The Sorting and Transport of the Cargo Protein CcSnc1 by the Retromer Complex Regulate the Growth, Development, and Pathogenicity of Corynespora cassiicola
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains and Growth Conditions
2.2. Sample Preparation
2.3. RNA Extraction and RNA Sequencing
2.4. Bioinformatic Analysis
2.5. qRT-PCR
2.6. Construction of Gene Deletion Mutants and Complementation
2.7. Fungal Growth and Pathogenicity Assays
2.8. Yeast Two-Hybrid (Y2H), Co-Immunoprecipitation (Co-IP) and Bimolecular Fluorescence Complementation (BiFC) Assays
2.9. Staining and Live Cell Imaging of C. cassiicola
2.10. Phylogenetic Analysis and Protein Structure Prediction
3. Results
3.1. Infection of Kiwifruit Leaves by C. cassiicola
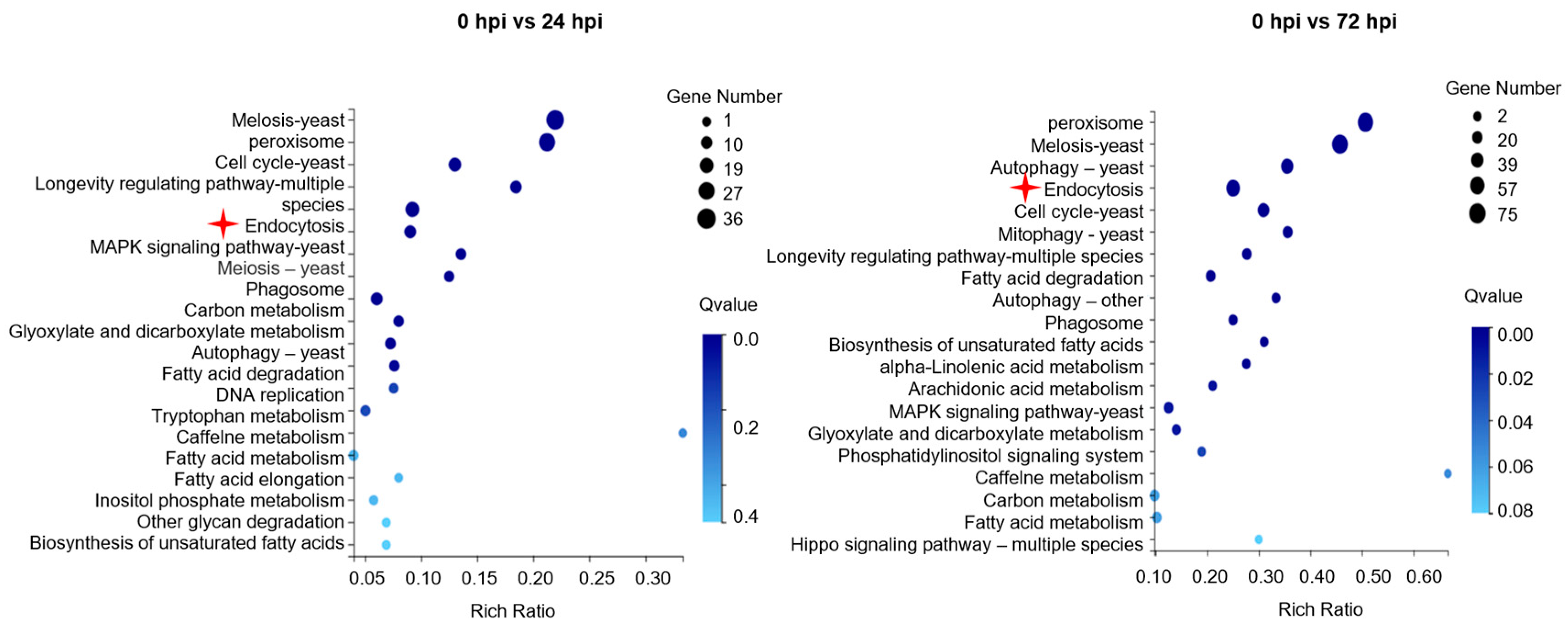
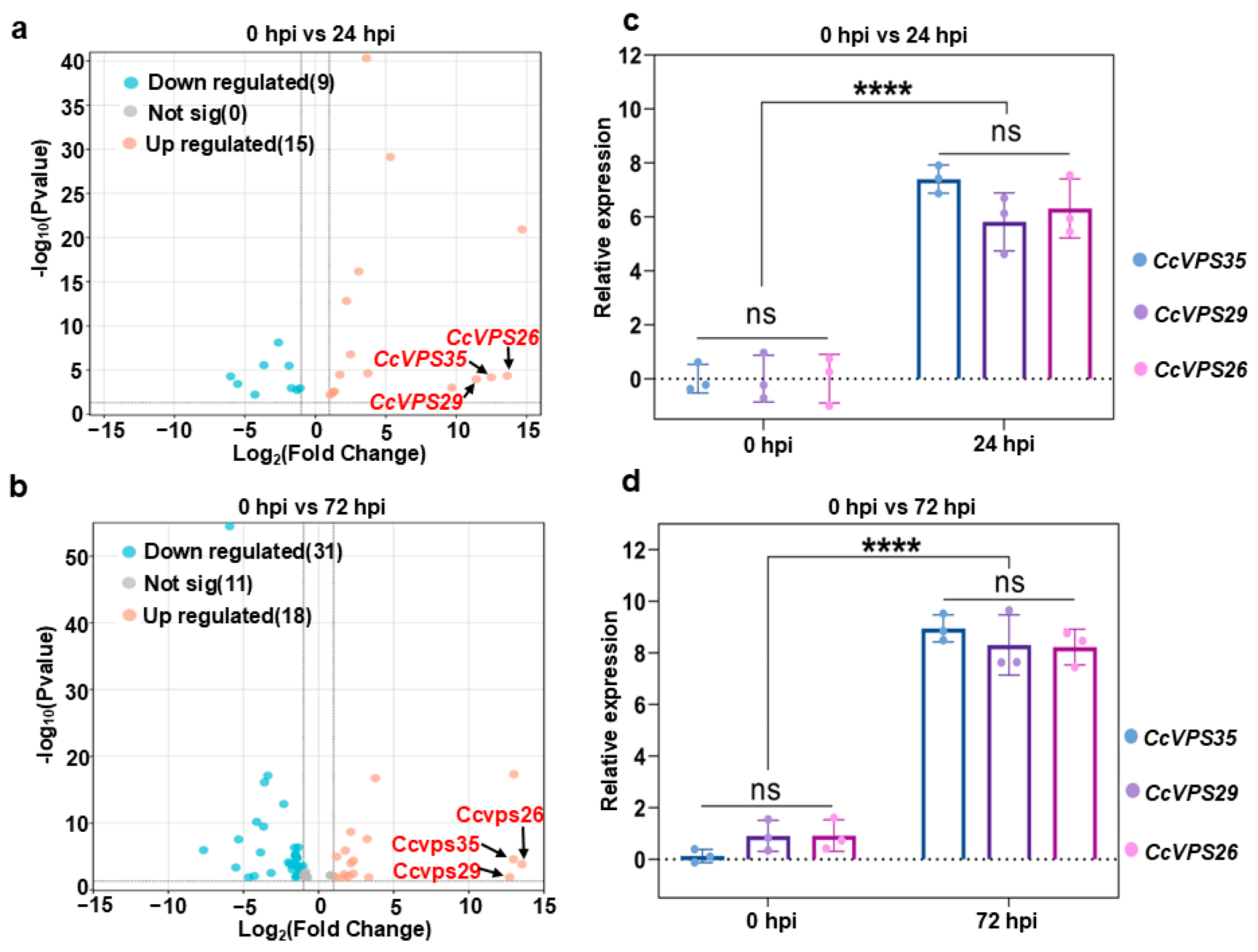
3.2. RNA-Seq Analysis and Validation
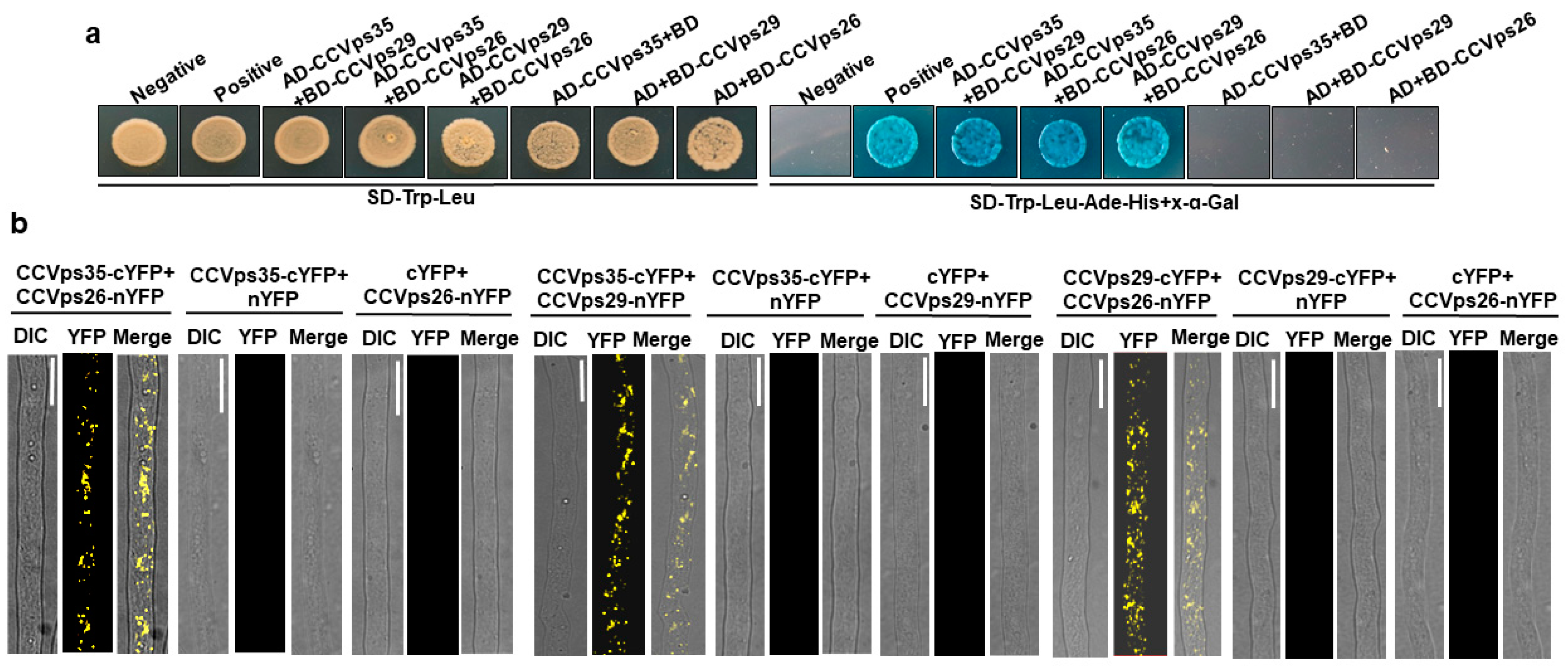
3.3. Retromer Components Are Conserved in C. cassiicola
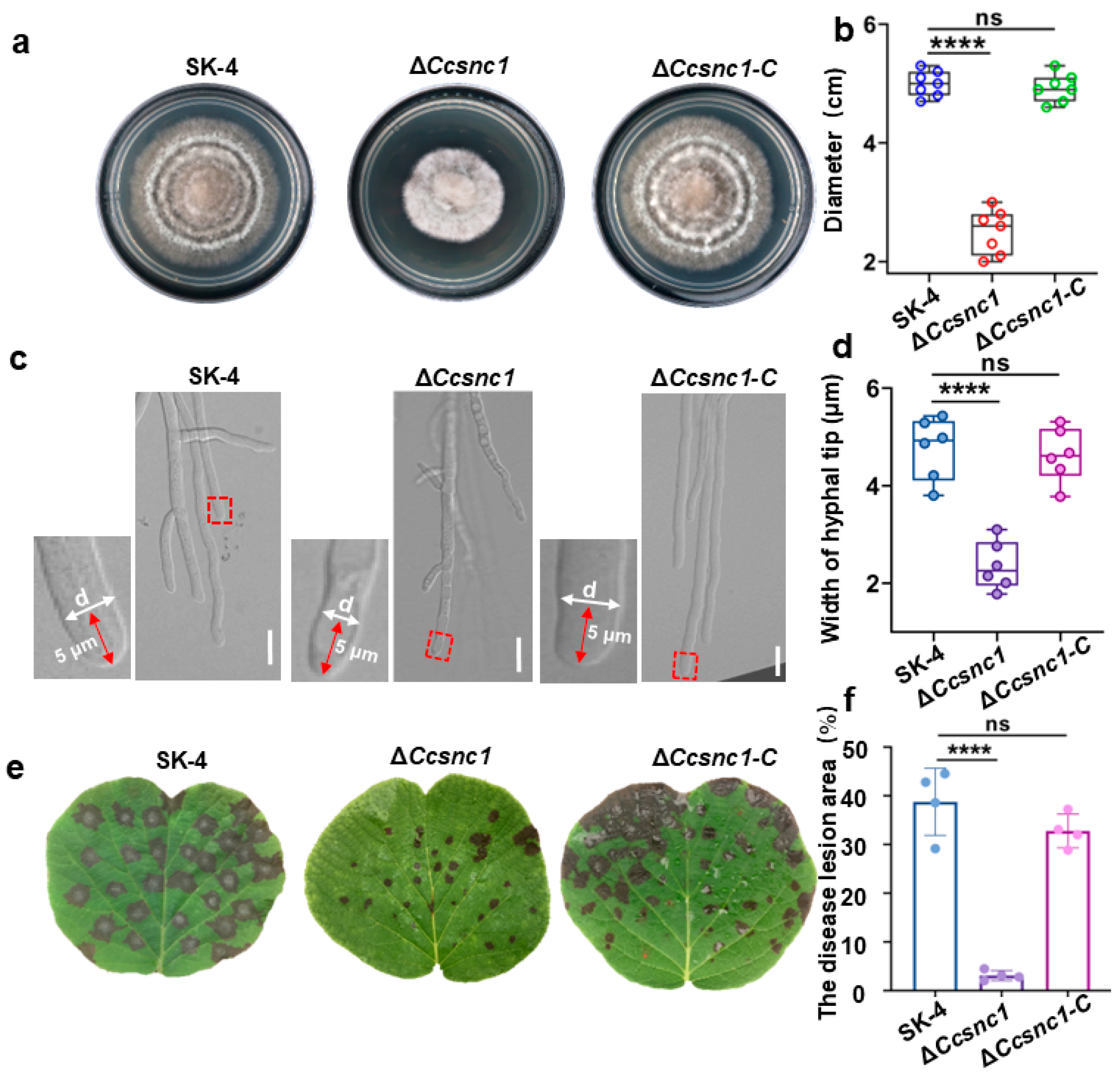
3.4. The Retromer Complex Is Essential for Hyphal Growth and Pathogenicity
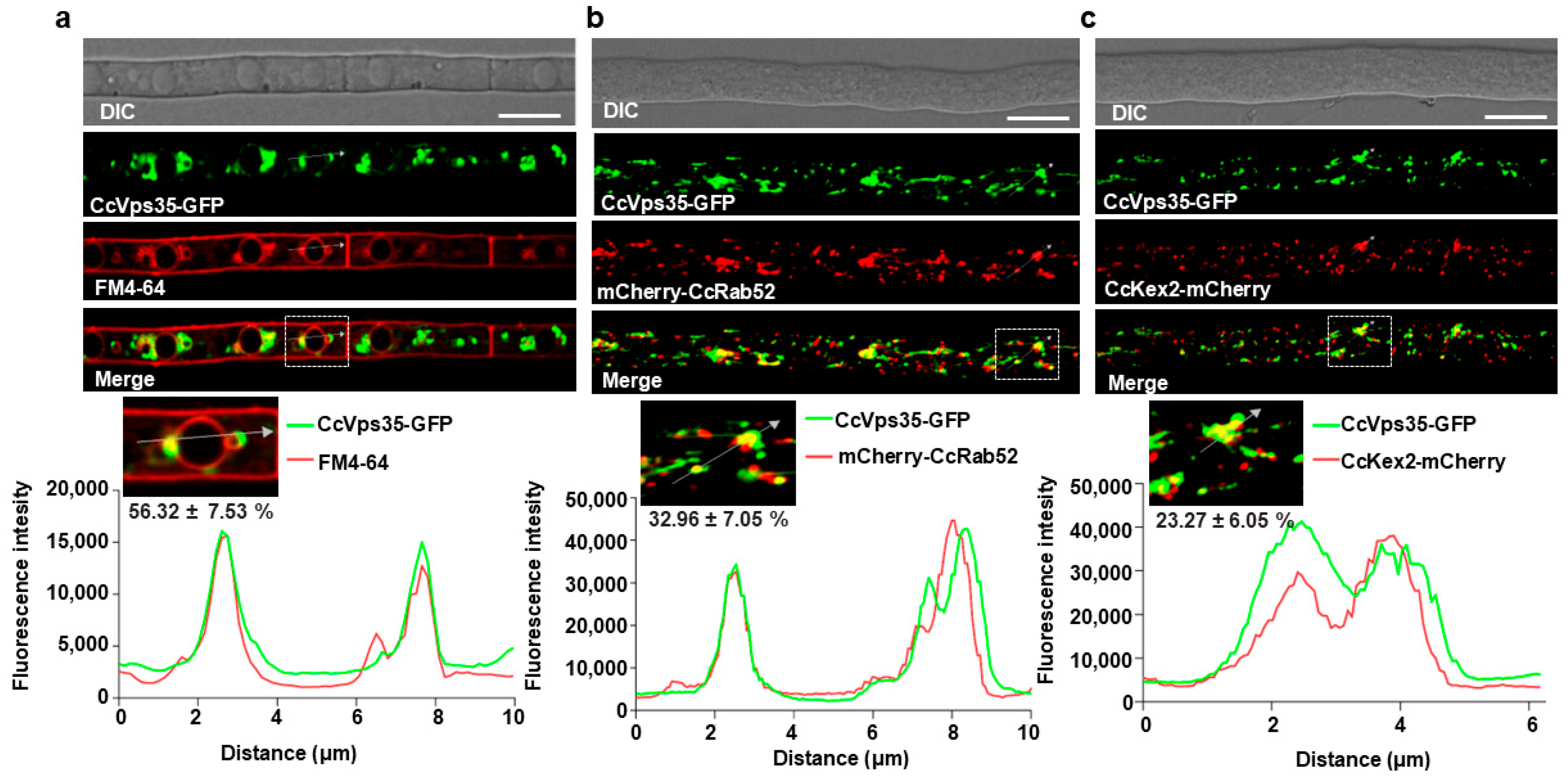
3.5. Retromer Complex Predominantly Localizes to the Vacuole Membrane
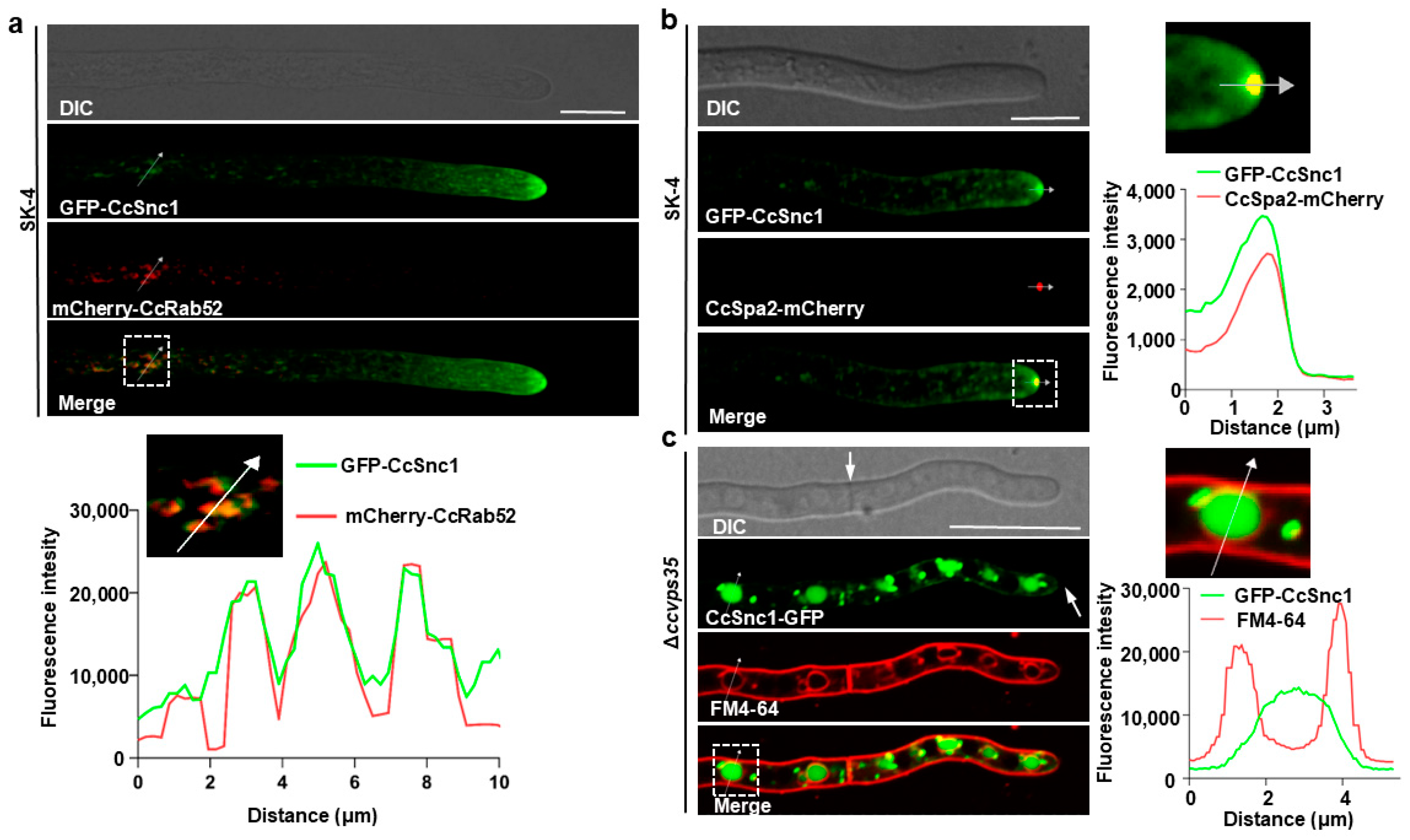
3.6. CcSnc1 Was Cargoes for Retromer-Mediated Trafficking Pathway
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conner, T.S.; Fletcher, B.D.; Haszard, J.J.; Pullar, J.M.; Spencer, E.; Mainvil, L.A.; Vissers, M.C.M. KiwiC for Vitality: Results of a Placebo-Controlled Trial Testing the Effects of Kiwifruit or Vitamin C Tablets on Vitality in Adults with Low Vitamin C Levels. Nutrients 2020, 12, 2898. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Gong, G.; Yu, X.; Xu, J.; Wen, X.; Zhang, M.; Chen, H.; Zheng, X.; Zhou, Y.; Chang, X. First Report of Brown Leaf Spot on Kiwifruit Caused by Corynespora cassiicola in Sichuan, China. Plant Dis. 2015, 99, 725. [Google Scholar] [CrossRef]
