Role of Squalene Epoxidase Gene (SQE1) in the Response of the Lichen Lobaria pulmonaria to Temperature Stress
Abstract
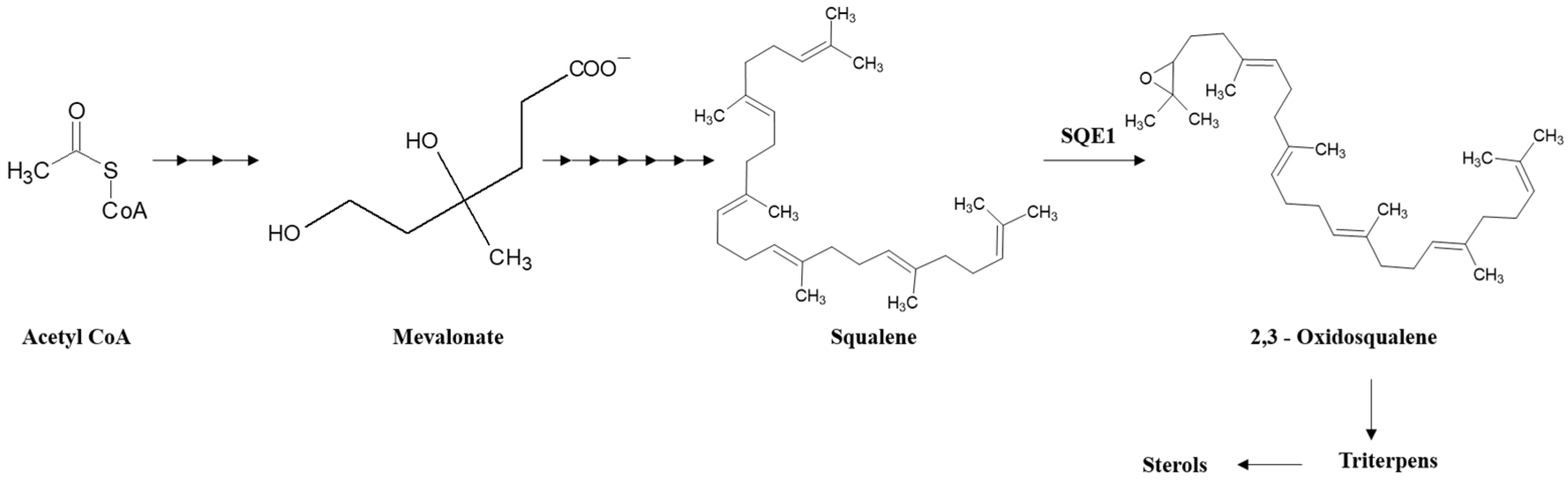
1. Introduction
2. Materials and Methods
2.1. Lichen Material
2.2. Stress Treatments
2.3. Determination of Respiration Rate
2.4. Measurement of Electrolyte Leakage
2.5. Chlorophyll Fluorescence
2.6. Gene Identification and Retrieval of Protein Sequences
2.7. Sequence Analysis
2.8. Phylogenetic and Comparative Analyses
2.9. RNA Extraction, cDNA Synthesis, and RT-qPCR
2.10. Statistical Analysis
3. Results
3.1. Analysis of the Physiological Status of L. pulmonaria under Different Temperatures
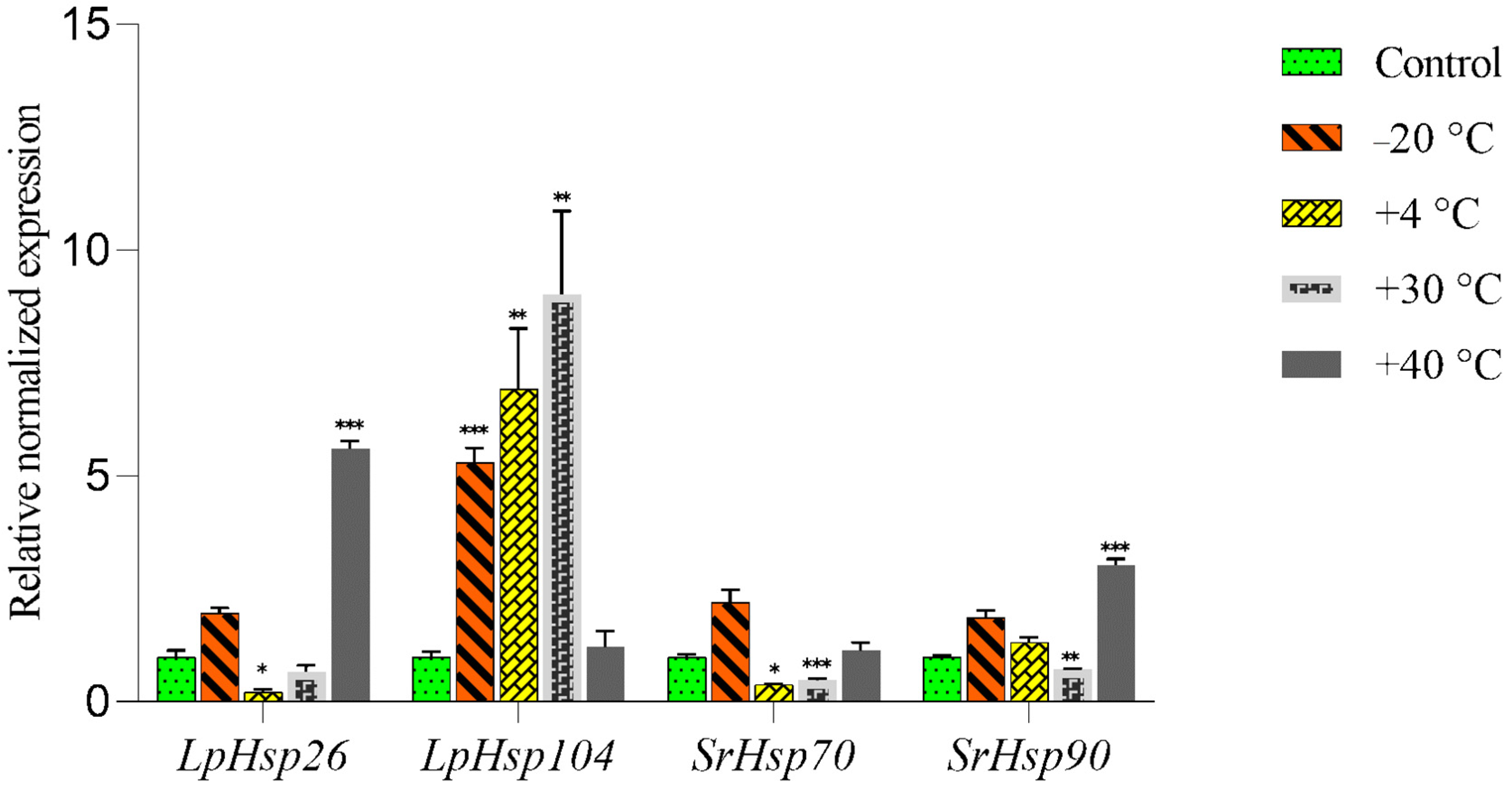
3.2. Effects of Low and Elevated Temperatures on the Expression of Hsp Genes in the Symbionts of L. pulmonaria
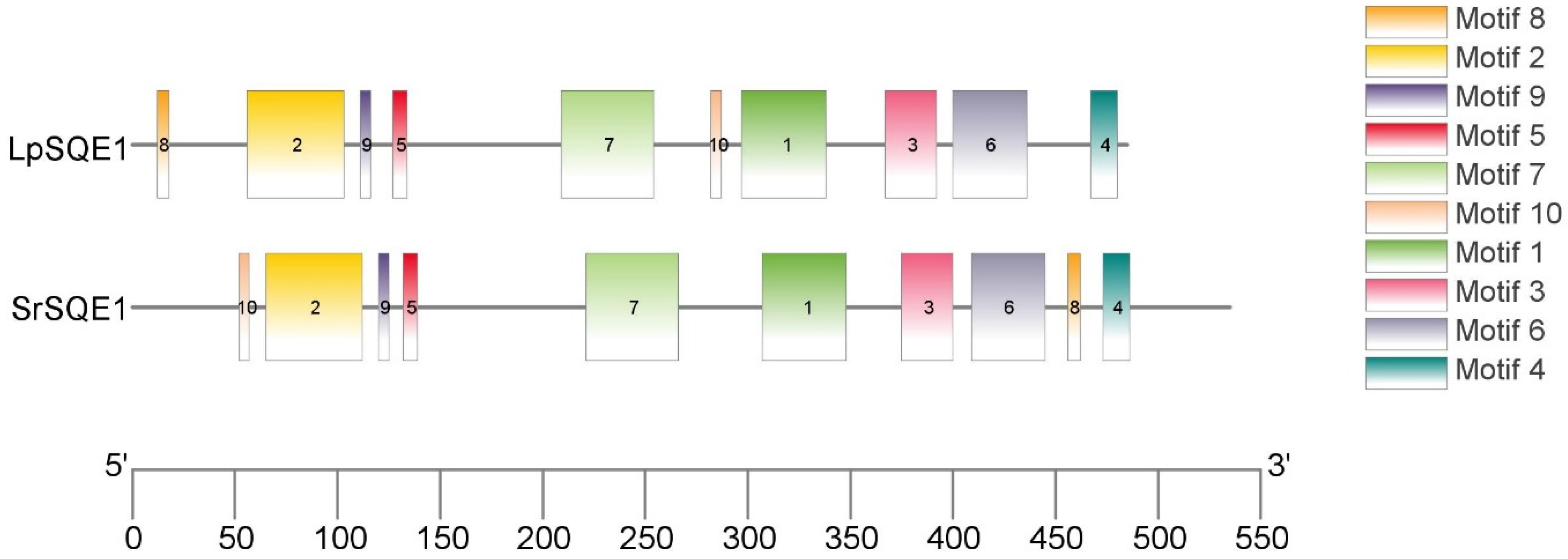
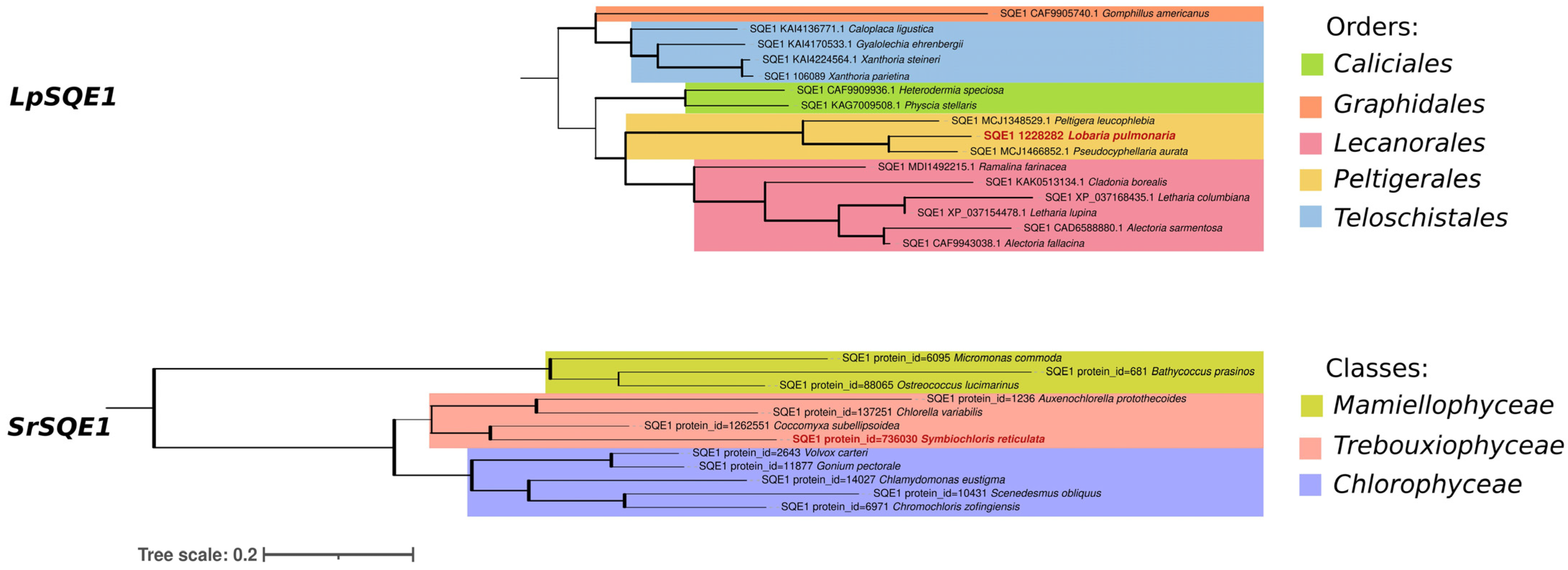
3.3. Characterization and of SQE1 Genes Using Phylogenetic and Comparative Analyses
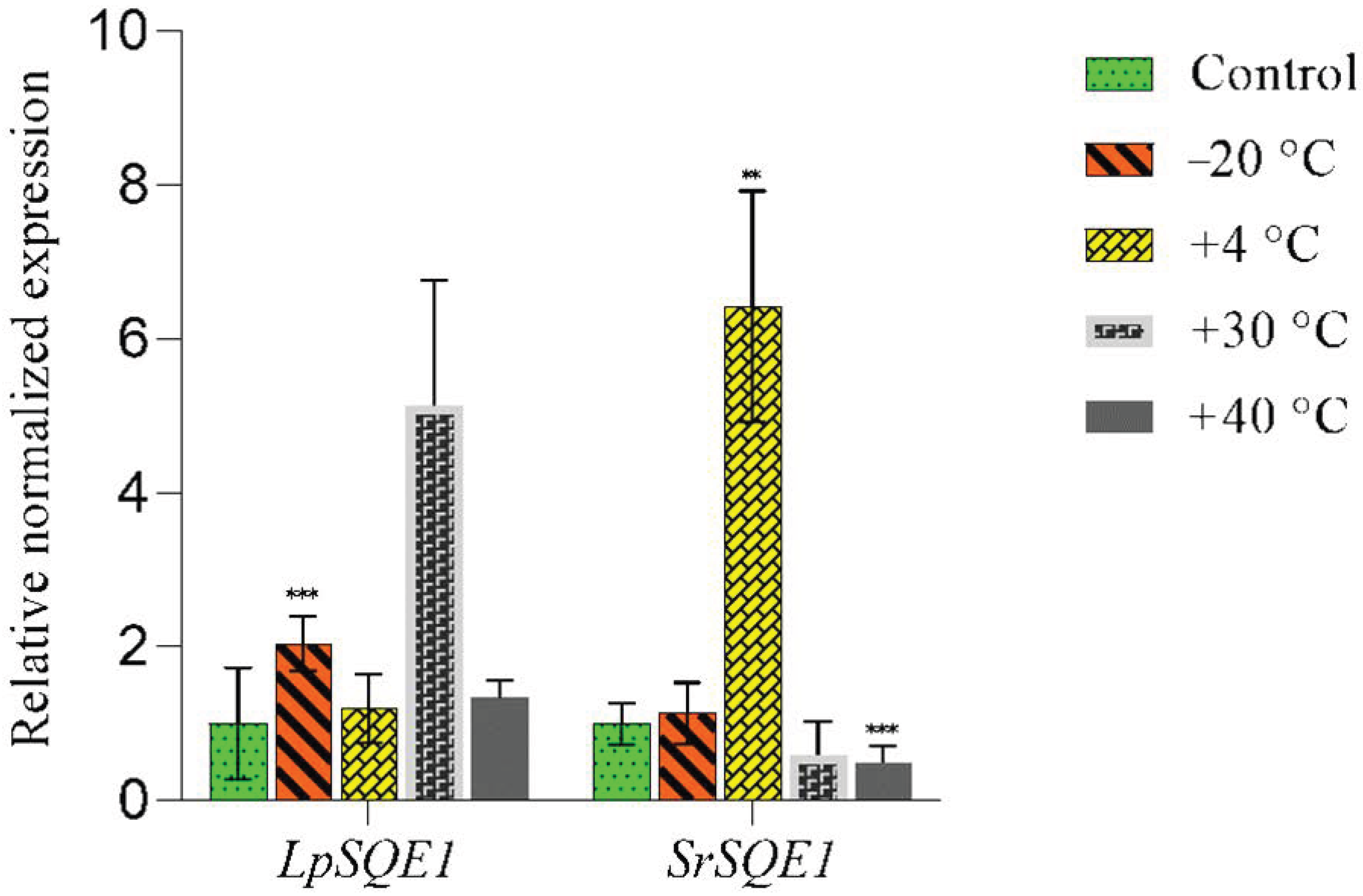
3.4. Effects of Low and Elevated Temperatures on the Expression of SQE1 Genes in the Symbionts of L. pulmonaria
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bellard, C.; Leclerc, C.; Courchamp, F. Impact of Sea Level Rise on the 10 Insular Biodiversity Hotspots. Glob. Ecol. Biogeogr. 2013, 23, 203–212. [Google Scholar] [CrossRef]
- Nascimbene, J.; Marini, L. Epiphytic Lichen Diversity along Elevational Gradients: Biological Traits Reveal a Complex Response to Water and Energy. J. Biogeogr. 2015, 42, 1222–1232. [Google Scholar] [CrossRef]
- Hawksworth, D.L.; Rose, F. Qualitative Scale for Estimating Sulphur Dioxide Air Pollution in England and Wales Using Epiphytic Lichens. Nature 1970, 227, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Green, T.G.A.; Sancho, L.G.; Pintado, A. Ecophysiology of Desiccation/Rehydration Cycles in Mosses and Lichens. In Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2011; pp. 89–120. [Google Scholar]
- Kraft, M.; Scheidegger, C.; Werth, S. Stressed out: The Effects of Heat Stress and Parasitism on Gene Expression of the Lichen-Forming Fungus Lobaria pulmonaria. Lichenologist 2022, 54, 71–83. [Google Scholar] [CrossRef]
