Fungal Keratitis in Northwestern Spain: Epidemiology, Risk Factors and Outcomes
Abstract
1. Introduction
2. Patients and Methods
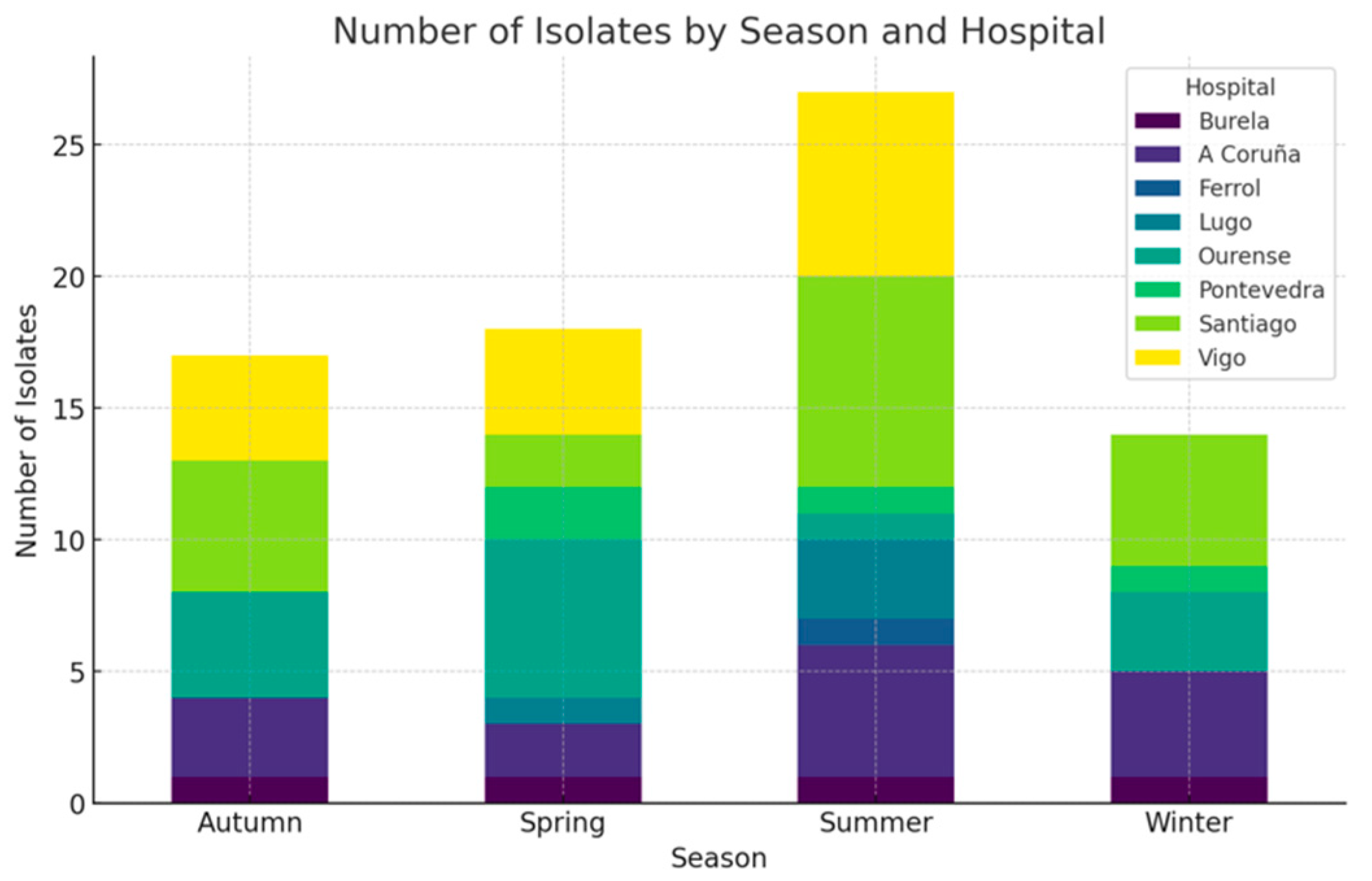
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, L.; Leck, A.K.; Gichangi, M.; Burton, M.J.; Denning, D.W. The Global Incidence and Diagnosis of Fungal Keratitis. Lancet Infect. Dis. 2021, 21, e49–e57. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.A. Fungal Infections of the Cornea. Eye 2003, 17, 852–862. [Google Scholar] [CrossRef]
- Niu, L.; Liu, X.; Ma, Z.; Yin, Y.; Sun, L.; Yang, L.; Zheng, Y. Fungal Keratitis: Pathogenesis, Diagnosis and Prevention. Microb. Pathog. 2020, 138, 103802. [Google Scholar] [CrossRef]
- Leal, S.M.; Pearlman, E. The Role of Cytokines and Pathogen Recognition Molecules in Fungal Keratitis—Insights from Human Disease and Animal Models. Cytokine 2012, 58, 107–111. [Google Scholar] [CrossRef]
- Rhee, M.K.; Ahmad, S.; Amescua, G.; Cheung, A.Y.; Choi, D.S.; Jhanji, V.; Lin, A.; Mian, S.I.; Viriya, E.T.; Mah, F.S.; et al. Bacterial Keratitis Preferred Practice Pattern®. Ophthalmology 2024, 131, P87–P133. [Google Scholar] [CrossRef]
- Wagner, K.; Springer, B.; Pires, V.P.; Keller, P.M. Molecular Detection of Fungal Pathogens in Clinical Specimens by 18S rDNA High-Throughput Screening in Comparison to ITS PCR and Culture. Sci. Rep. 2018, 8, 6964. [Google Scholar] [CrossRef] [PubMed]
- Prajna, N.V.; Krishnan, T.; Mascarenhas, J.; Rajaraman, R.; Prajna, L.; Srinivasan, M.; Raghavan, A.; Oldenburg, C.E.; Ray, K.J.; Zegans, M.E.; et al. The Mycotic Ulcer Treatment Trial. JAMA Ophthalmol. 2013, 131, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Sahay, P.; Goel, S.; Nagpal, R.; Maharana, P.K.; Sinha, R.; Agarwal, T.; Sharma, N.; Titiyal, J.S. Infectious Keratitis Caused by Rare and Emerging Micro-Organisms. Curr. Eye Res. 2020, 45, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Gintjee, T.J.; Donnelley, M.A.; Thompson, G.R. Aspiring Antifungals: Review of Current Antifungal Pipeline Developments. J. Fungi 2020, 6, 28. [Google Scholar] [CrossRef]
- Sharma, N.; Bagga, B.; Singhal, D.; Nagpal, R.; Kate, A.; Saluja, G.; Maharana, P.K. Fungal Keratitis: A Review of Clinical Presentations, Treatment Strategies and Outcomes. Ocul. Surf. 2022, 24, 22–30. [Google Scholar] [CrossRef]