- Smith, L.J. Host Range, Phylogenetic, and Pathogenic Diversity of Corynespora cassiicola (Berk. & Curt.) Wei. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 2008. [Google Scholar]
- Gourgues, M.; Brunet-Simon, A.; Lebrun, M.-H.; Levis, C. The tetraspanin BcPls1 is required for appressorium-mediated penetration of Botrytis cinerea into host plant leaves. Mol. Microbiol. 2004, 51, 619–629. [Google Scholar] [CrossRef]
- Dague, E.; Alsteens, D.; Latgé, J.-P.; Dufrêne, Y.F. High-Resolution Cell Surface Dynamics of Germinating Aspergillus fumigatus Conidia. Biophys. J. 2008, 94, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, M.; He, X.; Cai, S.; He, X.; Lu, X. Transcriptome sequencing and characterization of ungerminated and germinated spores of Nosema bombycis. Acta Biochim. Biophys. Sin. 2016, 48, 246–256. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, X.; Luo, J.; Ye, B.; Zhou, Y.; Zhou, L.; Lai, T. Identification of differentially expressed genes involved in spore germination of Penicillium expansum by comparative transcriptome and proteome approaches. Microbiologyopen 2018, 7, e00562. [Google Scholar] [CrossRef]
- Zhi-Heng, L.; Ye, Q.I.; Xin-Yang, H.; Hong, Y.; Yue, H.; Rui, Z.J.C.V. Conditions and Activity Analysis of Cell Wall Degrading Enzymes Produced from Corynespora cassiicola of Brown Spot of Cucumber. Chin. Veg. 2011, 1, 76–80. [Google Scholar]
- Li, B.; Yang, Y.; Cai, J.; Liu, X.; Shi, T.; Li, C.; Chen, Y.; Xu, P.; Huang, G.J.J.o.F. Genomic Characteristics and Comparative Genomics Analysis of Two Chinese Corynespora cassiicola Strains Causing Corynespora Leaf Fall (CLF) Disease. J. Fungi 2021, 7, 485. [Google Scholar] [CrossRef] [PubMed]