- Kershaw, K.A. Physiological Ecology of Lichens. x, 293 pp. Cambridge University Press, 1985. J. Mar. Biol. Assoc. UK 1986, 66, 267. [Google Scholar] [CrossRef]
- Gauslaa, Y.; Solhaug, K.A. High-Light Damage in Air-Dry Thalli of the Old Forest Lichen Lobaria pulmonaria-Interactions of Irradiance, Exposure Duration and High Temperature. J. Exp. Bot. 1999, 50, 697–705. [Google Scholar] [CrossRef]
- Bjerke, J.W.; Zielke, M.; Solheim, B. Long-term Impacts of Simulated Climatic Change on Secondary Metabolism, Thallus Structure and Nitrogen Fixation Activity in Two Cyanolichens from the Arctic. New Phytol. 2003, 159, 361–367. [Google Scholar] [CrossRef]
- Lange, O.L. Hitze-Und Trockenresistenz Der Flechten in Beziehung Zu Ihrer Verbreitung. Flora Allg. Bot. Ztg. 1953, 140, 39–97. [Google Scholar] [CrossRef]
- MacFarlane, J.D.; Kershaw, K.A. Thermal Sensitivity in Lichens. Science 1978, 201, 739–741. [Google Scholar] [CrossRef]
- Macfarlane, J.D.; Kershaw, K.A. Physiological-Environmental Interactions in Lichens. New Phytol. 1980, 84, 669–685. [Google Scholar] [CrossRef]
- Osyczka, P.; Myśliwa-Kurdziel, B. Do the Expected Heatwaves Pose a Threat to Lichens?: Linkage between a Passive Decline in Water Content in Thalli and Response to Heat Stress. Plant Cell Environ. 2024, 47, 3999–4013. [Google Scholar] [CrossRef] [PubMed]
- Chowaniec, K.; Latkowska, E.; Skubała, K. Effect of Thallus Melanisation on the Sensitivity of Lichens to Heat Stress. Sci. Rep. 2023, 13, 5083. [Google Scholar] [CrossRef] [PubMed]
- Nascimbene, J.; Casazza, G.; Benesperi, R.; Catalano, I.; Cataldo, D.; Grillo, M.; Isocrono, D.; Matteucci, E.; Ongaro, S.; Potenza, G.; et al. Climate Change Fosters the Decline of Epiphytic lobaria Species in Italy. Biol. Conserv. 2016, 201, 377–384. [Google Scholar] [CrossRef]
- Škaloud, P.; Friedl, T.; Hallmann, C.; Beck, A.; Grande, F.D. Taxonomic Revision and Species Delimitation of Coccoid Green Algae Currently Assigned to the Genus Dictyochloropsis (Trebouxiophyceae, Chlorophyta). J. Phycol. 2016, 52, 599–617. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, C.; Scheidegger, C. New Morphological Aspects of Cephalodium Formation in the lichen Lobaria pulmonaria (Lecanorales, Ascomycota). Lichenologist 2013, 45, 77–87. [Google Scholar] [CrossRef]
