Sugar and Soluble Protein of Tobacco
3.7. Analysis of Endophytic Fungi on the Antioxidant Properties of Tobacco
3.8. Effects of Endophytic Fungi on the Expression of Tobacco Growth-Related Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, S.; Chen, S.; Wang, B.; Li, Q.; Zu, J.; Yu, J.; Ding, Z.; Zhou, F. Screening of Endophytic Fungi from Cremastra appendiculata and Their Potential for Plant Growth Promotion and Biological Control. Folia Microbiol. 2023, 68, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Fontana, D.C.; de Paula, S.; Torres, A.G.; de Souza, V.H.M.; Pascholati, S.F.; Schmidt, D.; Dourado Neto, D. Endophytic Fungi: Biological Control and Induced Resistance to Phytopathogens and Abiotic Stresses. Pathogens 2021, 10, 570. [Google Scholar] [CrossRef] [PubMed]
- Ņečajeva, J.; Borodušķe, A.; Nikolajeva, V.; Seņkovs, M.; Kalniņa, I.; Roga, A.; Skinderskis, E.; Fridmanis, D. Epiphytic and Endophytic Fungi Colonizing Seeds of Two Poaceae Weed Species and Fusarium spp. Seed Degradation Potential In Vitro. Microorganisms 2023, 11, 184. [Google Scholar] [CrossRef] [PubMed]
- Eo, J.-K.; Choi, J.W.; Eom, A.H. Diversity, Distribution, and Host Plant of Endophytic Fungi: A Focus on Korea. Mycobiology 2022, 50, 399–407. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Babalola, O.O. Biotechnological Overview of Agriculturally Important Endophytic Fungi. Hortic. Environ. Biotechnol. 2021, 62, 507–520. [Google Scholar] [CrossRef]
- Anand, U.; Pal, T.; Yadav, N.; Singh, V.K.; Tripathi, V.; Choudhary, K.K.; Shukla, A.K.; Sunita, K.; Kumar, A.; Bontempi, E. Current Scenario and Future Prospects of Endophytic Microbes: Promising Candidates for Abiotic and Biotic Stress Management for Agricultural and Environmental Sustainability. Microb. Ecol. 2023, 86, 1455–1486. [Google Scholar] [CrossRef]
- Al-Hosni, K.; Shahzad, R.; Latif, K.A.; Muhammad, I.Q.; Al, H.A.; Al, R.A.; Asaf, S.; Kang, S.M.; Yun, B.W.; Lee, I.J. Preussia sp. BSL-10 Producing Nitric Oxide, Gibberellins, and Indole Acetic Acid and Improving Rice Plant Growth. J. Plant Interact. 2018, 13, 112–118. [Google Scholar] [CrossRef]
- Nutaratat, P.; Srisuk, N.; Arunrattiyakorn, P.; Limtong, S. Indole-3-Acetic Acid Biosynthetic Pathways in the Basidiomycetous Yeast Rhodosporidium paludigenum. Arch. Microbiol. 2016, 198, 429–437. [Google Scholar] [CrossRef]
- Vacheron, J.; Desbrosses, G.; Bouffaud, M.L.; Touraine, B.; Moënne-Loccoz, Y.; Muller, D.; Legendre, L.; Wisniewski-Dyé, F.; Prigent-Combaret, C. Plant Growth-Promoting Rhizobacteria and Root System Functioning. Front. Plant Sci. 2013, 4, 356. [Google Scholar] [CrossRef]
- Poveda, J.; Eugui, D.; Abril-Urías, P.; Velasco, P. Endophytic Fungi as Direct Plant Growth Promoters for Sustainable Agricultural Production. Symbiosis 2021, 85, 1–19. [Google Scholar] [CrossRef]
- Suebrasri, T.; Harada, H.; Jogloy, S.; Ekprasert, J.; Boonlue, S. Auxin-Producing Fungal Endophytes Promote Growth of Sunchoke. Rhizosphere 2020, 16, 100271. [Google Scholar] [CrossRef]