- Déon, M.; Bourré, Y.; Gimenez, S.; Berger, A.; Bieysse, D.; Lamotte, F.d.; Poncet, J.L.; Roussel, V.; Bonnot, F.O.; Oliver, G. Characterization of a cassiicolin-encoding gene from Corynespora cassiicola, pathogen of rubber tree (Hevea brasiliensis). Plant Sci. 2012, 185-186, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cao, S.; Zhang, H.; Wei, Y.; Pu, J. CCK1, a PMK1-type MAP kinase is required for hyphal growth, pigmentation, conidiation, enzyme activity, osmotic stress response, and pathogenicity in Corynespora cassiicola. Eur. J. Plant Pathol. 2017, 149, 313–323. [Google Scholar] [CrossRef]
- Ribeiro, S.; Label, P.; Garcia, D.; Montoro, P.; Pujade-Renaud, V. Transcriptome profiling in susceptible and tolerant rubber tree clones in response to cassiicolin Cas1, a necrotrophic effector from Corynespora cassiicola. PLoS ONE 2021, 16, e0254541. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Burd, C.G. Retrograde trafficking and plasma membrane recycling pathways of the budding yeast Saccharomyces cerevisiae. Traffic 2020, 21, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, Y.S.; Sadiq, I.Z.; Aarti, A.; Wang, Z.; Zheng, W. Interplay of transport vesicles during plant-fungal pathogen interaction. Stress Biol. 2023, 3, 35. [Google Scholar] [CrossRef]
- Vagnozzi, A.N.; Praticò, D. Endosomal sorting and trafficking, the retromer complex and neurodegeneration. Mol. Psychiatry 2019, 24, 857–868. [Google Scholar] [CrossRef]
- Chen, X.; Selvaraj, P.; Lin, L.; Fang, W.; Wu, C.; Yang, P.; Zhang, J.; Abubakar, Y.S.; Yang, F.; Lu, G.; et al. Rab7/Retromer-based endolysosomal trafficking is essential for proper host invasion in rice blast. New Phytol. 2023, 239, 1384–1403. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zheng, H.; Zhao, X.; Zhang, Y.; Xie, Q.; Lin, X.; Chen, A.; Yu, W.; Lu, G.; Shim, W.B.; et al. Retrograde trafficking from the endosome to the trans-Golgi network mediated by the retromer is required for fungal development and pathogenicity in Fusarium graminearum. New Phytol. 2016, 210, 1327–1343. [Google Scholar] [CrossRef]
