The Potential of Microorganisms for the Control of Grape Downy Mildew—A Review
Abstract
1. Introduction
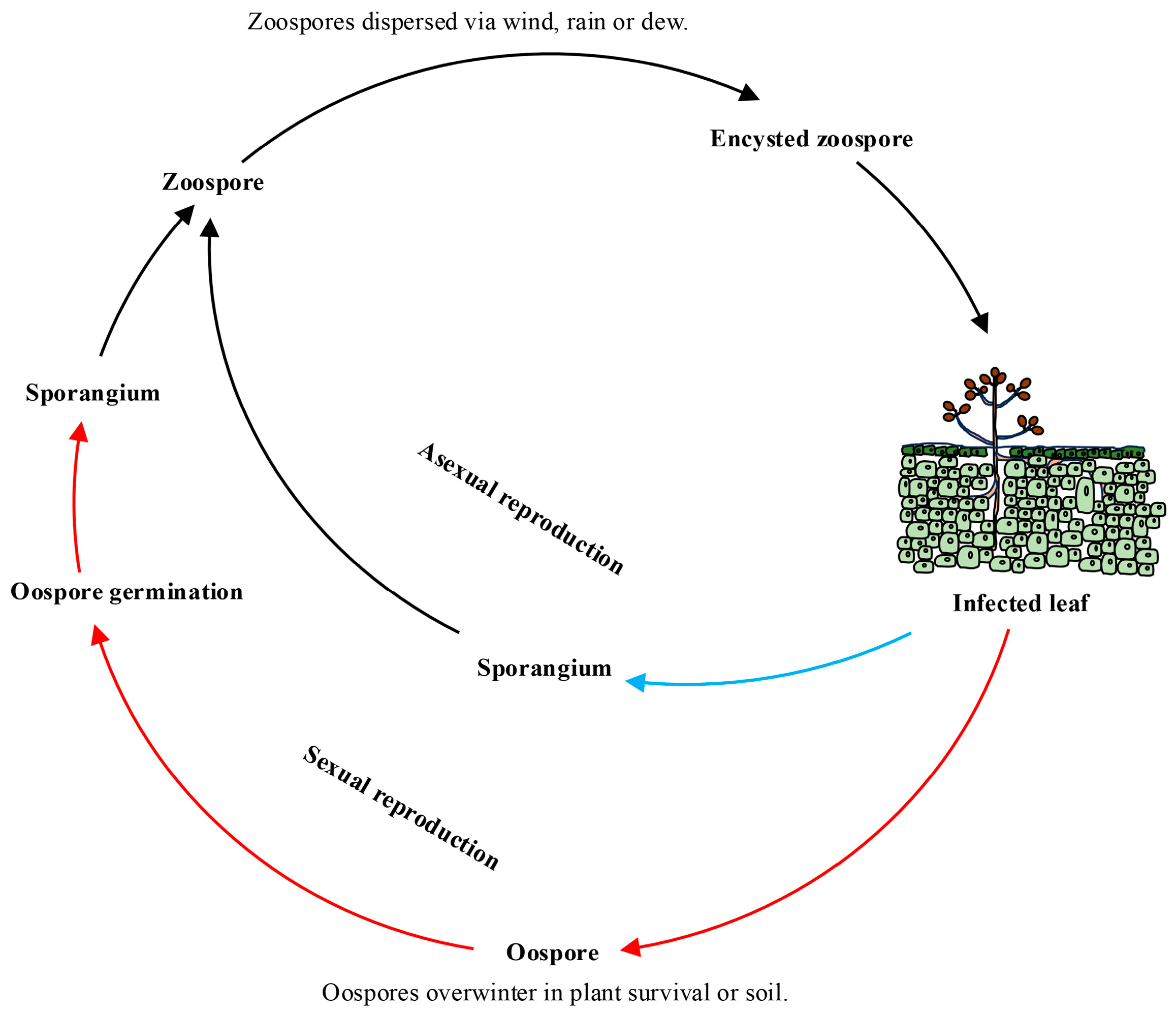
2. Biological Characteristics of Plasmopara viticola
3. Biocontrol Agents
3.1. Screening Biocontrol Agents
3.2. Bacteria as Control Agents for Grape Downy Mildew
| Biocontrol Microorganisms | Strain Name | Isolation Source | Application Scale | Application Manner | Application Types | Application Concentration | Control Efficiency |
|---|---|---|---|---|---|---|---|
| Ochrobactrum sp. | SY286 [74] | Grapevines leaves | Field | Spray | Live organisms | 108 cfu/mL | 91.05% |
| Paenibacillus polymyxa | PB-2 [72] | - | Field | Spray | Live organisms | 109 cfu/g | 46.91% |
| Paenibacillus sp. | B2 [73] | Sorghum mycorrhizosphere | Leaf discs | - | Metabolites | 0, 5, 10, 50 μg/mL | - |
| Pseudomonas aeruginosa | HB135 [69] | Grapevines leaves | Detached leaf | - | Live organisms | 107 cfu/mL | 91.27% |
| Pseudomonas fluorescens | PTA-CT2 [71] | Healthy filed-grown grape-vine | Greenhouse | Spray | Live organisms | 107 cfu/mL | - |
| Pseudomonas azotoformans | L-B-4 [70] | Grapevines leaves | Detached leaf | - | Live organisms | 1 × 108 cfu/mL | 97.2% |
| Microbacterium testaceum | N6 [50] | Grapevines leaves | Leaf discs | - | Live organisms | 1 × 107 cfu/mL | 74.2% |
| Lysobacter capsici | AZ78 [76] | Tobacco rhizosphere | Field | Spray | Live organisms | 108 cfu/mL | - |
| Streptomyces atratus | PY-1 [63] | Soil | Field | Spray | Fermentation solution | - | 90.08% |
| Streptomyces microflavus | QH94 [65] | Soil | Field | Spray | Fermentation solution | - | 82.53% |
| Streptomyces lydicus | A02 [52] | Soil | Field | Spray | Metabolites | - | 95.7% |
| Streptomyces viridosporus | HH1 [68] | - | - | Spray | Live organisms | 3 × 107/mL | - |
| Streptomyces violatus | HH5 [68] | - | - | Spray | Live organisms | 3 × 107/mL | - |
| Streptomyces corchorusii | NF0919 [67] | - | - | Spray | Fermentation supernatant | - | 71.55% |
| Streptomyces sp. | ANK313 [64] | Soil | - | - | Metabolites | - | - |
| Bacillus subtilis | KS1 [55] | Grape berry skins | Field | Spray | Live organisms | 1 × 108 cells/mL | - |
| Bacillus subtilis | GLB191 [53] | Grapevines leaves | Field | Spray | Live organisms | 108 cfu/mL | - |
| Bacillus subtilis | B-FS01 [56] | Oilseed rape stalk | Field | Spray | Live organisms | 107/mL | 88.25% |
| Bacillus subtilis | HMB-20428 [57] | Grapevines leaves | Field | Spray | Live organisms | 1 × 108 cfu/mL | 54.66% |
| Bacillus subtilis | JL4 [78] | Grapevines leaves | Greenhouse | Spray | Live organisms | 8 × 108 cfu/mL | 88% |
| Bacillus subtilis | DJ-6 WP [67] | - | Field | Spray | Live organisms | 1 × 1011 cfu/g | 70.71% |
| Bacillus pumilus | GLB197 [53] | Grapevines leaves | Field | Spray | Live organisms | 108 cfu/mL | - |
| Bacillus megaterium | BMJBN02 [58] | Soil | Plot | Spray | Metabolites crude extract | - | - |
| Bacillus velezensis | KOF112 [59] | Grapevine shoot xylem | Leaf discs | - | Live organisms | 1 × 108 cfu/mL | 100% |
| Bacillus amyloliquefaciens | YTB1407 [60] | American ginseng root | Leaf discs | - | Fermentation liquor | - | 58.05% |
| Bacillus amyloliquefaciens | N22 [61] | - | Field | Spray | Live organisms | 1 ×107/mL | 76.94% |
| Bacillus amyloliquefaciens | CS5 [53] | - | Detached leaf | - | Live organisms | 108 cfu/mL | 96.23% |
| Bacillus methylotrophicus | T3 [62] | Soil | Field | Spray | Live organisms | 2 × 108 cfu/mL | 55.4% |
| Bacillus sp. | BCJB01 [79] | - | Field | Spray | Live organisms | 3 × 109 cfu/mL | 84.05% |
3.3. Fungi as Control Agents for Grape Downy Mildew
| Biocontrol Microorganisms | Strain Name | Isolation Source | Application Scale | Application Manner | Application Types | Application Concentration | Control Efficiency |
|---|---|---|---|---|---|---|---|
| Acremonium byssoides | A21 [96] | Grapevines leaves | - | - | Culture filtrates, crude extracts | - | - |
| Acremonium sclerotigenum | A59 [97] | Grapevines seed | - | - | Culture filtrates | - | - |