- Jia, M.; Chen, L.; Xin, H.L.; Zheng, C.J.; Rahman, K.; Han, T.; Qin, L.P. A Friendly Relationship between Endophytic Fungi and Medicinal Plants: A Systematic Review. Front. Microbiol. 2016, 7, 906. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, L.; Tian, P.; Xiang, W.; Lu, X.; Huang, R.; Li, L. Fungal Endophytes from Medicinal Plant Bletilla striata (Thunb.) Reichb. F. Promote the Host Plant Growth and Phenolic Accumulation. S. Afr. J. Bot. 2021, 143, 25–32. [Google Scholar] [CrossRef]
- Acuña-Rodríguez, I.S.; Ballesteros, G.I.; Atala, C.; Gundel, P.E.; Molina-Montenegro, M.A. Hardening Blueberry Plants to Face Drought and Cold Events by the Application of Fungal Endophytes. Agronomy 2022, 12, 1000. [Google Scholar] [CrossRef]
- Ismail; Hamayun, M.; Hussain, A.; Iqbal, A.; Khan, S.A.; Lee, I.J. Endophytic Fungus Aspergillus japonicus Mediates Host Plant Growth under Normal and Heat Stress Conditions. BioMed Res. Int. 2018, 2018, 7696831. [Google Scholar] [CrossRef]
- Mao, W.; Wu, Y.; Li, F.; Tang, W.; Gong, W.; Han, X.; White, J.F.; Ji, X.; Li, H. Seed Endophytes and Their Roles in Host Plant Stress Resistance. J. Soil. Sci. Plant Nutr. 2023, 23, 2927–2937. [Google Scholar] [CrossRef]
- Chen, M.; Ding, Z.; Zhou, M.; Shang, Y.; Li, C.; Li, Q.; Bu, T.; Tang, Z.; Chen, H. The Diversity of Endophytic Fungi in Tartary Buckwheat (Fagopyrum tataricum) and Its Correlation with Flavonoids and Phenotypic Traits. Front. Microbiol. 2024, 15, 1360988. [Google Scholar] [CrossRef]
- Ye, B.; Wu, Y.; Zhai, X.; Zhang, R.; Wu, J.; Zhang, C.; Rahman, K.; Qin, L.; Han, T.; Zheng, C. Beneficial Effects of Endophytic Fungi from the Anoectochilus and Ludisia Species on the Growth and Secondary Metabolism of Anoectochilus roxburghii. ACS Omega 2020, 5, 3487–3497. [Google Scholar] [CrossRef]
- Durán-López, M.E.; Caroca-Cáceres, R.; Jahreis, K.; Narváez-Vera, M.; Ansaloni, R.; Cazar, M.E. The Micorryzal Fungi Ceratobasidium sp. and Sebacina vermifera Promote Seed Germination and Seedling Development of the Terrestrial Orchid Epidendrum secundum Jacq. S. Afr. J. Bot. 2019, 125, 54–61. [Google Scholar] [CrossRef]
- Sun, X.; Wang, N.; Li, P.; Jiang, Z.; Liu, X.; Wang, M.; Su, Z.; Zhang, C.; Lin, F.; Liang, Y. Endophytic Fungus Falciphora oryzae Promotes Lateral Root Growth by Producing Indole Derivatives after Sensing Plant Signals. Plant Cell Environ. 2020, 43, 358–373. [Google Scholar] [CrossRef]
- Tang, M.J.; Lu, F.; Yang, Y.; Sun, K.; Zhu, Q.; Xu, F.J.; Zhang, W.; Dai, C.C. Benefits of Endophytic Fungus Phomopsis liquidambaris Inoculation for Improving Mineral Nutrition, Quality, and Yield of Rice Grains Under Low Nitrogen and Phosphorus Condition. J. Plant Growth Regul. 2022, 41, 2499–2513. [Google Scholar] [CrossRef]
