Elucidating the Fundamental Process of Methyl-(5hydroxymethyl) Furan-2-Carboxylate Toxin Biosynthesis in Curvularia lunata Causing Maize Leaf Spot
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
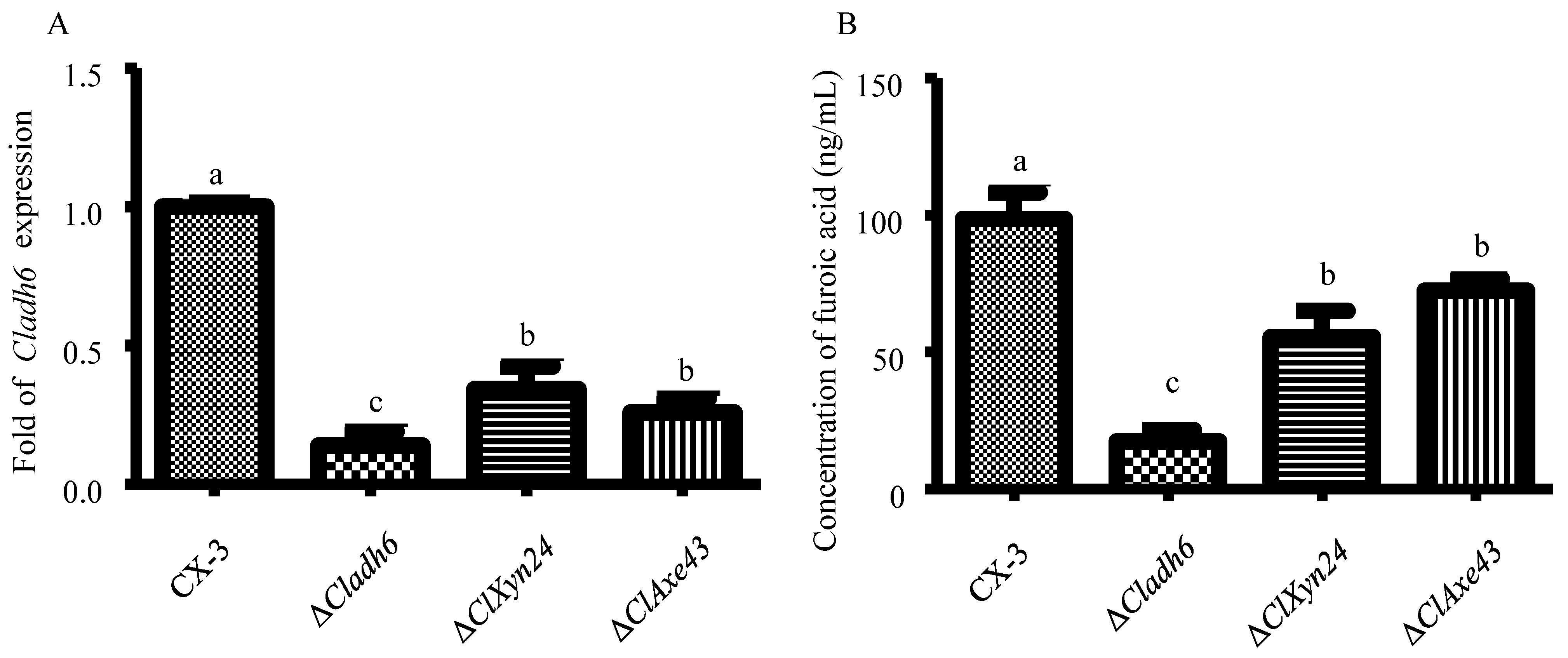
2.2.1. Detection of Expression Levels of Cladh6 in Δclxyn24, Δclaxe43 and CX-3 Strains
2.2.2. Detection of Furoic Acid Content in Δclxyn24, Δclaxe43, and CX-3 Strains
2.2.3. Xylose Anaplerosis Experiment
- (1)
- Liquid phase conditions:
- (2)
- Mass spectrometry conditions
2.2.4. Construction of Cladh6 Prokaryotic Expression System
2.2.5. Determination of ClADH6 Activity
2.2.6. Construction of Cladh6 Deletion Mutant Strain
2.2.7. Determination of Toxin Synthesis Ability of ΔCladh6 and ΔCladh6-C Strains
3. Results
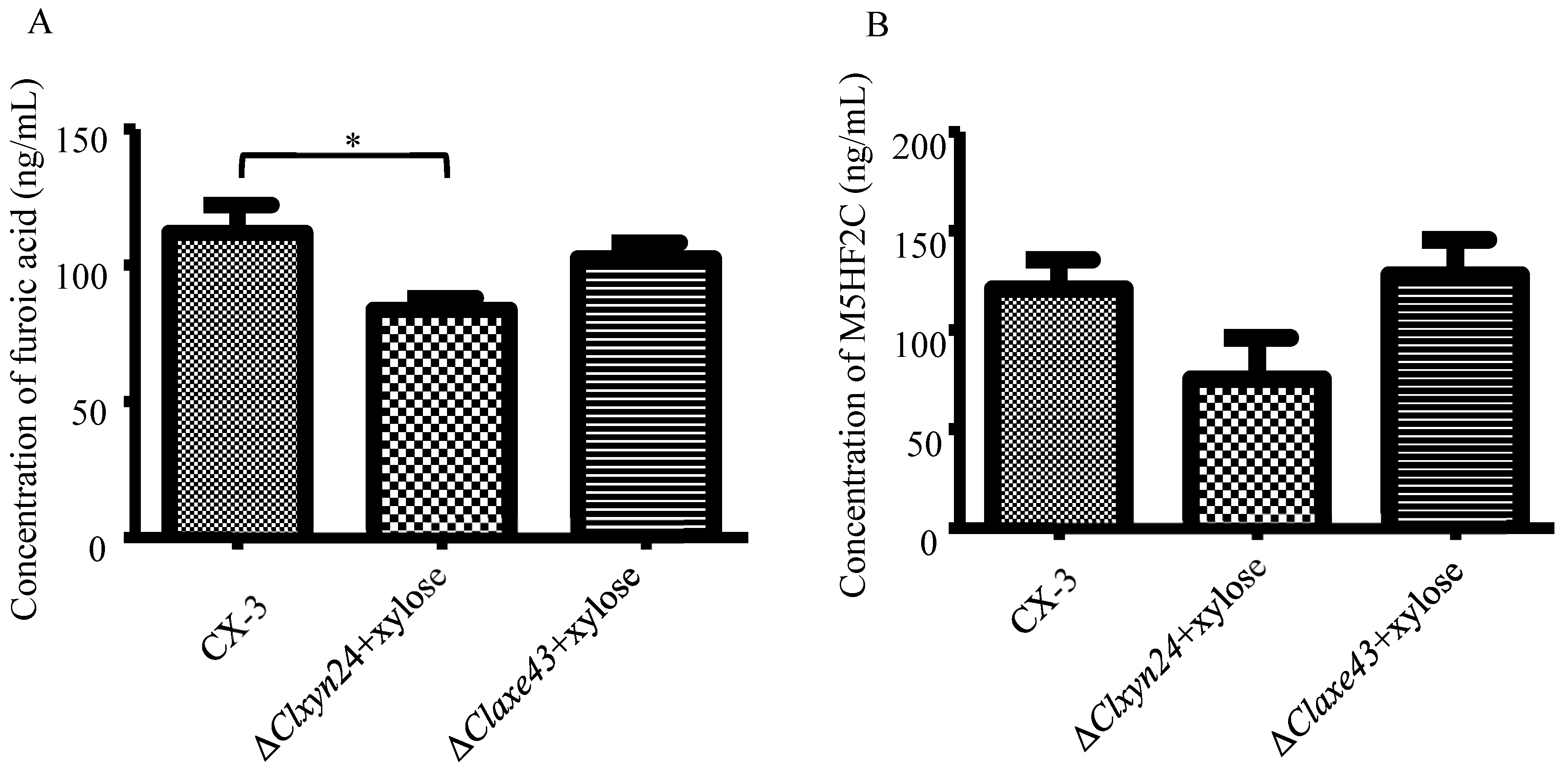
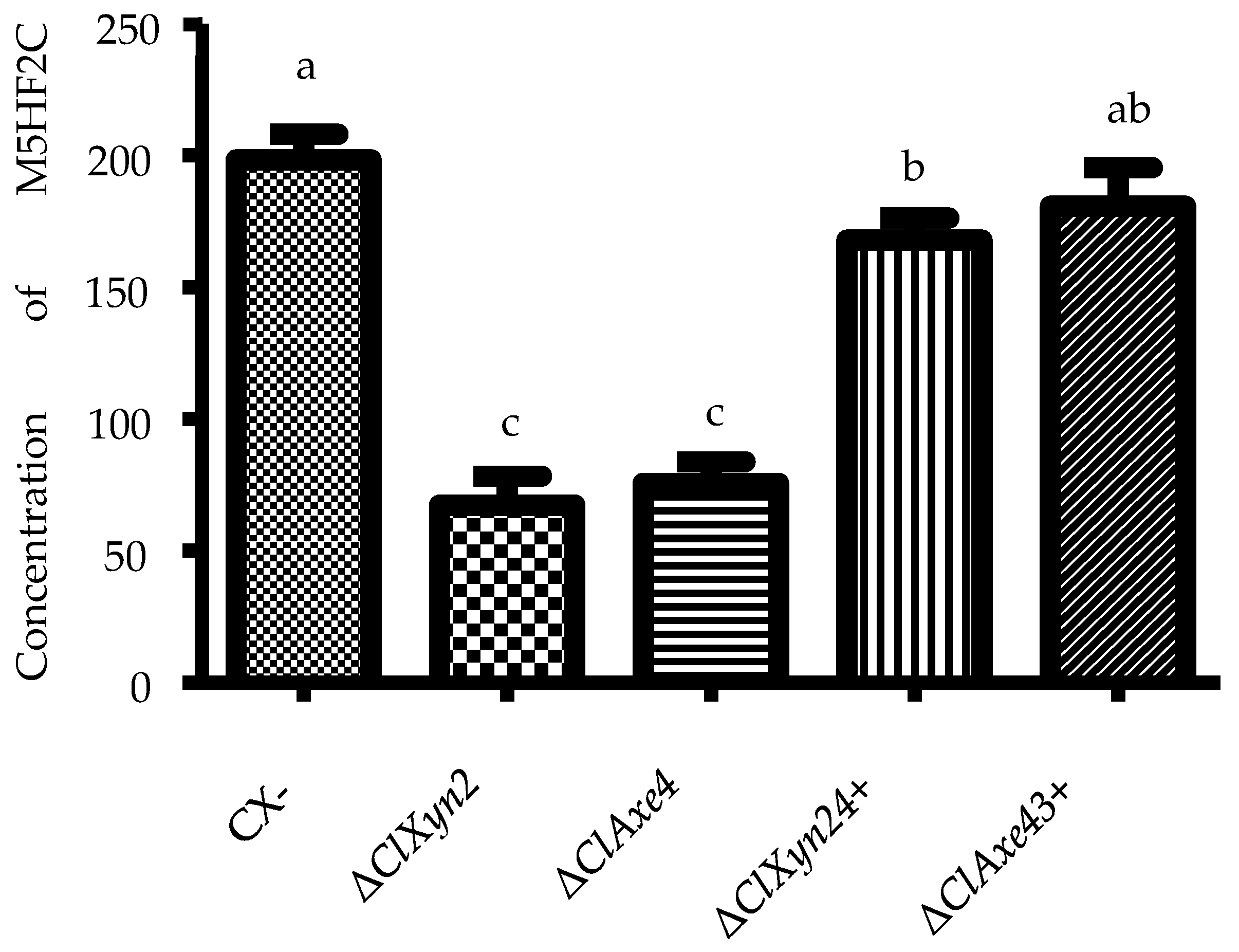
3.1. Effects of Xylose on Furoic Acid Production
3.2. Xylose Is Used as a Raw Material for the Biosynthesis of Furoic Acid
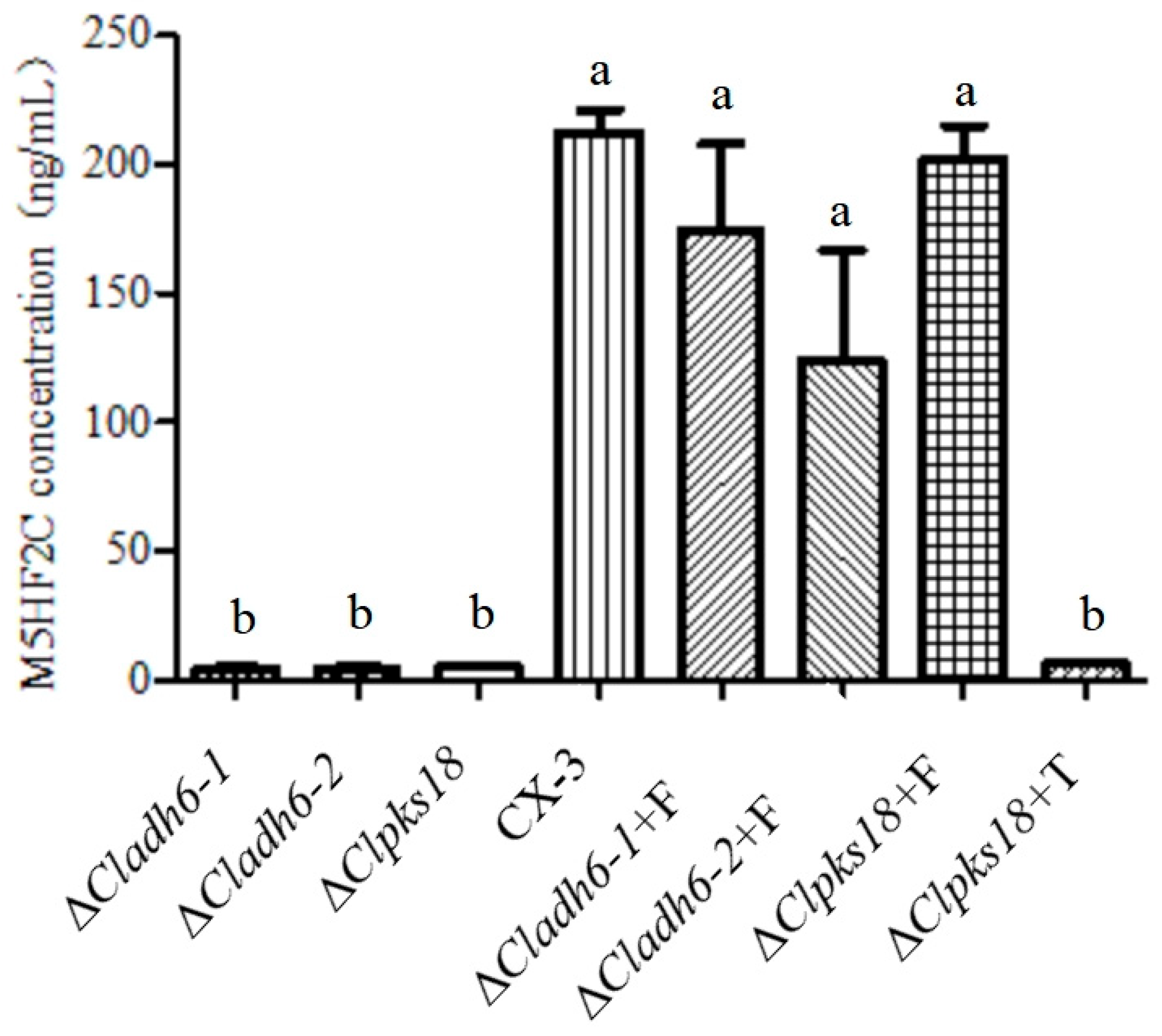