| Acremonium persicinum | A3 [97] | Grapevines leaves | - | - | Culture filtrates | - | - |
| Penicillium chrysogenum | - [88] | - | Field | Spray | Metabolites | 45 g/L | 90% |
| Alternaria alternata | - [90] | Grapevines leaves | Greenhouse | Spray | Metabolites | 10−3, 10−4, 10−5, 10−6 M | 100% |
| Fusarium delphinoides | M1 [86] | P. viticola sporangiophore | Leaf discs | - | Culture extract | 10 μg/mL | - |
| Fusarium brachygibbosum | M2 [86] | P. viticola sporangiophore | Leaf discs | - | Culture extract | 10 μg/mL | - |
| Fusarium pseudonygamai | M10 [86] | P. viticola sporangiophore | Leaf discs | - | Culture extract | 10 μg/mL | - |
| Fusarium pseudonygamai | M12_1 [86] | P. viticola sporangiophore | Leaf discs | - | Culture extract | 10 μg/mL | - |
| Fusarium sp. | M12_2 [86] | P. viticola sporangiophore | Leaf discs | - | Culture extract | 10 μg/mL | - |
| Fusarium proliferatum | G6 [84] | Atypical grape downy mildew lesions | Field | Spray | Live organisms | 1 × 106 microconidia/mL | - |
| Fusarium proliferatum | F3 [85] | Abnormal lesions of grape downy mildew | Detached leaf | - | Live organisms | 1.7 × 107 spores/mL | 88.9% |
| Pythium oligandrum | Po37 [94] | Grapevine roots | Foliar discs | - | Live organisms | 2 × 104 oospores/mL | - |
| Beauveria bassiana | ATP01 [94] | Maize stem borer | Greenhouse | Spray | Live organisms | 1 × 108 conidia/mL | - |
| Beauveria bassiana | ATP05 [92] | Sorghum chafer | Greenhouse | Spray | Live organisms | 1 × 108 conidia/mL | - |
| Beauveria bassiana | EABb04/01-Tip [92] | Dead Timaspis papaveris (Kieffer) larvae | Greenhouse | Spray | Live organisms | 1 × 108 conidia/mL | - |
| Beauveria bassiana | ATCC 74040 [92] | - | Greenhouse | Spray | Live organisms | 1.4 × 107 conidia/mL | 89.03% |
| Saccharomyces cerevisiae | - [100] | - | - | Spray | Live organisms | 1.5 L/ha | - |
| Saccharomyces cerevisiae | - [68] | - | - | Spray | Live organisms | 1 × 109 cell/mL | - |
| Aureobasidium pullulans | - [99] | - | Greenhouse | Spray | Live organisms | 1.0% | - |
| Leptosphaerulina australis | Y29 [51] | Grapevines leaves | Leaf discs | - | Live organisms | 1 × 107 spores/mL | 72.9% |
| Epicoccum nigrum | - [98] | P. viticola infected leaves | - | - | Live organisms | - | - |
| Phomopsis sp. | CAFT69 [89] | Leaves or stems bark of Endodesmia calophylloides | - | - | Metabolites | 10, 30, 50 μg/mL | - |
| Rhizophagus irregularis | - [95] | - | - | - | Live organisms | - | - |
| Trichoderma harzianum | TriH_JSB36 [81] | Soil | Field | - | Live organisms | 108 spores/mL | 82.9% |
| Trichoderma harzianum | HL1 [68] | Soil | - | Spray | Live organisms | 3 × 107/mL | - |
| Trichoderma harzianum | HL14 [82] | Bean rhizosphere | Field | Spray | Live organisms | 108 spores/mL | 69.7% |
| Trichoderma harzianum | T39 [80] | - | Greenhouse | Spray | Live organisms | 1 × 107 conidia/mL | - |
| Trichoderma viride | HL5 [68] | Soil | - | Spray | Live organisms | 3 × 107/mL | - |
| Trichoderma asperellum | ICC012 [83] | - | Laboratory | Spray | Live organisms | 2.5 kg/hl | - |
| Trichoderma gamsii | ICC080 [83] | Laboratory | Spray | Live organisms | 2.5 kg/hl | - |
4. Mechanisms of Action of Biocontrol Agents against Plasmopara viticola
| Biocontrol Microorganisms | Mechanisms | References |
|---|---|---|
| Beauveria bassiana ATCC74040 | Competition; induce the expression of defense-related gene PR-1-like in grape plants | [92] |
| Pythium oligandrum Po37 | Induce the expression of defenses genes (PR1, GLU, PR5, LOX2, PAL and STS) | [94] |
| Fusarium proliferatum F3 | Mycoparasitism | [85] |
| Fusarium proliferatum G6 | Mycoparasitism | [84] |
| Fusarium delphinoides M1 | Mycoparasitism; production of metabolites, fusaric acid, etc. | [86,87] |
| Fusarium brachygibbosum M2 | Mycoparasitism; production of metabolites, fusaric acid, etc. | [86,87] |
| Fusarium pseudonygamai M10 | Mycoparasitism; production of metabolites, fusaric acid, etc. | [86,87] |