- Bilal, L.; Asaf, S.; Hamayun, M.; Gul, H.; Iqbal, A.; Ullah, I.; Lee, I.J.; Hussain, A. Plant Growth Promoting Endophytic Fungi Asprgillus fumigatus TS1 and Fusarium proliferatum BRL1 Produce Gibberellins and Regulates Plant Endogenous Hormones. Symbiosis 2018, 76, 117–127. [Google Scholar] [CrossRef]
- Yang, J.; Feng, X.L.; Yu, Y.; Wang, Q.; Zou, J.; Wang, C.X.; Mu, Z.Q.; Yao, X.S.; Gao, H. Novel Phthalide Derivatives Identified from Ligusticum chuanxiong (Chuanxiong). Chin. Med. 2016, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Yi, T.; Fang, J.Y.; Zhu, L.; Tang, Y.N.; Ji, H.; Zhang, Y.Z.; Yu, J.C.; Zhang, X.J.; Yu, Z.L.; Zhao, Z.Z. The Variation in the Major Constituents of the Dried Rhizome of Ligusticum chuanxiong (Chuanxiong) after Herbal Processing. Chin. Med. 2016, 11, 26. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Zhou, H.; Liang, X.; Zhang, M.; Tang, Y.; Wang, J.; Mao, J. The Isolation, Structural Features and Biological Activities of Polysaccharide from Ligusticum chuanxiong: A Review. Carbohydr. Polym. 2022, 285, 118971. [Google Scholar] [CrossRef]
- Tang, Z.; Qin, Y.; Chen, W.; Zhao, Z.; Lin, W.; Xiao, Y.; Chen, H.; Liu, Y.; Chen, H.; Bu, T. Diversity, Chemical Constituents, and Biological Activities of Endophytic Fungi Isolated from Ligusticum chuanxiong Hort. Front. Microbiol. 2021, 12, 771000. [Google Scholar] [CrossRef]
- Tang, Z.; Qin, Y.; Wang, Y.; Lin, W.; Wang, Q.; Shen, N.; Xiao, Y.; Chen, H.; Chen, H.; Bu, T. The Endophytic Fungus Penicillium oxalicum Isolated from Ligusticum chuanxiong Hort Possesses DNA Damage-Protecting Potential and Increases Stress Resistance Properties in Caenorhabditis elegans. Front. Pharmacol. 2022, 13, 983716. [Google Scholar] [CrossRef]
- Kang, L.; He, D.; Wang, H.; Han, G.; Lv, H.; Xiao, W.; Zhang, Z.; Yan, Z.; Huang, L. “Breeding on Mountains” Resulted in the Reorganization of Endophytic Fungi in Asexually Propagated Plants (Ligusticum chuanxiong Hort.). Front. Plant Sci. 2021, 12, 740456. [Google Scholar] [CrossRef]
- Khalil, A.M.A.; Hassan, S.E.D.; Alsharif, S.M.; Eid, A.M.; Ewais, E.E.D.; Azab, E.; Gobouri, A.A.; Elkelish, A.; Fouda, A. Isolation and Characterization of Fungal Endophytes Isolated from Medicinal Plant Ephedra pachyclada as Plant Growth-Promoting. Biomolecules 2021, 11, 140. [Google Scholar] [CrossRef]
- Lee, S.H.; Jeon, S.H.; Park, J.Y.; Kim, D.S.; Kim, J.A.; Jeong, H.Y.; Kang, J.W. Isolation and Evaluation of the Antagonistic Activity of Cnidium Officinale Rhizosphere Bacteria against Phytopathogenic Fungi (Fusarium solani). Microorganisms 2023, 11, 1555. [Google Scholar] [CrossRef]