3.3. Cladh6 Plays an Important Role in M5HF2C Toxin Synthesis
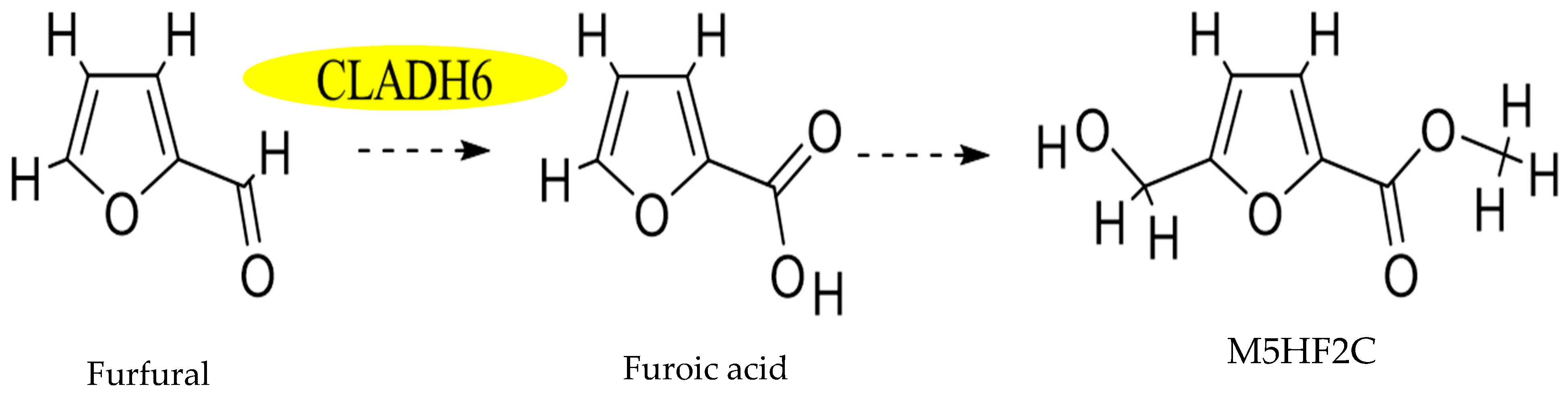
3.4. Demonstration of Furfural Transformed into M5HF2C Toxin in C. lunata
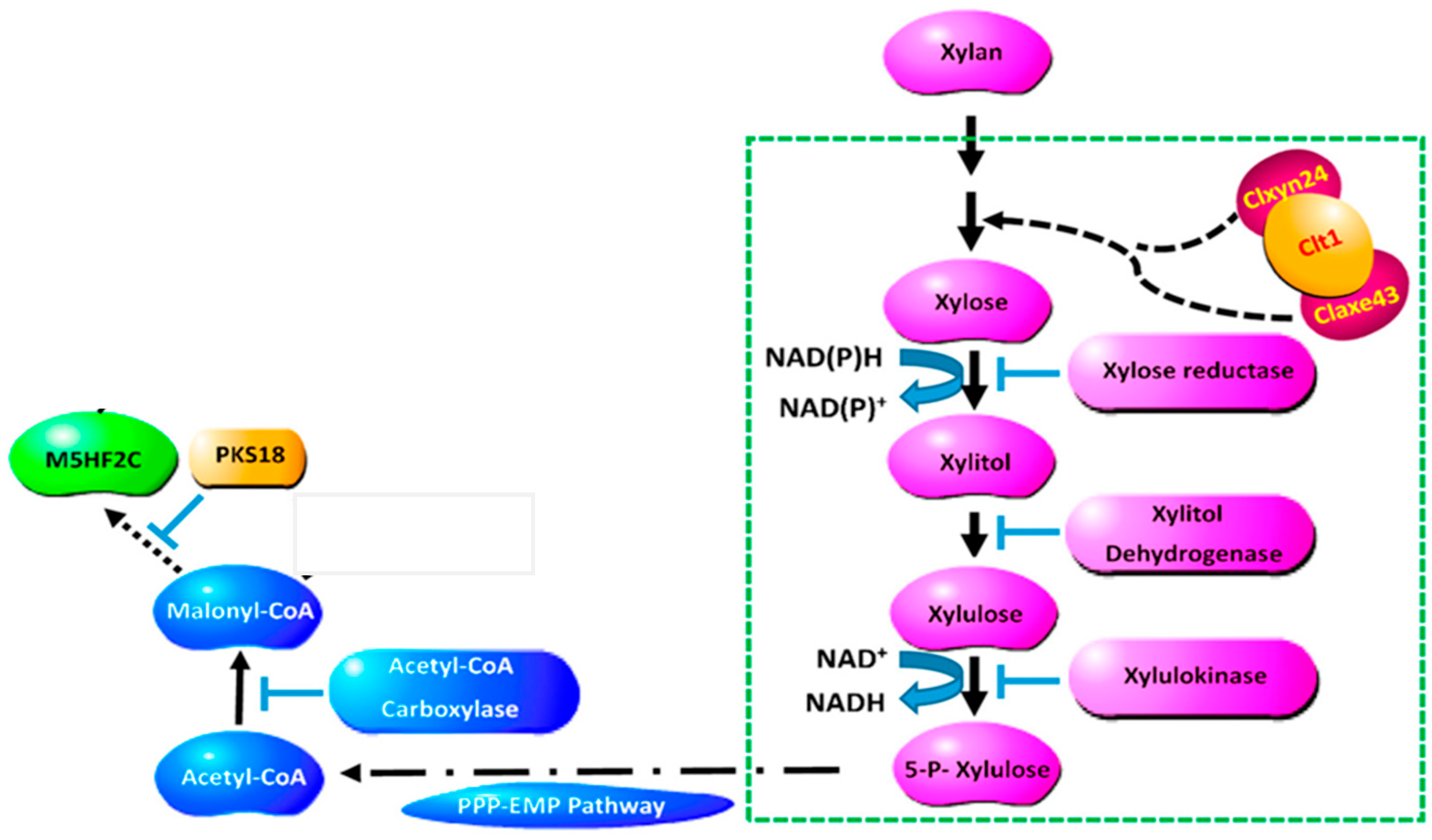
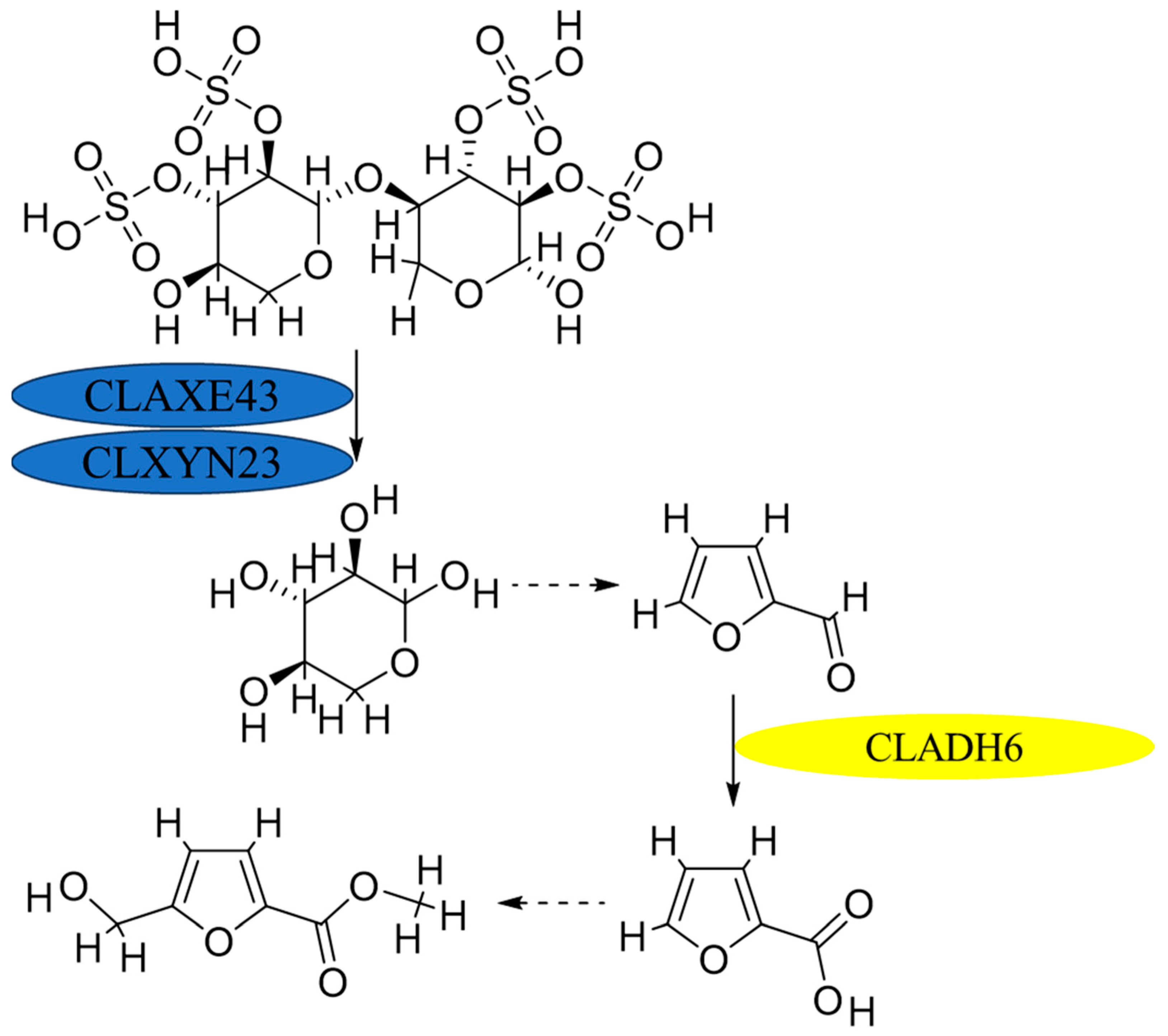
3.5. Synthesis Pathway from Xylose to M5HF2C Toxin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Gao, J.; Gao, S.; Liu, T.; Lu, Z.; Li, Y.; Chen, J. Research progress on maize Curvularia leaf spot caused by Curvularia lunata. Acta Phytopathol. Sin. 2019, 49, 433–444. [Google Scholar]
- Wang, S.; Lu, Z.; Lang, B.; Wang, X.; Li, Y.; Chen, J. Curvularia lunata and Curvularia Leaf Spot of Maize in China. ACS Omega 2022, 7, 47462–47470. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, L.; Jiang, X.; Huang, X.; Chen, J. A new furanoid toxin produced by Curvularia lunata, the causal agent of maize Curvularia leaf spot. Can. J. Plant Pathol. 2009, 31, 22–27. [Google Scholar] [CrossRef]