| Fusarium pseudonygamai M12_1 | Mycoparasitism; production of metabolites, fusaric acid, etc. | [86,87] |
| Fusarium sp. M12_2 | Mycoparasitism; production of metabolites, fusaric acid, etc. | [86,87] |
| Alternaria alternata | Production of three dipeptides, belonging to the family of diketopiperazines; competition | [90,91] |
| Penicillium chrysogenum | Production of Pen, an aqueous extract of the dry mycelium | [88] |
| Epicoccum nigrum | Mycoparasitism | [98] |
| Phomopsis sp. CAFT69 | Production of metabolites, excelsional etc. | [89] |
| Trichoderma harzianum T39 | Induce the expression of defenses genes PR-2, PR-4, OSM-1, etc. | [80,101] |
| Trichoderma harzianum TriH_JSB36 | Induce the activities of the defense enzymes, peroxidase, etc. | [81] |
| Trichoderma asperellum ICC012/Trichoderma gamsii ICC080 | Induce the production of jasmonic acid | [83] |
| Paenibacillus sp. B2 | Production of paenimyxin, an antagonistic peptide | [73] |
| Pseudomonas fluorescens PTA-CT2 | Induce the expression of defenses genes in SA and HR response | [71] |
| Lysobacter capsici AZ78 | Production of cyclo (L-Pro-l-Tyr) | [77] |
| Streptomyces atratus PY-1 | Production of metabolites (5-acetoxycycloheximide and cycloheximide) | [63] |
| Streptomyces sp. ANK313 | Production of metabolites (Khatmiamycin) | [64] |
| Bacillus subtilis GLB191 | Production of cyclic lipopeptides fengycin and surfactin; secretion of autolysins | [54,102] |
| Bacillus megaterium BMJBN02 | Induce the content of salicylic acid and expression of defenses genes (PR genes) | [58] |
| Bacillus velezensis KOF112 | Induce the expression of defenses genes (class IV chitinase and β-1,3-glucanase) | [59] |
4.1. Mycoparasitism
4.2. Competition
4.3. Secretion of Enzymes and Peptides
4.4. Production of Metabolites
4.5. Induction of Plant Systemic Resistance
5. Conclusions and Future Prospects
- (1)
- Screening for novel biocontrol microbial resources: There are currently still too few microbial resources able to control grape downy mildew. Novel biocontrol resources can be isolated from the soil, rhizosphere, or grape tissues using traditional isolation and purification methods, followed by being screened to determine their biocontrol efficacy. Alternatively, known microbial resources with broad-spectrum antimicrobial properties can be used to evaluate their control capabilities against grape downy mildew.
- (2)
- Improving the control efficiency of existing biocontrol agents: Improvement mainly involves optimizing cultivation or application conditions, in addition to improving the ability of biocontrol agents to produce enzymes and secondary metabolites. Moreover, a synergistic combination of different biocontrol agents, or the integration of biocontrol agents with pesticides, will further improve control efficiency.
- (3)
- Constructing biocontrol genetic engineering strains: The transformation of effective biocontrol genes into biocontrol agents to construct genetic engineering strains, thereby improving biocontrol efficiency against grape downy mildew.
- (4)
- Elucidating the control mechanism of biocontrol agents: Clarifying the control mechanism of biocontrol agents is crucial for further improving the control efficiency of biocontrol agents. Transcriptomics can be used to screen biocontrol-related genes in biocontrol agents that are significantly differentially expressed during the process of controlling grape downy mildew. Thereafter, gene knockout, silencing, or overexpression could be used to verify the function of differentially expressed genes in controlling grape downy mildew.
- (5)
- Elucidating the pathogenic mechanism of P. viticola: Clarifying the pathogenic mechanism of P. viticola could provide potential targets for biocontrol agents. Transcriptomics can be used to screen the differentially expressed genes during the infection process of P. viticola on grape tissues; thereafter, the function of these differentially expressed genes in P. viticola infection can be studied.