- Lü, Z.W.; Liu, H.Y.; Wang, C.L.; Chen, X.; Huang, Y.X.; Zhang, M.M.; Huang, Q.L.; Zhang, G.F. Isolation of Endophytic Fungi from Cotoneaster multiflorus and Screening of Drought-Tolerant Fungi and Evaluation of Their Growth-Promoting Effects. Front. Microbiol. 2023, 14, 1267404. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Pan, Y.; Shi, Y.; Chen, J.; Gong, D.; Li, Y.; Hao, G.; Han, D. Root Morphogenesis of Arabidopsis thaliana Tuned by Plant Growth-Promoting Streptomyces Isolated from Root-Associated Soil of Artemisia Annua. Front. Plant Sci. 2022, 12, 802737. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ren, J.; Wang, W.; Chen, B.; Li, E.; Chen, S. Siderophore and Indolic Acid Production by Paenibacillus triticisoli BJ-18 and Their Plant Growth-Promoting and Antimicrobe Abilities. PeerJ 2020, 8, e9403. [Google Scholar] [CrossRef] [PubMed]
- Taheri, P.; Kaida, R.; Dastogeer, K.M.G.; Appiah, K.S.; Yasuda, M.; Tanaka, K.; Mardani Korrani, H.; Azizi, M.; Okazaki, S.; Fujii, Y. Isolation and Functional Characterization of Culture-Dependent Endophytes Associated with Vicia villosa Roth. Agronomy 2022, 12, 2417. [Google Scholar] [CrossRef]
- Aallam, Y.; Maliki, B.E.; Dhiba, D.; Lemriss, S.; Souiri, A.; Haddioui, A.; Tarkka, M.; Hamdali, H. Multiple Potential Plant Growth Promotion Activities of Endemic Streptomyces spp. from Moroccan Sugar Beet Fields with Their Inhibitory Activities against Fusarium spp. Microorganisms 2021, 9, 1429. [Google Scholar] [CrossRef]
- Ding, C.; Wang, Q.B.; Guo, S.; Wang, Z. The Improvement of Bioactive Secondary Metabolites Accumulation in Rumex gmelini Turcz through Co-Culture with Endophytic Fungi. Braz. J. Microbiol. 2018, 49, 362–369. [Google Scholar] [CrossRef]
- Wang, J.; Chen, T.; White, J.F.; Wei, X.; Li, X.; Li, C. Pantoea agglomerans, a Seed-Borne Plant Pathogenic Bacterium, Decreased Seed Germination, Seedling Growth and Seed Quality of Oat. Eur. J. Plant Pathol. 2022, 162, 667–679. [Google Scholar] [CrossRef]
- Gateta, T.; Nacoon, S.; Seemakram, W.; Ekprasert, J.; Theerakulpisut, P.; Sanitchon, J.; Suwannarach, N.; Boonlue, S. The Potential of Endophytic Fungi for Enhancing the Growth and Accumulation of Phenolic Compounds and Anthocyanin in Maled Phai Rice (Oryza sativa L.). J. Fungi 2023, 9, 937. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper Enzymes in Isolated Chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, H.; Li, P.; Jin, J.; Li, Z. The Bacterial Consortia Promote Plant Growth and Secondary Metabolite Accumulation in Astragalus mongholicus under Drought Stress. BMC Plant Biology 2022, 22, 475. [Google Scholar] [CrossRef]
- Priyadharsini, P.; Muthukumar, T. The Root Endophytic Fungus Curvularia geniculata from Parthenium hysterophorus Roots Improves Plant Growth through Phosphate Solubilization and Phytohormone Production. Fungal Ecol. 2017, 27, 69–77. [Google Scholar] [CrossRef]
- Mehmood, A.; Hussain, A.; Irshad, M.; Hamayun, M.; Iqbal, A.; Khan, N. In Vitro Production of IAA by Endophytic Fungus Aspergillus awamori and Its Growth Promoting Activities in Zea mays. Symbiosis 2019, 77, 225–235. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Liu, L.; Li, S.; Xie, J.; Xue, X.; Jiang, Y. Screening of Phosphate-Solubilizing Bacteria and Their Abilities of Phosphorus Solubilization and Wheat Growth Promotion. BMC Microbiol. 2022, 22, 296. [Google Scholar] [CrossRef] [PubMed]