- Zheng, W.; Zhou, J.; He, Y.; Xie, Q.; Chen, A.; Zheng, H.; Shi, L.; Zhao, X.; Zhang, C.; Huang, Q.; et al. Retromer Is Essential for Autophagy-Dependent Plant Infection by the Rice Blast Fungus. PLoS Genet. 2015, 11, e1005704. [Google Scholar] [CrossRef]
- Buser, D.P.; Spang, A. Protein sorting from endosomes to the TGN. Front. Cell Dev. Biol. 2023, 11, 1140605. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, Y.S.; Qiu, H.; Fang, W.; Zheng, H.; Lu, G.; Zhou, J.; Wang, Z.; Zheng, W. FgRab5 and FgRab7 are essential for endosomes biogenesis and non-redundantly recruit the retromer complex to the endosomes in Fusarium graminearum. Stress Biol. 2021, 1, 17. [Google Scholar] [CrossRef]
- Seaman, M.N.J. The Retromer Complex: From Genesis to Revelations. Trends Biochem. Sci. 2021, 46, 608–620. [Google Scholar] [CrossRef]
- Gurunathan, S.; Marash, M.; Weinberger, A.; Gerst, J.E. t-SNARE phosphorylation regulates endocytosis in yeast. Mol. Biol. Cell 2002, 13, 1594–1607. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Berkopec, A. HyperQuick algorithm for discrete hypergeometric distribution. J. Discret. Algorithms 2007, 5, 341–347. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Hou, Z.; Xue, C.; Peng, Y.; Katan, T.; Kistler, H.C.; Xu, J.R. A mitogen-activated protein kinase gene (MGV1) in Fusarium graminearum is required for female fertility, heterokaryon formation, and plant infection. Mol. Plant-Microbe Interact. 2002, 15, 1119–1127. [Google Scholar] [CrossRef]
- Yu, J.H.; Hamari, Z.; Han, K.H.; Seo, J.A.; Reyes-Domínguez, Y.; Scazzocchio, C. Double-joint PCR: A PCR-based molecular tool for gene manipulations in filamentous fungi. Fungal Genet. Biol. 2004, 41, 973–981. [Google Scholar] [CrossRef]