- (6)
- Developing biocontrol products: Although some biocontrol agents have been reported, very few biocontrol agents have been developed into commercial products and widely used in field control. Therefore, the development of more commercial biocontrol products and the improvement of their shelf life will be of great value for the sustainable application of biocontrol agents.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, M.; Brun, F.; Raynal, M.; Makowski, D. Timing of grape downy mildew onset in bordeaux vineyards. Phytopathology 2019, 109, 787–795. [Google Scholar] [CrossRef]
- Li, Z.; Dos Santos, R.F.; Gao, L.; Chang, P.; Wang, X. Current status and future prospects of grapevine anthracnose caused by Elsinoe ampelina: An important disease in humid grape-growing regions. Mol. Plant Pathol. 2021, 22, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Godana, E.A.; Sui, Y.; Yang, Q.; Zhang, X.; Zhao, L. Biological control as an alternative to synthetic fungicides for the management of grey and blue mould diseases of table grapes: A review. Crit. Rev. Microbiol. 2020, 46, 450–462. [Google Scholar] [CrossRef]
- Su, K.; Guo, Y.; Zhao, Y.; Gao, H.; Liu, Z.; Li, K.; Ma, L.; Guo, X. Candidate genes for grape white rot resistance based on SMRT and Illumina sequencing. BMC Plant Biol. 2019, 19, 501. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Yang, J.; Zhang, K.; Yin, K.; Xiang, G.; Yin, X.; Liu, G.; Xu, Y. Plasmopara viticola effector PvCRN11 induces disease resistance to downy mildew in grapevine. Plant J. 2024, 117, 873–891. [Google Scholar] [CrossRef] [PubMed]
- Li, B.Y.; Ran, L.X.; Shi, L.M.; Zhang, K.C.; Ge, B.B. Research progress of grape downy mildew. Chin. Agric. Sci. Bull. 2023, 39, 125–131. [Google Scholar]
- Capriotti, L.; Baraldi, E.; Mezzetti, B.; Limera, C.; Sabbadini, S. Biotechnological approaches: Gene overexpression, gene silencing, and genome editing to control fungal and oomycete diseases in grapevine. Int. J. Mol. Sci. 2020, 21, 5701. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.X.; Feng, J.; Yi, T.Y. Toxicity determination of azoxystrobin and dimethomorph against Plasmopara viticola. J. Agric. 2016, 6, 20–23. [Google Scholar]
- Xin, Z.M.; Xu, D.K.; Zhang, M.; Wu, B.; Jiang, S.S.; Xin, X.Q. Allied toxicity determination of azoxystrobin and polyhexamethylene biguanidine hydrochloride combined with dimethomorph on Plasmopara viticola. Shandong Agric. Sci. 2016, 48, 117–120. [Google Scholar]
- Cheng, Y.P.; Pan, S.F.; Li, Y.L.; Zhang, W.L.; Gao, C.L.; Cheng, Y. Comparison on effect of six fungicides on grape downy mildew (Plasmopara viticola) in laboratory and field. J. Anhui Agric. Sci. 2018, 46, 109–111. [Google Scholar]
- Djennane, S.; Gersch, S.; Le-Bohec, F.; Piron, M.C.; Baltenweck, R.; Lemaire, O.; Merdinoglu, D.; Hugueney, P.; Nogué, F.; Mestre, P. CRISPR/Cas9 editing of downy mildew resistant 6 (DMR6-1) in grapevine leads to reduced susceptibility to Plasmopara viticola. J. Exp. Bot. 2024, 75, 2100–2112. [Google Scholar] [CrossRef] [PubMed]
- González-Centeno, M.R.; Chira, K.; Miramont, C.; Escudier, J.L.; Samson, A.; Salmon, J.M.; Ojeda, H.; Teissedre, P.L. Disease resistant bouquet vine varieties: Assessment of the phenolic, aromatic, and sensory potential of their wines. Biomolecules 2019, 9, 793. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Cai, X.; Yang, C.; Xie, L.; Qin, G.; Zhang, M.; Huang, Y.; Gong, G.; Chang, X.; Chen, H. Studies on the control effect of Bacillus subtilis on wheat powdery mildew. Pest. Manag. Sci. 2021, 77, 4375–4382. [Google Scholar] [CrossRef]
- Kiani Dehkian, Z.; Taheri, H.; Pakdaman Sardrood, B.; Farkhari, M. Controlling tomato Fusarium wilt disease through Bacillus thuringiensis-mediated defense primining. Iran. J. Biotechnol. 2024, 22, e3690. [Google Scholar]
- Sawant, S.S.; Song, J.; Seo, H.J. Characterization of Bacillus velezensis RDA1 as a biological control agent against white root rot disease caused by Rosellinia necatrix. Plants 2022, 11, 2486. [Google Scholar] [CrossRef]
- Yamamoto, S.; Shiraishi, S.; Kawagoe, Y.; Mochizuki, M.; Suzuki, S. Impact of Bacillus amyloliquefaciens S13-3 on control of bacterial wilt and powdery mildew in tomato. Pest. Manag. Sci. 2015, 71, 722–727. [Google Scholar] [CrossRef]
- Akram, W.; Waqar, S.; Hanif, S.; Anjum, T.; Aftab, Z.E.; Li, G.; Ali, B.; Rizwana, H.; Hassan, A.; Rehman, A.; et al. Comparative effect of seed coating and biopriming of Bacillus aryabhattai Z-48 on seedling growth, growth promotion, and suppression of Fusarium wilt disease of tomato plants. Microorganisms 2024, 12, 792. [Google Scholar] [CrossRef] [PubMed]
- Hariprasad, P.; Chandrashekar, S.; Singh, S.B.; Niranjana, S.R. Mechanisms of plant growth promotion and disease suppression by Pseudomonas aeruginosa strain 2apa. J. Basic. Microbiol. 2014, 54, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Srivastava, S.; Karnwal, A.; Malik, T. Streptomyces as a promising biological control agents for plant pathogens. Front. Microbiol. 2023, 14, 1285543. [Google Scholar] [CrossRef]
- Son, S.H.; Khan, Z.; Kim, S.G.; Kim, Y.H. Plant growth-promoting rhizobacteria, Paenibacillus polymyxa and Paenibacillus lentimorbus suppress disease complex caused by root-knot nematode and fusarium wilt fungus. J. Appl. Microbiol. 2009, 107, 524–532. [Google Scholar] [CrossRef]