- Alamzeb, M. Inamullah Management of Phosphorus Sources in Combination with Rhizobium and Phosphate Solubilizing Bacteria Improve Nodulation, Yield and Phosphorus Uptake in Chickpea. Gesunde Pflanz. 2023, 75, 549–564. [Google Scholar] [CrossRef]
- Favre-Godal, Q.; Schwob, P.; Lecoultre, N.; Hofstetter, V.; Gourguillon, L.; Riffault-Valois, L.; Lordel-Madeleine, S.; Gindro, K.; Choisy, P. Plant-Microbe Features of Dendrobium fimbriatum (Orchidaceae) Fungal Community. Symbiosis 2021, 85, 31–46. [Google Scholar] [CrossRef]
- Chowdappa, S.; Jagannath, S.; Konappa, N.; Udayashankar, A.C.; Jogaiah, S. Detection and Characterization of Antibacterial Siderophores Secreted by Endophytic Fungi from Cymbidium aloifolium. Biomolecules 2020, 10, 1412. [Google Scholar] [CrossRef]
- Khan, A.; Singh, P.; Srivastava, A. Synthesis, Nature and Utility of Universal Iron Chelator—Siderophore: A Review. Microbiol. Res. 2018, 212, 103–111. [Google Scholar] [CrossRef]
- Banik, A.; Dash, G.K.; Swain, P.; Kumar, U.; Mukhopadhyay, S.K.; Dangar, T.K. Application of Rice (Oryza Sativa L.) Root Endophytic Diazotrophic Azotobacter sp. Strain Avi2 (MCC 3432) Can Increase Rice Yield under Green House and Field Condition. Microbiol. Res. 2019, 219, 56–65. [Google Scholar] [CrossRef]
- Liu, Q.; Li, L.; Chen, Y.; Wang, S.; Xue, L.; Meng, W.; Jiang, J.; Cao, X. Diversity of Endophytic Microbes in Taxus yunnanensis and Their Potential for Plant Growth Promotion and Taxane accumulation. Microorganisms 2023, 11, 1645. [Google Scholar] [CrossRef]
- Emami, S.; Alikhani, H.A.; Pourbabaei, A.A.; Etesami, H.; Sarmadian, F.; Motessharezadeh, B. Effect of Rhizospheric and Endophytic Bacteria with Multiple Plant Growth Promoting Traits on Wheat Growth. Environ. Sci. Pollut. Res. 2019, 26, 19804–19813. [Google Scholar] [CrossRef]
- Fodor, J.; Kámán-Tóth, E.; Dankó, T.; Schwarczinger, I.; Bozsó, Z.; Pogány, M. Description of the Nicotiana benthamiana−Cercospora Nicotianae Pathosystem. Phytopathology® 2018, 108, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ye, Z.X.; Wang, Y.Z.; Wang, X.J.; Chen, J.P.; Huang, H.J. Transcriptomic Analysis of Tobacco Plants in Response to Whitefly Infection. Genes 2023, 14, 1640. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, X.; Li, J.; Huang, J.; Bao, S.; He, C.; Zhang, M.; Xiang, T. Metabolites of Zearalenone and Phytohormones Secreted by Endophytic Fungus Strain TH15 Regulating the Root Development in Tetrastigma hemsleyanum. Plant Cell Tiss. Organ. Cult. 2022, 150, 683–694. [Google Scholar] [CrossRef]
- Zhao, D.; Jiao, J.; Du, B.; Liu, K.; Wang, C.; Ding, Y. Volatile Organic Compounds from Lysinibacillus macroides Regulating the Seedling Growth of Arabidopsis thaliana. Physiol. Mol. Biol. Plants 2022, 28, 1997–2009. [Google Scholar] [CrossRef] [PubMed]