- Zheng, H.; Li, L.; Yu, Z.; Yuan, Y.; Zheng, Q.; Xie, Q.; Li, G.; Abubakar, Y.S.; Zhou, J.; Wang, Z.; et al. FgSpa2 recruits FgMsb3, a Rab8 GAP, to the polarisome to regulate polarized trafficking, growth and pathogenicity in Fusarium graminearum. New Phytol. 2021, 229, 1665–1683. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Lin, Y.; Fang, W.; Zhao, X.; Lou, Y.; Wang, G.; Zheng, H.; Liang, Q.; Abubakar, Y.S.; Olsson, S.; et al. The endosomal recycling of FgSnc1 by FgSnx41-FgSnx4 heterodimer is essential for polarized growth and pathogenicity in Fusarium graminearum. New Phytol. 2018, 219, 654–671. [Google Scholar] [CrossRef] [PubMed]
- Bryant, P.; Pozzati, G.; Elofsson, A. Improved prediction of protein-protein interactions using AlphaFold2. Nat. Commun. 2022, 13, 1265. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Carosi, J.M.; Denton, D.; Kumar, S.; Sargeant, T.J. Receptor Recycling by Retromer. Mol. Cell. Biol. 2023, 43, 317–334. [Google Scholar] [CrossRef]
- Purushothaman, L.K.; Ungermann, C. Cargo induces retromer-mediated membrane remodeling on membranes. Mol. Biol. Cell 2018, 29, 2709–2719. [Google Scholar] [CrossRef]
- Bonifacino, J.S.; Hurley, J.H. Retromer. Curr. Opin. Cell Biol. 2008, 20, 427–436. [Google Scholar] [CrossRef]
- Seaman, M.N.J. Retromer and Its Role in Regulating Signaling at Endosomes. Prog. Mol. Subcell. Biol. 2018, 57, 137–149. [Google Scholar] [CrossRef]
- Zheng, H.; Zheng, W.; Wu, C.; Yang, J.; Xi, Y.; Xie, Q.; Zhao, X.; Deng, X.; Lu, G.; Li, G.; et al. Rab GTPases are essential for membrane trafficking-dependent growth and pathogenicity in Fusarium graminearum. Environ. Microbiol. 2015, 17, 4580–4599. [Google Scholar] [CrossRef]
- Zheng, H.; Miao, P.; Lin, X.; Li, L.; Wu, C.; Chen, X.; Abubakar, Y.S.; Norvienyeku, J.; Li, G.; Zhou, J.; et al. Small GTPase Rab7-mediated FgAtg9 trafficking is essential for autophagy-dependent development and pathogenicity in Fusarium graminearum. PLoS Genet. 2018, 14, e1007546. [Google Scholar] [CrossRef] [PubMed]