- Chavarria-Pizarro, T.; Resl, P.; Janjic, A.; Werth, S. Gene Expression Responses to Thermal Shifts in the Endangered Lichen Lobaria pulmonaria. Mol. Ecol. 2021, 31, 839–858. [Google Scholar] [CrossRef]
- Pratt, W.B.; Toft, D.O. Regulation of Signaling Protein Function and Trafficking by the Hsp90/Hsp70-Based Chaperone Machinery. Exp. Biol. Med. 2003, 228, 111–133. [Google Scholar] [CrossRef]
- Sato, Y.; Yokoya, S. Enhanced Tolerance to Drought Stress in Transgenic Rice Plants Overexpressing a Small Heat-Shock Protein, sHSP17.7. Plant Cell Rep. 2007, 27, 329–334. [Google Scholar] [CrossRef]
- Du, Y.; Fu, X.; Chu, Y.; Wu, P.; Liu, Y.; Ma, L.; Tian, H.; Zhu, B. Biosynthesis and the Roles of Plant Sterols in Development and Stress Responses. Int. J. Mol. Sci. 2022, 23, 2332. [Google Scholar] [CrossRef]
- Elix, J.A.; Stocker-Wörgötter, E. Biochemistry and Secondary Metabolites; Cambridge University Press eBooks: Cambridge, UK, 2008; pp. 104–133. [Google Scholar]
- Valitova, J.N.; Khabibrakhmanova, V.R.; Babaev, V.M.; Uvaeva, V.L.; Khairullina, A.F.; Rakhmatullina, D.F.; Galeeva, E.I.; Swid, M.A.; Minibayeva, F.V. Sterol Composition of Lichen Peltigera canina When Exposed to Unfavorable Temperatures. Russ. J. Plant Physiol. 2023, 70, 887–897. [Google Scholar] [CrossRef]
- Espenshade, P.J.; Hughes, A.L. Regulation of Sterol Synthesis in Eukaryotes. Annu. Rev. Genet. 2007, 41, 401–427. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, H.; Coates, H.W.; Chua, N.K.; Hashimoto, Y.; Brown, A.J.; Ohgane, K. A Key Mammalian Cholesterol Synthesis Enzyme, Squalene Monooxygenase, Is Allosterically Stabilized by Its Substrate. Proc. Natl. Acad. Sci. USA 2020, 117, 7150–7158. [Google Scholar] [CrossRef]
- Ryder, N.S. Squalene Epoxidase as the Target of Antifungal Allylamines. Pestic. Sci. 1987, 21, 281–288. [Google Scholar] [CrossRef]