- Arunga, S.; Kintoki, G.M.; Gichuhi, S.; Onyango, J.; Newton, R.; Leck, A.; Macleod, D.; Hu, V.H.; Burton, M.J. Delay Along the Care Seeking Journey of Patients with Microbial Keratitis in Uganda. Ophthalmic Epidemiol. 2019, 26, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Said, D.G.; Rallis, K.I.; Al-Aqaba, M.A.; Ting, D.S.J.; Dua, H.S. Surgical Management of Infectious Keratitis. Ocul. Surf. 2023, 28, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Lalitha, P.; Prajna, N.V.; Manoharan, G.; Srinivasan, M.; Mascarenhas, J.; Das, M.; D’Silva, S.S.; Porco, T.C.; Keenan, J.D. Trends in Bacterial and Fungal Keratitis in South India, 2002–2012. Br. J. Ophthalmol. 2015, 99, 192–194. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, L.; Han, L.; Yin, W. Study of Pathogens of Fungal Keratitis and the Sensitivity of Pathogenic Fungi to Therapeutic Agents with the Disk Diffusion Method. Curr. Eye Res. 2015, 40, 1095–1101. [Google Scholar] [CrossRef]
- Hoffman, J.J.; Burton, M.J.; Leck, A. Mycotic Keratitis—A Global Threat from the Filamentous Fungi. J. Fungi 2021, 7, 273. [Google Scholar] [CrossRef]
- Ting, D.S.J.; Ho, C.S.; Cairns, J.; Gopal, B.P.; Elsahn, A.; Al-Aqaba, M.; Boswell, T.; Said, D.G.; Dua, H.S. Seasonal Patterns of Incidence, Demographic Factors and Microbiological Profiles of Infectious Keratitis: The Nottingham Infectious Keratitis Study. Eye 2021, 35, 2543–2549. [Google Scholar] [CrossRef]
- Moledina, M.; Roberts, H.W.; Mukherjee, A.; Spokes, D.; Pimenides, D.; Stephenson, C.; Bassily, R.; Rajan, M.S.; Myerscough, J. Analysis of Microbial Keratitis Incidence, Isolates and in-Vitro Antimicrobial Susceptibility in the East of England: A 6-Year Study. Eye 2023, 37, 2716–2722. [Google Scholar] [CrossRef]
- Lin, C.C.; Prajna, L.; Srinivasan, M.; Prajna, N.V.; McLeod, S.D.; Acharya, N.R.; Lietman, T.M.; Porco, T.C. Seasonal Trends of Microbial Keratitis in South India. Cornea 2012, 31, 1123–1127. [Google Scholar] [CrossRef]
- Ting, D.S.J.; Galal, M.; Kulkarni, B.; Elalfy, M.S.; Lake, D.; Hamada, S.; Said, D.G.; Dua, H.S. Clinical Characteristics and Outcomes of Fungal Keratitis in the United Kingdom 2011–2020: A 10-Year Study. J. Fungi 2021, 7, 966. [Google Scholar] [CrossRef]
- Keay, L.J.; Gower, E.W.; Iovieno, A.; Oechsler, R.A.; Alfonso, E.C.; Matoba, A.; Colby, K.; Tuli, S.S.; Hammersmith, K.; Cavanagh, D.; et al. Clinical and Microbiological Characteristics of Fungal Keratitis in the United States, 2001–2007: A Multicenter Study. Ophthalmology 2011, 118, 920–926. [Google Scholar] [CrossRef]
- Trinh, T.; Emami, S.; Gould, J.; Mimouni, M.; Cohen, E.; Rootman, D.S.; Slomovic, A.R.; Chan, C.C. Clinical and Microbiological Analysis of Fungal Keratitis in Toronto, Canada: A 20-Year Study. Med. Mycol. 2022, 60, myac047. [Google Scholar] [CrossRef]
- Masoumi, A.; Soleimani, M.; Azizkhani, M.; Izadi, A.; Cheraqpour, K.; Tabatabaei, S.A.; Khodavaisy, S.; Aminzadeh, M. Clinical Features, Risk Factors, and Management of Candida Keratitis. Ocul. Immunol. Inflamm. 2024, 32, 1169–1174. [Google Scholar] [CrossRef] [PubMed]