- Mahmoud, G.A.; Abdel-Sater, M.A.; Al-Amery, E.; Hussein, N.A. Controlling Alternaria cerealis MT808477 tomato phytopathogen by Trichoderma harzianum and tracking the plant physiological changes. Plants 2021, 10, 1846. [Google Scholar] [CrossRef] [PubMed]
- Tomah, A.A.; Alamer, I.S.A.; Khattak, A.A.; Ahmed, T.; Hatamleh, A.A.; Al-Dosary, M.A.; Ali, H.M.; Wang, D.; Zhang, J.; Xu, L.; et al. Potential of Trichoderma virens HZA14 in controlling Verticillium wilt disease of eggplant and analysis of its genes responsible for microsclerotial degradation. Plants 2023, 12, 3761. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Zeng, L.; Jiangm, H.; Mei, L.; Wang, Y. Trichoderma atroviride LZ42 releases volatile organic compounds promoting plant growth and suppressing Fusarium wilt disease in tomato seedlings. BMC Microbiol. 2022, 22, 88. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Leng, J.; Yu, L.; Bai, H.; Li, X.; Wisniewski, M.; Liu, J.; Sui, Y. Efficacy of the biocontrol agent Trichoderma hamatum against Lasiodiplodia theobromae on macadamia. Front. Microbiol. 2022, 13, 994422. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, N.; Pang, Q.; Khan, R.A.A.; Xu, Q.; Wu, C.; Liu, T. A salt-tolerant strain of Trichoderma longibrachiatum HL167 is effective in alleviating salt stress, promoting plant growth, and managing Fusarium wilt disease in cowpea. J. Fungi 2023, 9, 304. [Google Scholar] [CrossRef]
- Sun, Z.B.; Li, S.D.; Ren, Q.; Xu, J.L.; Lu, X.; Sun, M.H. Biology and applications of Clonostachys rosea. J. Appl. Microbiol. 2020, 129, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Partridge, D.E.; Sutton, T.B.; Jordan, D.L.; Curtis, V.L.; Bailey, J.E. Management of Sclerotinia blight of peanut with the biological control agent Coniothyrium minitans. Plant Dis. 2006, 90, 957–963. [Google Scholar] [CrossRef]
- Ownley, B.H.; Griffin, M.R.; Klingeman, W.E.; Gwinn, K.D.; Moulton, J.K.; Pereira, R.M. Beauveria bassiana: Endophytic colonization and plant disease control. J. Invertebr. Pathol. 2008, 98, 267–270. [Google Scholar] [CrossRef]
- Sarven, M.S.; Hao, Q.; Deng, J.; Yang, F.; Wang, G.; Xiao, Y.; Xiao, X. Biological control of tomato gray mold caused by Botrytis Cinerea with the entomopathogenic fungus Metarhizium Anisopliae. Pathogens 2020, 9, 213. [Google Scholar] [CrossRef]
- Hashem, A.; Tabassum, B.; Abd_Allah, E.F. Bacillus subtilis: A plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi. J. Biol. Sci. 2019, 26, 1291–1297. [Google Scholar] [CrossRef]
- Vurukonda, S.S.K.P.; Giovanardi, D.; Stefani, E. Plant growth promoting and biocontrol activity of Streptomyces spp. as endophytes. Int. J. Mol. Sci. 2018, 19, 952. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Xie, J.; Li, Y.; Gao, T.; Zhang, X.; Wang, Q. Comprehensive genomic analysis of the endophytic Bacillus altitudinis strain GLB197, a potential biocontrol agent of grape downy mildew. Front. Genet. 2021, 12, 729603. [Google Scholar] [CrossRef]
- Palmieri, M.C.; Perazzolli, M.; Matafora, V.; Moretto, M.; Bachi, A.; Pertot, I. Proteomic analysis of grapevine resistance induced by Trichoderma harzianum T39 reveals specific defence pathways activated against downy mildew. J. Exp. Bot. 2012, 63, 6237–6251. [Google Scholar] [CrossRef]
- Gouveia, C.; Santos, R.B.; Paiva-Silva, C.; Buchholz, G.; Malhó, R.; Figueiredo, A. The pathogenicity of Plasmopara viticola: A review of evolutionary dynamics, infection strategies and effector molecules. BMC Plant Biol. 2024, 24, 327. [Google Scholar] [CrossRef] [PubMed]
- Gessler, C.; Pertot, I.; Perazzolli, M. Plasmopara viticola: A review of knowledge on downy mildew of grapevine and effective disease management. Phytopathol. Mediterr. 2011, 50, 3–44. [Google Scholar]
- Jürges, G.; Kassemeyer, H.H.; Dürrenberger, M.; Düggelin, M.; Nick, P. The mode of interaction between Vitis and Plasmopara viticola Berk. & Curt. Ex de Bary depends on the host species. Plant Biol. 2009, 11, 886–898. [Google Scholar]
- Buonassisi, D.; Colombo, M.; Migliaro, D.; Dolzani, C.; Peressotti, E.; Mizzotti, C.; Velasco, R.; Masiero, S.; Perazzolli, M.; Vezzulli, S. Breeding for grapevine downy mildew resistance: A review of “omics” approaches. Euphytica 2017, 213, 103. [Google Scholar] [CrossRef]
- Burruano, S. The life-cycle of Plasmopara viticola, cause of downy mildew of vine. Mycologist 2000, 14, 179–182. [Google Scholar] [CrossRef]
- Wang, L.L.; Sun, Y.K.; Xu, X.; Zhou, J.; Wang, Q.M.; Zhu, X.Z. Research progress on pathogensis and control of grape downy mildew. Hortic. Seed 2022, 42, 31–33. [Google Scholar]
- Velasquez-Camacho, L.; Otero, M.; Basile, B.; Pijuan, J.; Corrado, G. Current trends and perspectives on predictive models for mildew diseases in vineyards. Microorganisms 2022, 11, 73. [Google Scholar] [CrossRef]
- Fröbel, S.; Zyprian, E. Colonization of different grapevine tissues by Plasmopara viticola-a histological study. Front. Plant Sci. 2019, 10, 951. [Google Scholar] [CrossRef] [PubMed]
- Musetti, R.; Stringher, L.; Borselli, S.; Vecchione, A.; Zulini, L.; Pertot, I. Ultrastructural analysis of Vitis vinifera leaf tissues showing atypical symptoms of Plasmopara viticola. Micron 2005, 36, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Wang, X.; Wang, H.; Li, X.; Zhang, Q.; Wang, M.; Yan, J. Advances in understanding grapevine downy mildew: From pathogen infection to disease management. Mol. Plant Pathol. 2024, 25, e13401. [Google Scholar] [CrossRef] [PubMed]