- Upadhyay, S.; Jeena, G.S.; Kumar, S.; Shukla, R.K. Asparagus racemosus bZIP Transcription Factor-Regulated Squalene Epoxidase (ArSQE) Promotes Germination and Abiotic Stress Tolerance in Transgenic Tobacco. Plant Sci. 2020, 290, 110291. [Google Scholar] [CrossRef] [PubMed]
- Jordá, T.; Puig, S. Regulation of Ergosterol Biosynthesis in Saccharomyces cerevisiae. Genes 2020, 11, 795. [Google Scholar] [CrossRef] [PubMed]
- Almer, J.; Resl, P.; Gudmundsson, H.; Warshan, D.; Andrésson, Ó.S.; Werth, S. Symbiont-specific Responses to Environmental Cues in a Threesome Lichen Symbiosis. Mol. Ecol. 2023, 32, 1045–1061. [Google Scholar] [CrossRef]
- Munzi, S.; Pisani, T.; Loppi, S. The Integrity of Lichen Cell Membrane as a Suitable Parameter for Monitoring Biological Effects of Acute Nitrogen Pollution. Ecotoxicol. Environ. Saf. 2009, 72, 2009–2012. [Google Scholar] [CrossRef]
- Goltsev, V.N.; Kalaji, H.M.; Paunov, M.; Bąba, W.; Horaczek, T.; Mojski, J.; Kociel, H.; Allakhverdiev, S.I. Variable Chlorophyll Fluorescence and Its Use for Assessing Physiological Condition of Plant Photosynthetic Apparatus. Russ. J. Plant Physiol. 2016, 63, 869–893. [Google Scholar] [CrossRef]
- El-Gebali, S.; Mistry, J.; Bateman, A.; Eddy, S.R.; Luciani, A.; Potter, S.C.; Qureshi, M.; Richardson, L.J.; Salazar, G.A.; Smart, A.; et al. The Pfam Protein Families Database in 2019. Nucl. Acids Res. 2018, 47, D427–D432. [Google Scholar] [CrossRef]
- Mitchell, A.L.; Attwood, T.K.; Babbitt, P.C.; Blum, M.; Bork, P.; Bridge, A.; Brown, S.D.; Chang, H.-Y.; El-Gebali, S.; Fraser, M.I.; et al. InterPro in 2019: Improving Coverage, Classification and Access to Protein Sequence Annotations. Nucl. Acids Res. 2018, 47, D351–D360. [Google Scholar] [CrossRef]
- Wilkins, M.R.; Gasteiger, E.; Bairoch, A.; Sanchez, J.C.; Williams, K.L.; Appel, R.D.; Hochstrasser, D.F. Protein Identification and Analysis Tools in the ExPASY Server; Humana Press Ebooks: Totowa, NJ, USA, 2003; pp. 531–552. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, D.; Yao, Y.; Eubel, H.; Künzler, P.; Møller, I.M.; Xu, D. MULocDeep: A Deep-Learning Framework for Protein Subcellular and Suborganellar Localization Prediction with Residue-Level Interpretation. Comput. Struct. Biotechnol. J. 2021, 19, 4825–4839. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for Motif Discovery and Searching. Nucl. Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBTools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Geourjon, C.; Deléage, G. SOPMA: Significant Improvements in Protein Secondary Structure Prediction by Consensus Prediction from Multiple Alignments. Bioinformatics 1995, 11, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Capella-Gutiérrez, S.; Silla-Martínez, J.M.; Gabaldón, T. trimAl: A Tool for Automated Alignment Trimming in Large-Scale Phylogenetic Analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2014, 32, 268–274. [Google Scholar] [CrossRef]
- Minh, B.Q.; Nguyen, M.A.T.; Von Haeseler, A. Ultrafast Approximation for Phylogenetic Bootstrap. Mol. Biol. Evol. 2013, 30, 1188–1195. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v5: An Online Tool for Phylogenetic Tree Display and Annotation. Nucl. Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Bay, R.A.; Palumbi, S.R. Transcriptome Predictors of Coral Survival and Growth in a Highly Variable Environment. Ecol. Evol. 2017, 7, 4794–4803. [Google Scholar] [CrossRef]
- Hancock, A.M.; Brachi, B.; Faure, N.; Horton, M.W.; Jarymowycz, L.B.; Sperone, F.G.; Toomajian, C.; Roux, F.; Bergelson, J. Adaptation to Climate Across the Arabidopsis thaliana Genome. Science 2011, 334, 83–86. [Google Scholar] [CrossRef]
- Mulgrew, A.; Williams, P. Biomonitoring of Air Quality Using Plants. In Air Hygiene Report 10; Kings College: London, UK, 2000. [Google Scholar]
- Green, T.G.A.; Lange, O.L. Photosynthesis in Poikilohydric Plants: A Comparison of Lichens and Bryophytes. In Ecophysiology of Photosynthesis; Springer eBooks: Berlin/Heidelberg, Germany, 1995; pp. 319–341. [Google Scholar]
- Convey, P. The Influence of Environmental Characteristics on Life History Attributes of Antarctic Terrestrial Biota. Biol. Rev. Biol. Rev. Camb. Philos. Soc. 1996, 71, 191–225. [Google Scholar] [CrossRef]
- Smith, D.C. The Biology of Lichen Thalli. Biol. Rev. Biol. Rev. Camb. Philos. Soc. 1962, 37, 537–570. [Google Scholar] [CrossRef]