- Seaman, M.N.J. Retromer and the cation-independent mannose 6-phosphate receptor-Time for a trial separation? Traffic 2018, 19, 150–152. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fedoseienko, A.; Chen, B.; Burstein, E.; Jia, D.; Billadeau, D.D. Endosomal receptor trafficking: Retromer and beyond. Traffic 2018, 19, 578–590. [Google Scholar] [CrossRef] [PubMed]
- Best, J.T.; Xu, P.; McGuire, J.G.; Leahy, S.N.; Graham, T.R. Yeast synaptobrevin, Snc1, engages distinct routes of postendocytic recycling mediated by a sorting nexin, Rcy1-COPI, and retromer. Mol. Biol. Cell 2020, 31, 944–962. [Google Scholar] [CrossRef] [PubMed]
- Gallon, M.; Cullen, P.J. Retromer and sorting nexins in endosomal sorting. Biochem. Soc. Trans. 2015, 43, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Heucken, N.; Ivanov, R. The retromer, sorting nexins and the plant endomembrane protein trafficking. J. Cell Sci. 2018, 131, jcs203695. [Google Scholar] [CrossRef]
- Lucas, M.; Hierro, A. Retromer. Curr. Biol. 2017, 27, R687–R689. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Liu, Y.; Liu, L.; Li, J.; Yang, K.; Liu, M.; Zeng, W.; Qin, L.; Lin, R.; et al. The regulatory role of the Aspergillus flavus core retromer complex in aflatoxin metabolism. J. Biol. Chem. 2022, 298, 102120. [Google Scholar] [CrossRef]
- Alessi, D.R.; Cullen, P.J.; Cookson, M.; Merchant, K.M.; Small, S.A. Retromer-dependent lysosomal stress in Parkinson’s disease. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2024, 379, 20220376. [Google Scholar] [CrossRef]
- Daly, J.L.; Danson, C.M.; Lewis, P.A.; Zhao, L.; Riccardo, S.; Di Filippo, L.; Cacchiarelli, D.; Lee, D.; Cross, S.J.; Heesom, K.J.; et al. Multi-omic approach characterises the neuroprotective role of retromer in regulating lysosomal health. Nat. Commun. 2023, 14, 3086. [Google Scholar] [CrossRef]
- Reitz, C. Retromer Dysfunction and Neurodegenerative Disease. Curr. Genom. 2018, 19, 279–288. [Google Scholar] [CrossRef]