- Knutsson, K.A.; Iovieno, A.; Matuska, S.; Fontana, L.; Rama, P. Topical Corticosteroids and Fungal Keratitis: A Review of the Literature and Case Series. J. Clin. Med. 2021, 10, 1178. [Google Scholar] [CrossRef] [PubMed]
- Czajka, K.M.; Venkataraman, K.; Brabant-Kirwan, D.; Santi, S.A.; Verschoor, C.; Appanna, V.D.; Singh, R.; Saunders, D.P.; Tharmalingam, S. Molecular Mechanisms Associated with Antifungal Resistance in Pathogenic Candida Species. Cells 2023, 12, 2655. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J.; Gibbs, D.L.; Newell, V.A.; Ellis, D.; Tullio, V.; Rodloff, A.; Fu, W.; Ling, T.A. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: A 10.5-Year Analysis of Susceptibilities of Candida Species to Fluconazole and Voriconazole as Determined by CLSI Standardized Disk Diffusion. J. Clin. Microbiol. 2010, 48, 1366–1377. [Google Scholar] [CrossRef]
- Cowen, L.E.; Sanglard, D.; Howard, S.J.; Rogers, P.D.; Perlin, D.S. Mechanisms of Antifungal Drug Resistance. Cold Spring Harb. Perspect. Med. 2014, 5, a019752. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Robbins, N.; Cowen, L.E. Molecular Mechanisms Governing Antifungal Drug Resistance. NPJ Antimicrob. Resist. 2023, 1, 5. [Google Scholar] [CrossRef]
- Priyashantha, A.K.H.; Dai, D.-Q.; Bhat, D.J.; Stephenson, S.L.; Promputtha, I.; Kaushik, P.; Tibpromma, S.; Karunarathna, S.C. Plant–Fungi Interactions: Where It Goes? Biology 2023, 12, 809. [Google Scholar] [CrossRef]
- Song, A.; Deshmukh, R.; Lin, H.; Ang, M.; Mehta, J.S.; Chodosh, J.; Said, D.G.; Dua, H.S.; Ting, D.S.J. Post-Keratoplasty Infectious Keratitis: Epidemiology, Risk Factors, Management, and Outcomes. Front. Med. 2021, 8, 707242. [Google Scholar] [CrossRef]
- Dan, J.; Zhou, Q.; Zhai, H.; Cheng, J.; Wan, L.; Ge, C.; Xie, L. Clinical Analysis of Fungal Keratitis in Patients with and without Diabetes. PLoS ONE 2018, 13, e0196741. [Google Scholar] [CrossRef]
- Bharathi, M.J.; Ramakrishnan, R.; Vasu, S.; Meenakshi, R.; Palaniappan, R. Epidemiological Characteristics and Laboratory Diagnosis of Fungal Keratitis. A three-year study. Indian J. Ophthalmol. 2003, 51, 315–321. [Google Scholar] [PubMed]
- Atta, S.; Perera, C.; Kowalski, R.P.; Jhanji, V. Fungal Keratitis: Clinical Features, Risk Factors, Treatment, and Outcomes. J. Fungi 2022, 8, 962. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Gupta, A.; Rudramurthy, S.M.; Paul, S.; Hallur, V.K.; Chakrabarti, A. Fungal Keratitis in North India: Spectrum of Agents, Risk Factors and Treatment. Mycopathologia 2016, 181, 843–850. [Google Scholar] [CrossRef]