- Gao, J.; Chen, J. The Role of Clt1-Regulated Xylan Metabolism in Melanin and Toxin Formation for the Pathogenicity of Curvularia lunata in Maize. Mol. Plant-Microbe Interact. 2021, 34, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; López Granados, M. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Lu, Z. Functional Evaluation and Pathogenic Mechanism of Clpks 18 in Maize Leaf Spot Pathogen Curvularia lunata. Ph.D. Thesis, Shanghai Jiaotong University, Shanghai, China, 2023. [Google Scholar]
- Peng, B.; Ma, C.-L.; Zhang, P.-Q.; Wu, C.-Q.; Wang, Z.-W.; Li, A.-T.; He, Y.-C.; Yang, B. An effective hybrid strategy for converting rice straw to furoic acid by tandem catalysis via Sn-sepiolite combined with recombinant E. coli whole cells harboring horse liver alcohol dehydrogenase. Green Chem. 2019, 21, 5914–5923. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, C.; Huang, C.; Fu, Y.; Chang, J. Efficient Conversion of Xylose into Furfural Using Sulfonic Acid-Functionalized Metal–Organic Frameworks in a Biphasic System. Ind. Eng. Chem. Res. 2018, 57, 16628–16634. [Google Scholar] [CrossRef]
- Lin, Q.; Liao, S.; Li, L.; Li, W.; Yue, F.; Peng, F.; Ren, J. Solvent effect on xylose conversion under catalyst-free conditions: Insights from molecular dynamics simulation and experiments. Green Chem. 2020, 22, 532–539. [Google Scholar] [CrossRef]
- Yu, Z.; Cal, W.; Zhang, S. Synthesis of sulfonated Nb-MCM-41 catalyst and its application in hydrolysis of xylan. J. Dalian Polytech. Univ. 2019, 38, 4. [Google Scholar]
- Liu, T.; Xu, S.; Liu, L.; Zhou, F.; Hou, J.; Chen, J. Cloning and characteristics of Brn1 gene in Curvularia lunata causing leaf spot in maize. Eur. J. Plant Pathol. 2011, 131, 211–219. [Google Scholar] [CrossRef]
- Tsai, H.F.; Wheeler, M.H.; Chang, Y.C.; Kwon-Chung, K.J. A developmentally regulated gene cluster involved in conidial pigment biosynthesis in Aspergillus fumigatus. J. Bacteriol. 1999, 181, 6469–6477. [Google Scholar] [CrossRef] [PubMed]