- Cui, Y.; Yang, Z.; Flores-Rodriguez, N.; Follett, J.; Ariotti, N.; Wall, A.A.; Parton, R.G.; Teasdale, R.D. Formation of retromer transport carriers is disrupted by the Parkinson disease-linked Vps35 D620N variant. Traffic 2021, 22, 123–136. [Google Scholar] [CrossRef]
- Luo, A.D.; Xu, Z.C.; Liao, S.S. VPS35, the core component of the retromer complex, and Parkinson’s disease. Ibrain 2021, 7, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.A.; Stevens, T.H. Vps10p cycles between the late-Golgi and prevacuolar compartments in its function as the sorting receptor for multiple yeast vacuolar hydrolases. J. Cell Biol. 1996, 133, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Oliviusson, P.; Heinzerling, O.; Hillmer, S.; Hinz, G.; Tse, Y.C.; Jiang, L.; Robinson, D.G. Plant retromer, localized to the prevacuolar compartment and microvesicles in Arabidopsis, may interact with vacuolar sorting receptors. Plant Cell 2006, 18, 1239–1252. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, M.C.; Valent, B. Filamentous plant pathogen effectors in action. Nat. Rev. Microbiol. 2013, 11, 800–814. [Google Scholar] [CrossRef]
- Tariqjaveed, M.; Mateen, A.; Wang, S.; Qiu, S.; Zheng, X.; Zhang, J.; Bhadauria, V.; Sun, W. Versatile effectors of phytopathogenic fungi target host immunity. J. Integr. Plant Biol. 2021, 63, 1856–1873. [Google Scholar] [CrossRef]
- Rabouille, C.; Malhotra, V.; Nickel, W. Diversity in unconventional protein secretion. J. Cell Sci. 2012, 125, 5251–5255. [Google Scholar] [CrossRef]
- Mentlak, T.A.; Kombrink, A.; Shinya, T.; Ryder, L.S.; Otomo, I.; Saitoh, H.; Terauchi, R.; Nishizawa, Y.; Shibuya, N.; Thomma, B.P.; et al. Effector-mediated suppression of chitin-triggered immunity by magnaporthe oryzae is necessary for rice blast disease. Plant Cell 2012, 24, 322–335. [Google Scholar] [CrossRef]