- Culberson, W.L.; Ferry, B.W.; Baddeley, M.S.; Hawksworth, D.L. Air Pollution and Lichens. Bryologist 1974, 77, 101. [Google Scholar] [CrossRef]
- Shelyakin, M.; Zakhozhiy, I.; Golovko, T. The Effect of Temperature on Antarctic Lichen Cytochrome and Alternative Respiratory Pathway Rates. Polar Biol. 2020, 43, 2003–2010. [Google Scholar] [CrossRef]
- Feige, G.B. Problems of Lichen Physiology. Nova Hedwig. 1979, 30, 725–774. [Google Scholar] [CrossRef]
- Smyth, E.S. A Contribution to the Physiology and Ecology of Peltigera canina and P. polydactyla. Ann. Bot. 1934, 48, 781–818. [Google Scholar] [CrossRef]
- Bayram, Ö.; Feussner, K.; Dumkow, M.; Herrfurth, C.; Feussner, I.; Braus, G.H. Changes of Global Gene Expression and Secondary Metabolite Accumulation during Light-Dependent Aspergillus nidulans Development. Fungal Genet. Biol. 2016, 87, 30–53. [Google Scholar] [CrossRef]
- Lohman, B.K.; Stutz, W.E.; Bolnick, D.I. Gene Expression Stasis and Plasticity Following Migration into a Foreign Environment. Mol. Ecol. 2017, 26, 4657–4670. [Google Scholar] [CrossRef]
- Baniwal, S.K.; Chan, K.Y.; Scharf, K.-D.; Nover, L. Role of Heat Stress Transcription Factor HSFA5 as Specific Repressor of HSFA4. J. Biol. Chem. 2007, 282, 3605–3613. [Google Scholar] [CrossRef]
- Doyle, S.M.; Wickner, S. Hsp104 and ClpB: Protein Disaggregating Machines. Trends Biochem. Sci. 2009, 34, 40–48. [Google Scholar] [CrossRef]
- Steinhäuser, S.S.; Andrésson, Ó.S.; Pálsson, A.; Werth, S. Fungal and Cyanobacterial Gene Expression in a Lichen Symbiosis: Effect of Temperature and Location. Fungal Biol. 2016, 120, 1194–1208. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Liu, D.; Chong, K. Cold Signaling in Plants: Insights into Mechanisms and Regulation. J. Integr. Plant Biol. 2018, 60, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Leber, R.; Landl, K.; Zinser, E.; Ahorn, H.; Spök, A.; Kohlwein, S.D.; Turnowsky, F.; Daum, G. Dual Localization of Squalene Epoxidase, Erg1p, in Yeast Reflects a Relationship between the Endoplasmic Reticulum and Lipid Particles. Mol. Biol. Cell 1998, 9, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Yan, S.; Li, Y.; Feng, S.; Zhang, J.-J.; Li, J.