- Fincheira, P.; Venthur, H.; Mutis, A.; Parada, M.; Quiroz, A. Growth Promotion of Lactuca sativa in Response to Volatile Organic Compounds Emitted from Diverse Bacterial Species. Microbiol. Res. 2016, 193, 39–47. [Google Scholar] [CrossRef]
- Shearin, Z.R.C.; Filipek, M.; Desai, R.; Bickford, W.A.; Kowalski, K.P.; Clay, K. Fungal Endophytes from Seeds of Invasive, Non-Native Phragmites Australis and Their Potential Role in Germination and Seedling Growth. Plant Soil 2018, 422, 183–194. [Google Scholar] [CrossRef]
- Agbodjato, N.A.; Noumavo, P.A.; Adjanohoun, A.; Agbessi, L.; Baba-Moussa, L. Synergistic Effects of Plant Growth Promoting Rhizobacteria and Chitosan on In Vitro Seeds Germination, Greenhouse Growth, and Nutrient Uptake of Maize (Zea mays L.). Biotechnol. Res. Int. 2016, 2016, 7830182. [Google Scholar] [CrossRef]
- El-Nagar, D.; Salem, S.H.; El-Zamik, F.I.; El-Basit, H.M.I.A.; Galal, Y.G.M.; Soliman, S.; Aziz, H.A.; Rizk, M.A.; El-Sayed, E.-S.R. Bioprospecting Endophytic Fungi for Bioactive Metabolites with Seed Germination Promoting Potentials. BMC Microbiol. 2024, 24, 200. [Google Scholar] [CrossRef]
- Ahmad, R.Z.; Khalid, R.; Aqeel, M.; Ameen, F.; Li, C.J. Fungal Endophytes Trigger Achnatherum inebrians Germination Ability against Environmental Stresses. S. Afr. J. Bot. 2020, 134, 230–236. [Google Scholar] [CrossRef]
- Liu, S.; Lv, D.; Lu, C.; Xiao, Y.; Wang, S.; Zhou, W.; Niu, J.; Wang, Z. Tulasnella Can Contribute to Breakthrough in Germination of the Medicinal Orchid Bletilla striata via Producing Plant Hormones. Rhizosphere 2022, 22, 100527. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, W.; Zhao, Z.; Long, Y.; Fan, R. Biocontrol Potential and Growth-Promoting Effect of Endophytic Fungus Talaromyces muroii SD1-4 against Potato Leaf Spot Disease Caused by Alternaria alternata. BMC Microbiol. 2024, 24, 255. [Google Scholar] [CrossRef] [PubMed]
- Petkova, M.; Petrova, S.; Spasova-Apostolova, V.; Naydenov, M. Tobacco Plant Growth-Promoting and Antifungal Activities of Three Endophytic Yeast Strains. Plants 2022, 11, 751. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Peng, F.; Yu, J.; Li, Q. Identification and Characterization of a Plant Endophytic Fungus Paraphaosphaeria sp. JRF11 and Its Growth-Promoting Effects. J. Fungi 2024, 10, 120. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Mojahid, M.S.; Bidochka, M.J. Root Colonization of Industrial Hemp (Cannabis sativa L.) by the Endophytic Fungi Metarhizium and Pochonia Improves Growth. Ind. Crops Prod. 2023, 198, 116716. [Google Scholar] [CrossRef]
- Turjaman, M.; Herdyantara, B.; Faulina, S.A.; Agustini, L.; Irianto, R.S.B.; Hidayat, A.; Wahno, I.; Murdani; Tjahyono, B.; Indrayadi, H. Mycorrhizal Colonization of Indigenous Tropical Tree Species Grown in Peat Swamp Forests of Sumatera, Indonesia. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; Volume 308, p. 012049. [Google Scholar]