- Koledenkova, K.; Esmaeel, Q.; Jacquard, C.; Nowak, J.; Clément, C.; Ait Barka, E. Plasmopara viticola the causal agent of downy mildew of grapevine: From its taxonomy to disease management. Front. Microbiol. 2022, 13, 889472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yan, J.Y.; Liu, M.; Peng, J.B.; Xing, Q.K.; Li, X.H. Research progress on prediction and epidemic of grapevine downy mildew. China Fruits 2020, 3, 11–15. [Google Scholar]
- Toffolatti, S.L.; De Lorenzis, G.; Brilli, M.; Moser, M.; Shariati, V.; Tavakol, E.; Maddalena, G.; Passera, A.; Casati, P.; Pindo, M.; et al. Novel aspects on the interaction between grapevine and Plasmopara viticola: Dual-RNA-Seq analysis highlights gene expression dynamics in the pathogen and the plant during the battle for infection. Genes 2020, 11, 261. [Google Scholar] [CrossRef]
- Gómez-Zeledón, J.; Spring, O. Up-regulated RxLR effector genes of Plasmopara viticola in synchronized host-free stages and infected leaves of hosts with different susceptibility. Fungal Biol. 2018, 122, 1125–1133. [Google Scholar] [CrossRef]
- Fu, Q.; Wang, Y.; Yang, J.; Jiao, Y.; Li, W.; Yang, F.; Yin, X.; Shang, B.; Liu, R.; Zhang, Y.; et al. Plasmopara viticola RxLR effector PvAvh77 triggers cell death and governs immunity responses in grapevine. J. Exp. Bot. 2023, 74, 2047–2066. [Google Scholar] [CrossRef]
- Yin, L.; An, Y.; Qu, J.; Li, X.; Zhang, Y.; Dry, I.; Wu, H.; Lu, J. Genome sequence of Plasmopara viticola and insight into the pathogenic mechanism. Sci. Rep. 2017, 7, 46553. [Google Scholar] [CrossRef]
- Guo, J.P.; Ma, G.; Zhu, H.X.; Liu, C.Y.; Fu, J.F. Isolation, screening and identification of antagonistic bacteria strain N6 against grape downy mildew. Hubei Agric. Sci. 2014, 53, 4063–4065. [Google Scholar]
- Guo, J.P.; Ma, G.; Zhu, H.X.; Liu, C.Y.; Fu, J.F. Isolation, screening and identification of antagonistic fungi against grape downy mildew. J. Jilin Agric. Sci. 2014, 59, 72–75. [Google Scholar]
- Liu, X.; Yang, X.C.; Tao, Y.; Yu, X.; Li, H. Screening, identification and optimization of fermentation conditions for antagonistic bacteria against Plasmopara viticola on grape. J. Fruit Sci. 2015, 32, 681–688. [Google Scholar]
- Zhang, X.; Zhou, Y.Y.; Li, Y.; Fu, X.C.; Wang, Q. Screening and characterization of endophytic Bacillus for biocontrol of grapevine downy mildew. Crop Prot. 2017, 96, 173–179. [Google Scholar] [CrossRef]
- Li, Y.; Héloir, M.C.; Zhang, X.; Geissler, M.; Trouvelot, S.; Jacquens, L.; Henkel, M.; Su, X.; Fang, X.; Wang, Q.; et al. Surfactin and fengycin contribute to the protection of a Bacillus subtilis strain against grape downy mildew by both direct effect and defence stimulation. Mol. Plant Pathol. 2019, 20, 1037–1050. [Google Scholar] [CrossRef]
- Furuya, S.; Mochizuki, M.; Aoki, Y.; Kobayashi, H.; Takayanagi, T.; Shimizu, M.; Suzuki, S. Isolation and characterization of Bacillus subtilis KS1 for the biocontrol of grapevine fungal diseases. Biocontrol Sci. Techn. 2011, 21, 705–720. [Google Scholar] [CrossRef]
- Chen, H.; Hu, L.B.; Tang, C.P.; Hu, J.; Shi, Z.Q. Efficacy of the strain B-FS01 of Bacillus subtilis in suppression of the grape downy mildew caused by Plasmopara viticola. Plant Prot. 2011, 37, 194–197. [Google Scholar]
- Bi, Q.Y.; Han, X.Y.; Ma, Z.Q.; Zhao, J.J.; Wang, W.Q.; Jia, H.M. Inhibitory effects of Bacillus subtilis HMB-20428 interacted with chemical fungicides and decrement of chemical fungicides on oomycete pathogen Plasmopara viticola. J. Plant Prot. 2018, 45, 1396–1404. [Google Scholar]
- Xie, X.Y.; Han, X.Y.; Zhang, G.Z.; Fan, S.S.; Zhou, H.Z.; Zhang, X.J. Bacillus megaterium BMJBN02 induces the resistance of grapevine against downy mildew. Vitis 2022, 61, 101–109. [Google Scholar]
- Hamaoka, K.; Aoki, Y.; Suzuki, S. Isolation and characterization of endophyte Bacillus velezensis KOF112 from grapevine shoot xylem as biological control agent for fungal diseases. Plants 2021, 10, 1815. [Google Scholar] [CrossRef]
- Yu, X.L.; Qi, C.; Wang, P.S.; Li, B.Y.; Wang, Y.Z. Screening and identification of antagonistic bacteria against fungal pathogens in fruit trees and their antifungal mechanisms. J. Fruit Sci. 2016, 33, 734–743. [Google Scholar]
- Guo, J.P.; Ma, G.; Gao, X.K.; Fu, J.F. Preliminary report on screening and identification of antagonistic bacterium N22 against grape downy mildew and its controlling efficiency. China Plant Prot. 2016, 36, 9–14. [Google Scholar]
- Kang, X.J.; Shen, H.M.; Jia, Z.S.; Yang, J.Y.; Ran, L.X.; Zhen, Z.X. Identification of Bacillus methylotrophicus T3 and its control effect on grape downy mildew. Chin. J. Biol. Control 2016, 32, 775–782. [Google Scholar]
- Liang, C.H.; Zang, C.Q.; McDermott, M.I.; Zhao, K.H.; Yu, S.Y.; Huang, Y.Q. Two imide substances from a soil isolated Streptomyces atratus strain provide effective biocontrol activity against grapevine downy mildew. Biocontrol Sci. Technol. 2016, 10, 1337–1351. [Google Scholar] [CrossRef]
- Abdalla, M.A.; Win, H.Y.; Islam, M.T.; von Tiedemann, A.; Schüffler, A.; Laatsch, H. Khatmiamycin, a motility inhibitor and zoosporicide against the grapevine downy mildew pathogen Plasmopara viticola from Streptomyces sp. ANK313. J. Antibiot. 2011, 64, 655–659. [Google Scholar] [CrossRef]
- Jiang, X.T.; Zhao, K.H.; Liu, C.Y.; Liang, C.H.; Zang, C.Q. Screening and identifying of antagonistic actinomycete QH94 against Plasmopara viticola. J. Shenyang Agric. Univ. 2015, 46, 303–308. [Google Scholar]