- Rižner, T.L.; Wheeler, M.H. Melanin biosynthesis in the fungus Curvularia lunata (teleomorph: Cochliobolus lunatus). Can. J. Microbiol. 2003, 49, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.-X.; Chen, J. Involvement of a Polyketide Synthetase ClPKS18 in the Regulation of Vegetative Growth, Melanin and Toxin Synthesis, and Virulence in Curvularia lunata. Plant Pathol. J. 2017, 33, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Li, Y.; Gao, J.; Suo, Y.; Fu, K.; Li, Y.; Chen, J. Genome sequence and virulence variation-related transcriptome profiles of Curvularia lunata, an important maize pathogenic fungus. BMC Genom. 2014, 15, 627. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, A.; Kihara, J.; Kobayashi, T.; Tokunaga, T.; Arase, S.; Honda, Y. Insertional mutagenesis and characterization of a polyketide synthase gene (PKS1) required for melanin biosynthesis in Bipolaris oryzae. FEMS Microbiol. Lett. 2004, 238, 1–8. [Google Scholar] [PubMed]
- Blin, K.; Wolf, T.; Chevrette, M.G.; Lu, X.; Schwalen, C.J.; Kautsar, S.A.; Suarez Duran, H.G.; de los Santos Emmanuel, L.C.; Kim, H.U.; Nave, M. antiSMASH 4.0—Improvements in chemistry prediction and gene cluster boundary identification. Nucleic Acids Res. 2017, 45, W36–W41. [Google Scholar] [CrossRef] [PubMed]
- Fulton, T.R.; Ibrahim, N.; Losada, M.C.; Grzegorski, D.; Tkacz, J.S. A melanin polyketide synthase (PKS) gene from Nodulisporium sp. that shows homology to the pks1 gene of Colletotrichum lagenarium. Mol. Gen. Genet. MGG 1999, 262, 714–720. [Google Scholar] [CrossRef] [PubMed]


| Time (min) | A (%) | B (%) | Flow Rate (mL/min) |
|---|---|---|---|
| 0 | 95 | 5 | 0.4 |
| 1 | 95 | 5 | 0.4 |
| 3 | 40 | 60 | 0.4 |
| 4 | 5 | 95 | 0.4 |
| 6 | 5 | 95 | 0.4 |
| 6.1 | 95 | 5 | 0.4 |
| 8 | 95 | 5 | 0.4 |
| Precursor Ion | Daughter Ion | Declustering Voltage (V) | Collision Energy (V) |
|---|---|---|---|
| 157.0 | 139.0 | 70 | 20 |
| 79.0 | 70 | 25 |