- Mosquera, G.; Giraldo, M.C.; Khang, C.H.; Coughlan, S.; Valent, B. Interaction transcriptome analysis identifies Magnaporthe oryzae BAS1-4 as Biotrophy-associated secreted proteins in rice blast disease. Plant Cell 2009, 21, 1273–1290. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, M.C.; Dagdas, Y.F.; Gupta, Y.K.; Mentlak, T.A.; Yi, M.; Martinez-Rocha, A.L.; Saitoh, H.; Terauchi, R.; Talbot, N.J.; Valent, B. Two distinct secretion systems facilitate tissue invasion by the rice blast fungus Magnaporthe oryzae. Nat. Commun. 2013, 4, 1996. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, S.; Long, Y.; Zhang, X.; Liu, B.; Song, S.; Li, G.; Hu, Y.; Du, L.; Wang, Q.; Jiang, J.; et al. The Sorting and Transport of the Cargo Protein CcSnc1 by the Retromer Complex Regulate the Growth, Development, and Pathogenicity of Corynespora cassiicola. J. Fungi 2024, 10, 714. https://doi.org/10.3390/jof10100714
Cheng S, Long Y, Zhang X, Liu B, Song S, Li G, Hu Y, Du L, Wang Q, Jiang J, et al. The Sorting and Transport of the Cargo Protein CcSnc1 by the Retromer Complex Regulate the Growth, Development, and Pathogenicity of Corynespora cassiicola. Journal of Fungi. 2024; 10(10):714. https://doi.org/10.3390/jof10100714
Chicago/Turabian StyleCheng, Shuyuan, Yunfei Long, Xiaoyang Zhang, Bing Liu, Shuilin Song, Genghua Li, Yuzhuan Hu, Lei Du, Quanxing Wang, Junxi Jiang, and et al. 2024. "The Sorting and Transport of the Cargo Protein CcSnc1 by the Retromer Complex Regulate the Growth, Development, and Pathogenicity of Corynespora cassiicola" Journal of Fungi 10, no. 10: 714. https://doi.org/10.3390/jof10100714
APA StyleCheng, S., Long, Y., Zhang, X., Liu, B., Song, S., Li, G., Hu, Y., Du, L., Wang, Q., Jiang, J., & Xiong, G. (2024). The Sorting and Transport of the Cargo Protein CcSnc1 by the Retromer Complex Regulate the Growth, Development, and Pathogenicity of Corynespora cassiicola. Journal of Fungi, 10(10), 714. https://doi.org/10.3390/jof10100714