- Liu, T.; Tao, W.Q.; Wang, H.; Hua, Y.P.; Liu, W.C. The antifungal activity of the metabolite of biocontrol strain A02 against some fruit tree diseases. North. Horticult. 2011, 17, 13–16. [Google Scholar]
- Ji, M.X.; Yao, K.B.; Miao, K.; Chen, H.Z.; Wu, X.; Zhuang, Y.Q. Toxicity test and field control effects of 4 different fungicides on grape downy mildew. Agric. Sci. Technol. 2016, 17, 1654–1657, 1752. [Google Scholar]
- El-Sharkawy, H.H.A.; Abo-El-Wafa, T.S.A.; Aal Ibrahim, S.A. Biological control agents improve the productivity and induce the resistance against downy mildew of grapevine. J. Plant Pathol. 2018, 100, 33–42. [Google Scholar] [CrossRef]
- Wang, X.J.; Dong, C.H.; Liu, C.Y.; Zhao, K.H.; Huang, Y.F.; Jiang, X.T. Identification of endophytic bacteria HB135 from grape and primary studies on its extract. J. Shenyang Agric. Univ. 2014, 45, 538–542. [Google Scholar]
- Shi, X.M.; Zhang, S.Y.; Wang, Y.C.; Shen, H.M.; Ran, L.X. Resuscitation of grape endophytic bacteria and their inhibitive effects against Plasmopara viticola. Acta Phytopathol. Sin. 2021, 51, 951–961. [Google Scholar]
- Lakkis, S.; Trotel-Aziz, P.; Rabenoelina, F.; Schwarzenberg, A.; Nguema-Ona, E.; Clément, C.; Aziz, A. Strengthening grapevine resistance by Pseudomonas fluorescens PTA-CT2 relies on distinct defense pathways in susceptible and partially resistant genotypes to downy mildew and gray mold diseases. Front. Plant Sci. 2019, 10, 1112. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.D.; Wu, Y.Z.; Li, J.S.; Wei, Y.L.; Chen, Q.; Yang, H.T. The control effects of antagonistic Paenibacillus polymyxa PB-2 and its preparation against grape downy mildew. Shandong Sci. 2014, 27, 30–33. [Google Scholar]
- Hao, Z.P.; Tuinen, D.V.; Wipf, D.; Fayolle, L.; Chataignier, O.; Li, X.L.; Chen, B.D.; Gianinazzi, S.; Gianinazzi-Pearson, V.; Adrian, M. Biocontrol of grapevine aerial and root pathogens by Paenibacillus sp. strain B2 and paenimyxin in vitro and in planta. Biol. Control 2017, 109, 42–50. [Google Scholar] [CrossRef]
- Zang, C.Q.; Lin, Q.J.; Xie, J.H.; Lin, Y.; Zhao, K.H.; Liang, C.H. The biological control of the grapevine downy mildew disease using Ochrobactrum sp. Plant Protect. Sci. 2020, 56, 52–61. [Google Scholar] [CrossRef]
- Zang, C.Q.; Zhao, K.H.; Liu, C.Y.; Liang, C.H.; Liu, L.; Yu, S.Y. Inhibitory effect of SY286 strain against Plasmopara viticola. J. Shenyang Agric. Univ. 2014, 45, 221–224. [Google Scholar]
- Markellou, M.; Kapaxidi, E.; Karamaouna, F.; Samara, M.; Kyriakopoulou, K.; Anastasiadou, P.; Vavoulidou, E.; Meidanis, M.; Machera, K.; Mandoulaki, A.; et al. Evaluation of plant protection efficacy in field conditions and side effects of Lysobacter capsica AZ78, a biocontrol agent of Plasmopara viticola. Biocontrol Sci. Technol. 2022, 32, 930–951. [Google Scholar] [CrossRef]
- Puopolo, G.; Cimmino, A.; Palmieri, M.C.; Giovannini, O.; Evidente, A.; Pertot, I. Lysobacter capsici AZ78 produces cyclo(L-Pro-L-Tyr), a 2,5-diketopiperazine with toxic activity against sporangia of Phytophthora infestans and Plasmopara viticola. J. Appl. Microbiol. 2014, 117, 1168–1180. [Google Scholar] [CrossRef]
- Shen, H.M.; Li, Z.N.; Jia, Z.S.; Yang, J.Y.; Ran, L.X. Colonization of grape leaves by endophytic Bacillus subtilis JL4 and its control of grape downy mildew. Chin. J. Appl. Ecol. 2016, 27, 4022–4028. [Google Scholar]
- Wang, Y.L.; Li, J.S.; Wang, Y.Z.; Xin, X.Q.; Li, B.Y.; Xie, X.Y. Efficacy of Bacillus spp. BCJB01 and BMJBN02 on grape downy mildew. Northern Horticult. 2017, 17, 67–71. [Google Scholar]
- Banani, H.; Roatti, B.; Ezzahi, B.; Giovannini, O.; Gessler, G.; Pertot, I.; Perazzolli, M. Characterization of resistance mechanisms activated by Trichoderma harzianum T39 and benzothiadiazole to downy mildew in different grapevine cultivars. Plant Pathol. 2014, 63, 334–343. [Google Scholar] [CrossRef]
- Kamble, M.V.; Joshi, S.M.; Hadimani, S.; Jogaiah, S. Biopriming with rhizosphere Trichoderma harzianum elicit protection against grapevine downy mildew disease by triggering histopathological and biochemical defense responses. Rhizosphere 2021, 19, 100398. [Google Scholar] [CrossRef]
- El-Sharkawy, H.H.A.; Abo-El-Wafa, T.S.A.; Mostafa, N.A.; Yousef, S.A.M. Boosting biopesticide potential of Trichoderma harzianum for controlling the downy mildew and improving the growth and the productivity of King Ruby seedless grape. Egypt. J. Biol. Pest. Control 2023, 33, 61. [Google Scholar] [CrossRef]
- Parrilli, M.; Sommaggio, D.; Tassini, C.; Di Marco, S.; Osti, F.; Ferrari, R.; Metruccio, E.; Masetti, A.; Burgio, G. The role of Trichoderma spp. and silica gel in plant defence mechanisms and insect response in vineyard. Bull. Entomol. Res. 2019, 109, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Falk, S.P.; Pearson, R.C.; Gadoury, D.M.; Seem, R.C.; Sztejnberg, A. Fusarium proliferatum as a biocontrol agent against grape downy mildew. Phytopathology 1996, 86, 1010–1017. [Google Scholar] [CrossRef]
- Shen, H.M.; Li, Z.N.; Yang, J.Y.; Zhang, M.; Ran, L.X. Identification of the mycoparasitic strain F3 on Plasmopara viticola and its control effect on grape downy mildew. J. Plant Protect. 2017, 44, 643–649. [Google Scholar]
- Ghule, M.R.; Sawant, I.S.; Oulkar, D.; Hingmire, S.; Shabeer, A.; Holkar, S. Identification of secondary metabolites in mycoparasites Fusarium strains and antifungal activity of fusaric acid against Plasmopara viticola. Arch. Phytopathol. Plant Protect. 2022, 55, 1283–1297. [Google Scholar] [CrossRef]