| Time (min) | A (%) | B (%) | Flow Rate (mL/min) |
|---|---|---|---|
| 0 | 95 | 5 | 0.2 |
| 2 | 95 | 5 | 0.2 |
| 6 | 40 | 60 | 0.2 |
| 8 | 5 | 95 | 0.2 |
| 12 | 5 | 95 | 0.2 |
| 12.1 | 95 | 5 | 0.2 |
| 16 | 95 | 5 | 0.2 |
| Q1 Mass (Da) | Q3 Mass (Da) | Time (msec) | ID | DP (volts) | CE (volts) |
|---|---|---|---|---|---|
| negative ion | |||||
| 154.8 | 109.9 | 100 | ME | −42.09 | −42.09 |
| Primer Name | Sequence |
|---|---|
| Cladh6Uf | ACGACGGCCAGTGCCAAGCTTTCTGGAAGACGACCCTGGCGA |
| Cladh6Ur | GACCTGCAGGCATGCATTTGGCGGGTACAGATGTTG |
| 1300qh-F | GCATGCCTGCAGGTCGACTCT |
| Cladh6D + 1300qh-RB | GTGAATTAGTGTACGGAGCTCGGTACCCGGGGATC |
| Cladh6Df | CGTACACTAATTCACATATACGGCT |
| Cladh6Dr | TATGACCATGATTACGAATTCCTAGCCTAAGGTTGGTGAAGG |
| HygR1 | ACCGCAAGGAATCGGTCAAT |
| HygR2 | GATTTGTGTACGCCCGACAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; Lang, B.; Wang, S.; Liu, H.; Wang, X.; Chen, J. Elucidating the Fundamental Process of Methyl-(5hydroxymethyl) Furan-2-Carboxylate Toxin Biosynthesis in Curvularia lunata Causing Maize Leaf Spot. J. Fungi 2024, 10, 688. https://doi.org/10.3390/jof10100688
Lu Z, Lang B, Wang S, Liu H, Wang X, Chen J. Elucidating the Fundamental Process of Methyl-(5hydroxymethyl) Furan-2-Carboxylate Toxin Biosynthesis in Curvularia lunata Causing Maize Leaf Spot. Journal of Fungi. 2024; 10(10):688. https://doi.org/10.3390/jof10100688
Chicago/Turabian StyleLu, Zhixiang, Bo Lang, Shaoqing Wang, Hongyi Liu, Xinhua Wang, and Jie Chen. 2024. "Elucidating the Fundamental Process of Methyl-(5hydroxymethyl) Furan-2-Carboxylate Toxin Biosynthesis in Curvularia lunata Causing Maize Leaf Spot" Journal of Fungi 10, no. 10: 688. https://doi.org/10.3390/jof10100688
APA StyleLu, Z., Lang, B., Wang, S., Liu, H., Wang, X., & Chen, J. (2024). Elucidating the Fundamental Process of Methyl-(5hydroxymethyl) Furan-2-Carboxylate Toxin Biosynthesis in Curvularia lunata Causing Maize Leaf Spot. Journal of Fungi, 10(10), 688. https://doi.org/10.3390/jof10100688