- Soleimani, M.; Izadi, A.; Khodavaisy, S.; Santos, C.O.D.; Tehupeiory-Kooreman, M.C.; Ghazvini, R.D.; Hashemi, S.J.; Mousavi, S.A.A.; Aala, F.; Abdorahimi, M.; et al. Fungal Keratitis in Iran: Risk Factors, Clinical Features, and Mycological Profile. Front. Cell. Infect. Microbiol. 2023, 13, 1094182. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.N.; Karthik, H.; Proudmore, K.E.; Kidd, S.E.; Baird, R.W. Fungal Keratitis, Epidemiology and Outcomes in a Tropical Australian Setting. Trop. Med. Infect. Dis. 2024, 9, 127. [Google Scholar] [CrossRef]
- Cheikhrouhou, F.; Makni, F.; Neji, S.; Trigui, A.; Sellami, H.; Trabelsi, H.; Guidara, R.; Fki, J.; Ayadi, A. Epidemiological Profile of Fungal Keratitis in Sfax (Tunisia). J. De Mycol. Médicale 2014, 24, 308–312. [Google Scholar] [CrossRef]
- Sadik, N.; Elzeiny, S.M.; Ali, Y.E.; Sobeih, D. Fungal Keratitis in the Egyptian Delta: Epidemiology, Risk Factors, and Microbiological Diagnosis. Ophthalmic Epidemiol. 2022, 29, 198–205. [Google Scholar] [CrossRef]
- Cho, C.-H.; Lee, S.-B. Clinical Analysis of Microbiologically Proven Fungal Keratitis According to Prior Topical Steroid Use: A Retrospective Study in South Korea. BMC Ophthalmol. 2019, 19, 207. [Google Scholar] [CrossRef] [PubMed]
- Prajna, N.V.; Krishnan, T.; Rajaraman, R.; Patel, S.; Shah, R.; Srinivasan, M.; Das, M.; Ray, K.J.; Oldenburg, C.E.; McLeod, S.D.; et al. Predictors of Corneal Perforation or Need for Therapeutic Keratoplasty in Severe Fungal Keratitis: A Secondary Analysis of the Mycotic Ulcer Treatment Trial II. JAMA Ophthalmol. 2017, 135, 987. [Google Scholar] [CrossRef]
- Xuan, R.; Hong, S.C.; Trinh, T.; Coroneo, M.T.; Petsoglou, C. Case Series of Rare Fungal Keratitides: Experiences from a Quaternary Eye Hospital in Sydney, Australia. J. Fungi 2023, 9, 589. [Google Scholar] [CrossRef]
- Soleimani, M.; Esmaili, K.; Rahdar, A.; Aminizadeh, M.; Cheraqpour, K.; Tabatabaei, S.A.; Mirshahi, R.; Bibak, Z.; Mohammadi, S.F.; Koganti, R.; et al. From the Diagnosis of Infectious Keratitis to Discriminating Fungal Subtypes; a Deep Learning-Based Study. Sci. Rep. 2023, 13, 22200. [Google Scholar] [CrossRef] [PubMed]
| Associated Factor | Fungi | % | Bacteria and Amoebae | % | p-Value |
|---|---|---|---|---|---|
| Diabetes | 11 | 14.3 | 94 | 16.3 | 0.796 |
| Systemic immunosuppression | 5 | 6.5 | 24 | 4.2 | 0.926 |
| Systemic steroid use | 2 | 2.6 | 11 | 1.9 | 0.980 |
| Recent ocular surgery | 28 | 36.4 | 190 | 32.9 | 0.705 |
| Recent keratoplasty | 14 | 18.2 | 62 | 10.7 | 0.043 |
| Contact lens use | 21 | 27.3 | 137 | 23.7 | 0.563 |
| Recent ocular trauma | 15 | 19.5 | 60 | 10.4 | 0.033 |
| Contact with vegetable matter | 9 | 11.7 | 23 | 4.0 | 0.002 |