-R. Functional Characterization of Squalene Epoxidase and NADPH-Cytochrome P450 Reductase in Dioscorea zingiberensis. Biochem. Biophys. Res. Commun. 2019, 509, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-S.; Dou, Q.; Sui, C.; Yuan, G.; Zeng, B. Cloning, Subcellular Localization and Expression Analysis of Squalene Epoxidase Gene BsSE1 from Bletilla striata. Gene Expr. Patterns 2023, 47, 119298. [Google Scholar] [CrossRef]
- Yakimov, A.P.; Afanaseva, A.S.; Khodorkovskiy, M.A.; Petukhov, M.G. Design of Stable A-Helical Peptides and Thermostable Proteins in Biotechnology and Biomedicine. Acta Nat. 2016, 8, 70–81. [Google Scholar] [CrossRef]
- Huber, R. Conformational Flexibility and Its Functional Significance in Some Protein Molecules. In Biological Functions of Proteinases; Springer eBooks: Berlin/Heidelberg, Germany, 1979; pp. 1–16. [Google Scholar]
- Fielman, K.T.; Woodin, S.A.; Lincoln, D.E. Polychaete Indicator Species as a Source of Natural Halogenated Organic Compounds in Marine Sediments. Environ. Toxicol. Chem. 2001, 20, 738–747. [Google Scholar] [CrossRef]
- Potijun, S.; Yaisamlee, C.; Sirikhachornkit, A. Pigment Production under Cold Stress in the Green Microalga Chlamydomonas reinhardtii. Agriculture 2021, 11, 564. [Google Scholar] [CrossRef]
- Ranković, B.; Kosanić, M. Lichens as a Potential Source of Bioactive Secondary Metabolites. In Lichen Secondary Metabolites; Ranković, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Rasbery, J.M.; Shan, H.; LeClair, R.J.; Norman, M.; Matsuda, S.P.T.; Bartel, B. Arabidopsis thaliana Squalene Epoxidase 1 Is Essential for Root and Seed Development. J. Biol. Chem. 2007, 282, 17002–17013. [Google Scholar] [CrossRef]
- Suzuki, H.; Achnine, L.; Xu, R.; Matsuda, S.P.T.; Dixon, R.A. A Genomics Approach to the Early Stages of Triterpene Saponin Biosynthesis in Medicago truncatula. Plant J. 2002, 32, 1033–1048. [Google Scholar] [CrossRef]
- Ruckenstuhl, C.; Eidenberger, A.; Lang, S.; Turnowsky, F. Single Amino Acid Exchanges in FAD-Binding Domains of Squalene Epoxidase of Saccharomyces cerevisiae Lead to Either Loss of Functionality or Terbinafine Sensitivity. Biochem. Soc. Trans. 2005, 33, 1197. [Google Scholar] [CrossRef]