- Giordano, D.F.; Pastor, N.A.; Rouws, L.F.M.; de Freitas, K.M.; Erazo, J.G.; Del Canto, A.; da Silva Coelho, I.; Oddino, C.M.; Torres, A.M. Trichoderma harzianum ITEM 3636 Colonizes Peanut Roots as an Endophyte and Protects the Plants against Late Leaf Spot. Symbiosis 2023, 89, 337–352. [Google Scholar] [CrossRef]
- Begum, N.; Wang, L.; Ahmad, H.; Akhtar, K.; Roy, R.; Khan, M.I.; Zhao, T. Co-Inoculation of Arbuscular Mycorrhizal Fungi and the Plant Growth-Promoting Rhizobacteria Improve Growth and Photosynthesis in Tobacco Under Drought Stress by Up-Regulating Antioxidant and Mineral Nutrition Metabolism. Microb. Ecol. 2022, 83, 971–988. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Zhao, M.R.; Wang, J.Q.; Hu, B.Y.; Chen, Q.J.; Qin, Y.; Zhang, G.Q. Effects of Microbial Inoculants on Agronomic Characters, Physicochemical Properties and Nutritional Qualities of Lettuce and Celery in Hydroponic Cultivation. Sci. Hortic. 2023, 320, 112202. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, S.; Li, Y.; Shi, Y. Effect of Nano-Calcium Carbonate on Morphology, Antioxidant Enzyme Activity and Photosynthetic Parameters of Wheat (Triticum aestivum L.) Seedlings. Chem. Biol. Technol. Agric. 2023, 10, 31. [Google Scholar] [CrossRef]
- Li, X.; Lin, Y.; Qin, Y.; Han, G.; Wang, H.; Yan, Z. Beneficial Endophytic Fungi Improve the Yield and Quality of Salvia miltiorrhiza by Performing Different Ecological Functions. PeerJ 2024, 12, e16959. [Google Scholar] [CrossRef]
- Ortiz, J.; Soto, J.; Fuentes, A.; Herrera, H.; Meneses, C.; Arriagada, C. The Endophytic Fungus Chaetomium cupreum Regulates Expression of Genes Involved in the Tolerance to Metals and Plant Growth Promotion in Eucalyptus globulus Roots. Microorganisms 2019, 7, 490. [Google Scholar] [CrossRef]
- Xu, J.; Qin, L.; Xu, X.; Shen, H.; Yang, X. Bacillus paralicheniformis RP01 Enhances the Expression of Growth-Related Genes in Cotton and Promotes Plant Growth by Altering Microbiota inside and Outside the Root. Int. J. Mol. Sci. 2023, 24, 7227. [Google Scholar] [CrossRef] [PubMed]
- Aktar, N.; Hossain, M.S.; Amin, M.R.; Ahmed, R.; Ahmed, B.; Ullah, M.W.; Hossain, Q.M.; Islam, M.S. Endophytic Bacteria Improve Jute Growth through Induction of Phytohormones-Related Gene Expression. Physiol. Mol. Plant Pathol. 2023, 127, 102115. [Google Scholar] [CrossRef]
- Xu, L.; Wu, C.; Oelmüller, R.; Zhang, W. Role of Phytohormones in Piriformospora Indica-Induced Growth Promotion and Stress Tolerance in Plants: More Questions Than Answers. Front. Microbiol. 2018, 9, 1646. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.L.; Li, Y.; Tian, W.; Sun, Y.; Chen, F.; Zhang, Y.; Zhai, Y.; Zhang, J.; Su, H.; Wang, L. A Novel Dark Septate Fungal Endophyte Positively Affected Blueberry Growth and Changed the Expression of Plant Genes Involved in Phytohormone and Flavonoid Biosynthesis. Tree Physiol. 2020, 40, 1080–1094. [Google Scholar] [CrossRef]