| Foreign corneal body | 3 | 3.9 | 18 | 3.1 | 0.931 |
| Steroid eyedrop use | 23 | 29.9 | 100 | 17.3 | 0.004 |
| Glaucoma | 19 | 24.7 | 140 | 24.3 | 0.721 |
| Blepharitis | 12 | 15.6 | 121 | 21.0 | 0.350 |
| Eyelid disorders | 8 | 10.4 | 76 | 13.2 | 0.375 |
| Previous keratitis | 20 | 26.0 | 175 | 30.3 | 0.598 |
| Coefficient | Standard Error | Wald | Degrees of Freedom | Sig. | Odds Ratio (OR) | |
|---|---|---|---|---|---|---|
| Trauma | 0.187 | 0.449 | 0.174 | 1 | 0.677 | 1.206 |
| Recent keratoplasty | 0.553 | 0.345 | 2.569 | 1 | 0.109 | 1.738 |
| Previous topical steroids | 0.728 | 0.289 | 6.325 | 1 | 0.012 | 2.071 |
| Vegetable matter | 1.247 | 0.565 | 4.872 | 1 | 0.027 | 3.478 |
| Constant | −2.433 | 0.168 | 208.798 | 1 | 0.000 | 0.088 |
| Biomicroscopic Feature | N | % | |
|---|---|---|---|
| Epithelial defect | Small (<3 mm) | 36 | 46.8 |
| Large (>3 mm) | 32 | 41.6 | |
| Infiltrate number | 1 | 55 | 71.4 |
| 2 | 6 | 7.8 | |
| >2 | 9 | 11.7 | |
| Infiltrate depth | Superficial | 10 | 13.0 |
| Stromal | 49 | 63.6 | |
| Endothelial plaque | 8 | 10.4 | |
| Infiltrate localisation | Central 2 mm | 32 | 41.6 |
| Paracentral | 25 | 32.5 | |
| Peripheral 2 mm | 7 | 8.3 | |
| Corneal thinning | Thinning | 29 | 37.7 |
| Perforation | 19 | 24.7 | |
| Anterior chamber reaction | Tyndall | 15 | 19.5 |
| Hypopyon | 22 | 28.6 | |
| Endophthalmitis | 6 | 7.8 | |
| Isolate | No. of Isolates | % |
|---|---|---|
| Yeasts | ||
| Candida spp. | 43 | 55.8 |
| Non-dermatophyte moulds | ||
| Fusarium spp. | 13 | 16.9 |
| Aspergillus spp. | 6 | 7.8 |
| Paecilomyces spp. | 4 | 5.2 |
| Alternaria spp. | 3 | 3.9 |
| Acremonium spp. | 3 | 3.9 |
| Stemphylium spp. | 1 | 1.3 |
| Albifimbria spp. | 1 | 1.3 |
| Scedosporium spp. | 1 | 1.3 |
| Curvularia spp. | 1 | 1.3 |
| Not identified | 1 | 1.3 |
| Treatment Regimen | N | % | |
|---|---|---|---|
| Initial topical treatment | Fortified antibiotics | 36 | 46.8 |
| Commercial antibiotics | 34 | 44.2 | |
| Voriconazole | 3 | 3.9 | |
| Voriconazole + amphotericin B | 2 | 2.6 | |
| Voriconazole + amphotericin B + natamycin | 1 | 1.3 | |
| Amphotericin B | 1 | 1.3 | |
| Topical steroids | 37 | 48.0 | |
| 37 | Antifungals | 23 | 29.9 |
| Steroids | 6 | 7.8 | |
| Surgery | Penetrating keratoplasty | 17 | 22.1 |
| Deep anterior lamellar keratoplasty | 2 | 2.6 | |
| Amniotic membrane transplantation | 6 | 7.8 | |
| Acrylic glue application | 9 | 11.7 | |
| Stromal antifungal injection | 3 | 3.9 | |
| Evisceration | 14 | 18.2 | |
| Author | Region | Study Period | N | Contact Lens Use | Trauma | Ocular Surface Disease | Ocular Surgery | Topical Steroids | Diabetes | Most Common Fungal Isolate |
|---|---|---|---|---|---|---|---|---|---|---|