- Liu, W.; May, G.S.; Lionakis, M.S.; Lewis, R.E.; Kontoyiannis, D.P. Extra Copies of the Aspergillus fumigatus Squalene Epoxidase Gene Confer Resistance to Terbinafine: Genetic Approach to Studying Gene Dose-Dependent Resistance to Antifungals in A. fumigatus. Antimicrob. Agents Chemother. 2004, 48, 2490–2496. [Google Scholar] [CrossRef] [PubMed]
| Samples | MSI, % | Respiration Rate, µL/h/g Dry Mass | Photosynthesis | |
|---|---|---|---|---|
| FV/FM | rETR | |||
| Control | 97.5 ± 0.4 | 422 ± 26 | 0.71 ± 0.0 | 7.2 ± 0.2 |
| −20 °C | 94.9 ± 0.1 ** | 198 ± 65 *** | 0.70 ± 0.0 | 5.2 ± 0.9 |
| +4 °C | 94.9 ± 0.2 ** | 186 ± 35 *** | 0.71 ± 0.0 | 7.3 ± 0.3 |
| +30 °C | 96.0 ± 0.2 * | 361 ± 88 | 0.71 ± 0.0 | 7.4 ± 0.3 |
| +40 °C | 78.2 ± 0.2 *** | 97 ± 16 *** | 0.27 ± 0.0 *** | 0.4 ± 0.1 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onele, A.O.; Swid, M.A.; Leksin, I.Y.; Rakhmatullina, D.F.; Galeeva, E.I.; Beckett, R.P.; Minibayeva, F.V.; Valitova, J.N. Role of Squalene Epoxidase Gene (SQE1) in the Response of the Lichen Lobaria pulmonaria to Temperature Stress. J. Fungi 2024, 10, 705. https://doi.org/10.3390/jof10100705
Onele AO, Swid MA, Leksin IY, Rakhmatullina DF, Galeeva EI, Beckett RP, Minibayeva FV, Valitova JN. Role of Squalene Epoxidase Gene (SQE1) in the Response of the Lichen Lobaria pulmonaria to Temperature Stress. Journal of Fungi. 2024; 10(10):705. https://doi.org/10.3390/jof10100705
Chicago/Turabian StyleOnele, Alfred O., Moatasem A. Swid, Ilya Y. Leksin, Daniya F. Rakhmatullina, Ekaterina I. Galeeva, Richard P. Beckett, Farida V. Minibayeva, and Julia N. Valitova. 2024. "Role of Squalene Epoxidase Gene (SQE1) in the Response of the Lichen Lobaria pulmonaria to Temperature Stress" Journal of Fungi 10, no. 10: 705. https://doi.org/10.3390/jof10100705
APA StyleOnele, A. O., Swid, M. A., Leksin, I. Y., Rakhmatullina, D. F., Galeeva, E. I., Beckett, R. P., Minibayeva, F. V., & Valitova, J. N. (2024). Role of Squalene Epoxidase Gene (SQE1) in the Response of the Lichen Lobaria pulmonaria to Temperature Stress. Journal of Fungi, 10(10), 705. https://doi.org/10.3390/jof10100705