| Gene | Gene Accession | Sequence | Primers | Reference Gene |
|---|---|---|---|---|
| NtCYCD3 | XM_016602338.1 | F′ | AGAGAGGCCGTTGATTGGAT | Cell cycle-related |
| R′ | GAAAGACAGGACACAGCAGC | |||
| NtARF6 | XM_016594427.1 | F′ | CCCACTACTTATTTGCCAGCTT | Auxin response factors |
| R′ | AGATGCCTCCTTTTGCTCT | |||
| NtARF16 | XM_016609598.1 | F′ | ACCTGAGCTAAGTACCGTAGAA | Auxin response factors |
| R′ | GCTTGCGGTGAAGAAATTGAG | |||
| NtGA3ox-2 | EF471116 | F′ | TGCTCGGCCCTACAACTAAA | GA3 biosynthesis gene |
| R′ | AACCGTGACCCAACCATTTC | |||
| NtDWF4 | XM_016618664.1 | F′ | CTGCCAGCCTTTGGTAATTTG | BR biosynthesis-related genes |
| R′ | CACTTCAAACCGTTCGTCATTT | |||
| NtBIN2 | XM_016592263.1 | F′ | CTCATACATCTGCTCCCGGT | BR signalling genes |
| R′ | ATTTCCTCCCTTGTCGGTGT | |||
| NtYUCCA8 | XM_016592388 | F′ | ATGTGTATGGGTAAATGGTCC | IAA biosynthesis gene |
| R′ | CAGATTTTTCCAAGATTACAC | |||
| NtICS | XM_016616491.1 | F′ | ATGTATGCTGGTCCTGTTG | SA biosynthesis gene |
| R′ | AATCACTTCCTTCCACTATCC | |||
| β-Actin | AB158612 | F′ | GATCTTGCTGGTCGTGATCT | Reference gene |
| R′ | ACTTCCGGACATCTGAACCT |
| Strains | Siderophores | Nitrogen Fixation | Ammonia Production | Potassium Solubilization |
|---|---|---|---|---|
| ZZ2 | − | + | − | + |
| ZZ3 | − | − | − | − |
| ZZ5 | − | − | − | − |
| ZZ9 | + | + | − | + |
| ZZ10 | + | + | + | − |
| ZZ11 | + | + | − | − |
| ZZ12 | − | − | − | − |
| ZZ13 | + | + | + | − |
| YMG1 | + | + | − | + |
| YMY5 | + | + | + | − |
| YMY6 | + | + | + | − |
| YMJ10 | − | − | − | − |
| YMJ11 | + | − | − | − |
| YMJ13 | + | − | + | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Zhang, X.; Xie, Q.; Tao, J.; Jia, Y.; Xiao, Y.; Tang, Z.; Li, Q.; Yuan, M.; Bu, T. Exploring Plant Growth-Promoting Traits of Endophytic Fungi Isolated from Ligusticum chuanxiong Hort and Their Interaction in Plant Growth and Development. J. Fungi 2024, 10, 713. https://doi.org/10.3390/jof10100713
Wang Q, Zhang X, Xie Q, Tao J, Jia Y, Xiao Y, Tang Z, Li Q, Yuan M, Bu T. Exploring Plant Growth-Promoting Traits of Endophytic Fungi Isolated from Ligusticum chuanxiong Hort and Their Interaction in Plant Growth and Development. Journal of Fungi. 2024; 10(10):713. https://doi.org/10.3390/jof10100713
Chicago/Turabian StyleWang, Qing, Xinyu Zhang, Qiqi Xie, Jiwen Tao, Yujie Jia, Yirong Xiao, Zizhong Tang, Qingfeng Li, Ming Yuan, and Tongliang Bu. 2024. "Exploring Plant Growth-Promoting Traits of Endophytic Fungi Isolated from Ligusticum chuanxiong Hort and Their Interaction in Plant Growth and Development" Journal of Fungi 10, no. 10: 713. https://doi.org/10.3390/jof10100713
APA StyleWang, Q., Zhang, X., Xie, Q., Tao, J., Jia, Y., Xiao, Y., Tang, Z., Li, Q., Yuan, M., & Bu, T. (2024). Exploring Plant Growth-Promoting Traits of Endophytic Fungi Isolated from Ligusticum chuanxiong Hort and Their Interaction in Plant Growth and Development. Journal of Fungi, 10(10), 713. https://doi.org/10.3390/jof10100713