| America | ||||||||||
| Keay et al. [20] | USA | 2001–2007 | 733 | 37% | 25% | 29% | Candida spp. | |||
| Atta et al. [32] | Pittsburgh (USA) | 2015–2021 | 28 | 68% | 43% | 32% | 43% | 32% | Aspergillus spp. and Fusarium spp. | |
| Trinh et al. [21] | Toronto (Canada) | 2020–2021 | 46 | 39% | 9% | 70% | Candida spp. | |||
| Southeast Asia | ||||||||||
| Ghosh et al. [33] | Chandigarh (India) | 2005–2011 | 393 | 32% | 6% | 1% | Aspergillus spp. | |||
| Bharathi et al. [31] | Tamil Nadu (India) | 1999–2002 | 1095 | 92% | 7% | 1% | 15% | Fusarium spp. | ||
| Soleimani et al. [34] | Tehran (Iran) | 2019–2021 | 86 | 6% | 49% | 12% | 3% | 7% | Fusarium spp. | |
| West Pacific | ||||||||||
| Dan et al. [30] | Shandong (China) | 2010–2016 | 851 | 55% | 3% | Fusarium spp. | ||||
| Kim et al. [35] | Darwin (Australia) | 2014–2022 | 31 | 45% | 32% | Curvularia spp. | ||||
| Africa | ||||||||||
| Cheikhrouhou et al. [36] | Sfat (Tunisia) | 1995–2012 | 60 | 3% | 50% | 10% | 10% | 18% | 5% | Fusarium spp. |
| Sadik et al. [37] | Mansoura (Egypt) | 2018 | 171 | 4% | 32% | 25% | 23% | 2% | 7% | Aspergillus spp. |
| Europe | ||||||||||
| Ting et al. [19] | Nottingham (UK) | 2011–2020 | 51 | 45% | 9% | 76% | 27% | 21% | Candida spp. | |
| Lamas-Francis et al. (present study) | Galicia (Spain) | 2010–2020 | 77 | 27% | 19% | 36% | 30% | 14% | Candida spp. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamas-Francis, D.; Navarro, D.; Mansilla, R.; de-Rojas, V.; Moreno, C.; Dios, E.; Rigueiro, J.; Álvarez, D.; Crego, P.; Rodríguez-Ares, T.; et al. Fungal Keratitis in Northwestern Spain: Epidemiology, Risk Factors and Outcomes. J. Fungi 2024, 10, 689. https://doi.org/10.3390/jof10100689
Lamas-Francis D, Navarro D, Mansilla R, de-Rojas V, Moreno C, Dios E, Rigueiro J, Álvarez D, Crego P, Rodríguez-Ares T, et al. Fungal Keratitis in Northwestern Spain: Epidemiology, Risk Factors and Outcomes. Journal of Fungi. 2024; 10(10):689. https://doi.org/10.3390/jof10100689
Chicago/Turabian StyleLamas-Francis, David, Daniel Navarro, Raquel Mansilla, Victoria de-Rojas, Claudio Moreno, Enrique Dios, Jesús Rigueiro, Dolores Álvarez, Paloma Crego, Teresa Rodríguez-Ares, and et al. 2024. "Fungal Keratitis in Northwestern Spain: Epidemiology, Risk Factors and Outcomes" Journal of Fungi 10, no. 10: 689. https://doi.org/10.3390/jof10100689
APA StyleLamas-Francis, D., Navarro, D., Mansilla, R., de-Rojas, V., Moreno, C., Dios, E., Rigueiro, J., Álvarez, D., Crego, P., Rodríguez-Ares, T., & Touriño, R. (2024). Fungal Keratitis in Northwestern Spain: Epidemiology, Risk Factors and Outcomes. Journal of Fungi, 10(10), 689. https://doi.org/10.3390/jof10100689