- Ghule, M.R.; Sawant, I.S.; Sawant, S.D.; Sharma, R.; Shouche, Y.S. Identification of Fusarium species as putative mycoparasites of Plasmopara viticola causing downy mildew in grapevines. Australas. Plant Dis. Notes 2018, 13, 16. [Google Scholar] [CrossRef]
- Thuerig, B.; Binder, A.; Boller, T.; Guyer, U.; Jimenez, S.; Rentsch, C.; Tamm, L. An aqueous extract of the dry mycelium of Penicillium chrysogenum induces resistance in several crops under controlled and field conditions. Eur. J. Plant Pathol. 2006, 114, 185–197. [Google Scholar] [CrossRef]
- Talontsi, F.M.; Islam, M.T.; Facey, P.; Douanla-Meli, C.; von Tiedemann, A.; Laatsch, H. Depsidones and other constituents from Phomopsis sp. CAFT69 and its host plant Endodesmia calophylloides with potent inhibitory effect on motility of zoospores of grapevine pathogen Plasmopara viticola. Phytochem. Lett. 2012, 5, 657–664. [Google Scholar] [CrossRef]
- Musetti, R.; Polizzotto, R.; Vecchione, A.; Borselli, S.; Zulini, L.; D’Ambrosio, M.; di Toppi, L.S.; Pertot, I. Antifungal activity of diketopiperazines extracted from Alternaria alternata against Plasmopara viticola: An ultrastructural study. Micron 2007, 38, 643–650. [Google Scholar] [CrossRef]
- Musetti, R.; Vecchione, A.; Stringher, L.; Borselli, S.; Zulini, L.; Marzani, C.; D’Ambrosio, M.; di Toppi, L.S.; Pertot, I. Inhibition of sporulation and ultrastructural alterations of grapevine downy mildew by the endophytic fungus Alternaria alternata. Phytopathology 2006, 96, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Jaber, L.R. Grapevine leaf tissue colonization by the fungal entomopathogen Beauveria bassiana s.l. and its effect against downy mildew. BioControl 2015, 60, 103–112. [Google Scholar] [CrossRef]
- Rondot, Y.; Reineke, A. Endophytic Beauveria bassiana activates expression of defence genes in grapevine and prevents infections by grapevine downy mildew Plasmopara viticola. Plant Pathol. 2019, 68, 1719–1731. [Google Scholar] [CrossRef]
- Yacoub, A.; Haidar, R.; Mesguida, O.; Gerbore, J.; Hachicha, M.; Attard, E.; Guyoneaud, R.; Rey, P. Deciphering plant-induced responses toward Botrytis cinerea and Plasmopara viticola attacks in two grapevine cultivars colonized by the root biocontrol Oomycete, Pythium oligandrum. J. Fungi 2023, 9, 511. [Google Scholar] [CrossRef]
- Cruz-Silva, A.; Figueiredo, A.; Sebastiana, M. First insights into the effect of mycorrhizae on the expression of pathogen effectors during the infection of grapevine with Plasmopara viticola. Sustainability 2021, 13, 1226. [Google Scholar] [CrossRef]
- Burruano, S.; Alfonzo, A.; Lo Piccolo, S.; Conigliaro, G.; Mondello, V.C.; Torta, L.; Moretti, M.; Assante, G. Interaction between Acremonium byssoides and Plasmopara viticola in Vitis vinifera. Phytopathol. Mediterr. 2008, 47, 122–131. [Google Scholar]
- Lo Piccolo, S.; Alfonzo, A.; Giambra, S.; Conigliaro, G.; Lopez-Llorca, L.V.; Burruano, S. Identification of Acremonium isolates from grapevines and evaluation of their antagonism towards Plasmopara viticola. Ann. Microbiol. 2015, 65, 2393–2403. [Google Scholar] [CrossRef]
- Kortekamp, A. Epicoccum nigrum LINK: A biological control agent of Plasmopara viticola (BERK. et CuRT.) BERL. et DE ToNI? Vitis 1997, 36, 215–216. [Google Scholar]
- Harm, A.; Kassemeyer, H.H.; Seibicke, T.; Regner, F. Evaluation of chemical and natural resistance inducers against downy mildew (Plasmopara viticola) in grapevine. Am. J. Enol. Vitic. 2011, 62, 184–192. [Google Scholar] [CrossRef]
- Gomes, E.C.S.; Leite, R.P.; Silva, F.J.A.; Cavalcanti, L.S.; Nascimento, L.C.; Silva, S.M. Management of mildew and rust in grapes with resistance elicitors: Yield and postharvest quality. Trop. Plant Pathol. 2011, 36, 332–335. [Google Scholar]
- Perazzolli, M.; Roatti, B.; Bozza, E.; Pertot, I. Trichoderma harzianum T39 induces resistance against downy mildew by priming for defense without costs for grapevine. Biol. Control 2011, 58, 74–82. [Google Scholar] [CrossRef]
- Wang, L.; Huang, J.; Chen, S.; Su, X.; Zhang, X.; Wang, L.; Zhang, W.; Wang, Z.; Zeng, Q.; Wang, Q.; et al. Endogenous cell wall degrading enzyme LytD is important for the biocontrol activity of Bacillus subtilis. Front. Plant Sci. 2024, 15, 1381018. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.-B.; Song, H.-J.; Liu, Y.-Q.; Ren, Q.; Wang, Q.-Y.; Li, X.-F.; Pan, H.-X.; Huang, X.-Q. The Potential of Microorganisms for the Control of Grape Downy Mildew—A Review. J. Fungi 2024, 10, 702. https://doi.org/10.3390/jof10100702
Sun Z-B, Song H-J, Liu Y-Q, Ren Q, Wang Q-Y, Li X-F, Pan H-X, Huang X-Q. The Potential of Microorganisms for the Control of Grape Downy Mildew—A Review. Journal of Fungi. 2024; 10(10):702. https://doi.org/10.3390/jof10100702
Chicago/Turabian StyleSun, Zhan-Bin, Han-Jian Song, Yong-Qiang Liu, Qing Ren, Qi-Yu Wang, Xiao-Feng Li, Han-Xu Pan, and Xiao-Qing Huang. 2024. "The Potential of Microorganisms for the Control of Grape Downy Mildew—A Review" Journal of Fungi 10, no. 10: 702. https://doi.org/10.3390/jof10100702
APA StyleSun, Z.-B., Song, H.-J., Liu, Y.-Q., Ren, Q., Wang, Q.-Y., Li, X.-F., Pan, H.-X., & Huang, X.-Q. (2024). The Potential of Microorganisms for the Control of Grape Downy Mildew—A Review. Journal of Fungi, 10(10), 702. https://doi.org/10.3390/jof10100702






