Carbon and Nitrogen Sources Influence Parasitic Responsiveness in Trichoderma atroviride NI-1
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
2.2. NI-1 Strain Identification
2.3. Antagonism Assays by Nonvolatile Compounds
2.4. Antagonism Assays by Volatile Compounds
2.5. Parasitism Assays
2.6. Effect of Different Carbon Sources on GRM Expression
2.7. Effect of Different Nitrogen Sources on GRM Expression
2.8. Dual Cultures Assay to Analyze Gene Expression before-, during Contact, and Overgrowth
2.9. RNA Extraction and Manipulation
2.10. Complementary DNA Synthesis
2.11. End-Point PCR to Detect Transcripts
2.12. Semiquantitative Expression Analysis
2.13. Statistical Analysis
3. Results
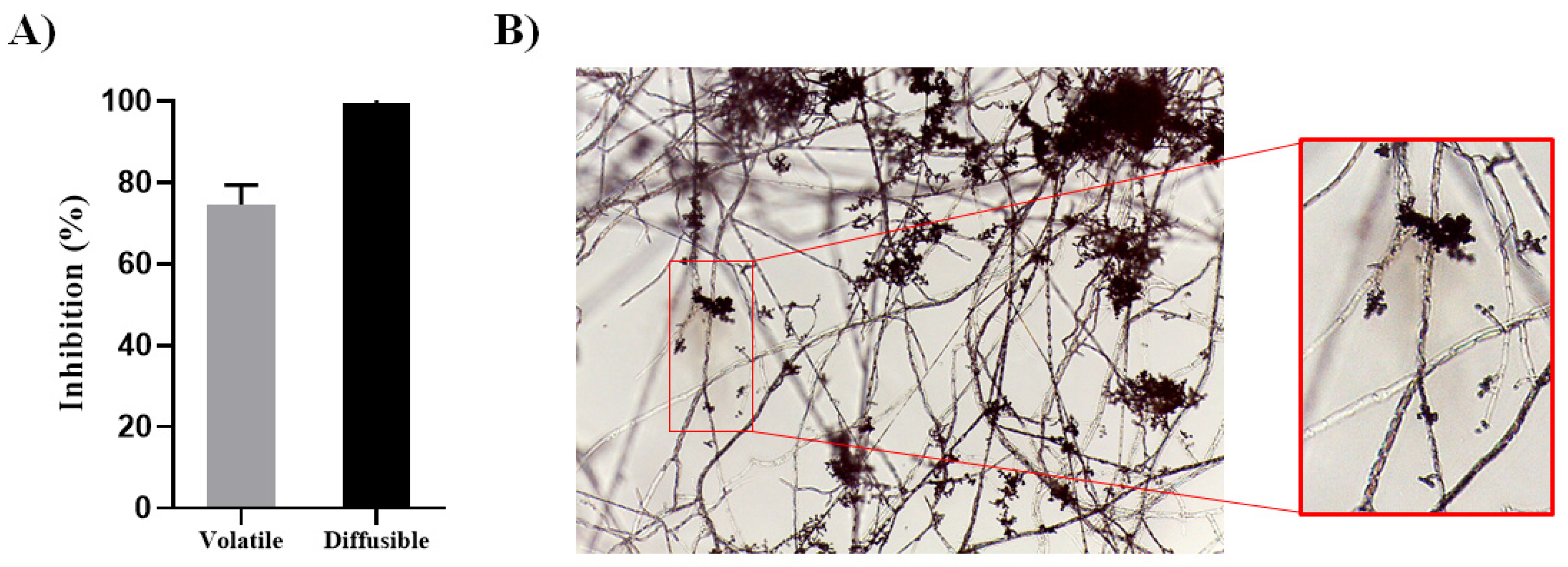
3.1. Trichoderma NI-1 Has a High Antagonistic Capacity against P. capsici D3
3.2. NI-1 Strain Is Phylogenetically Related to Trichoderma atroviride
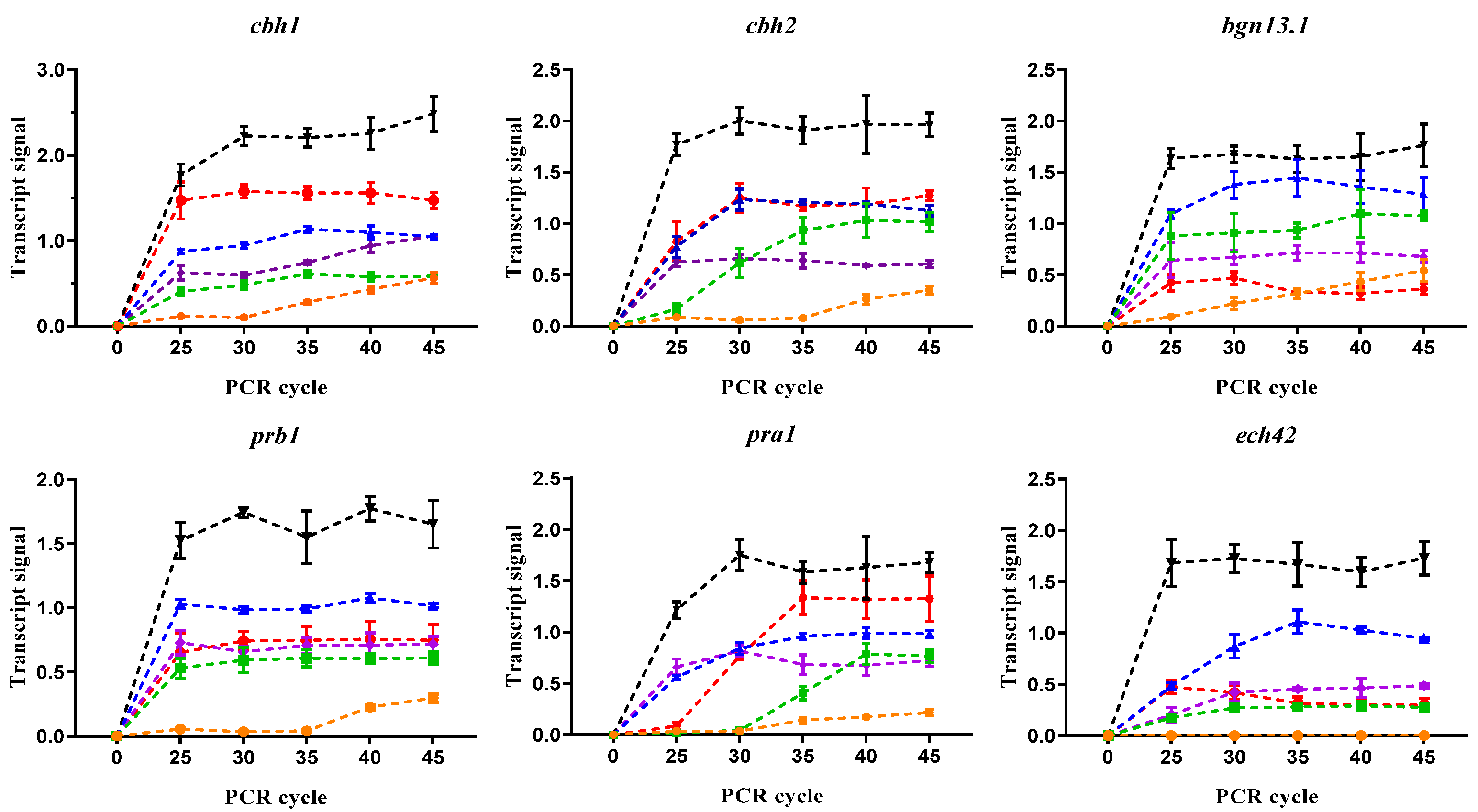
3.3. Carbon Sources Influence GRM Expression
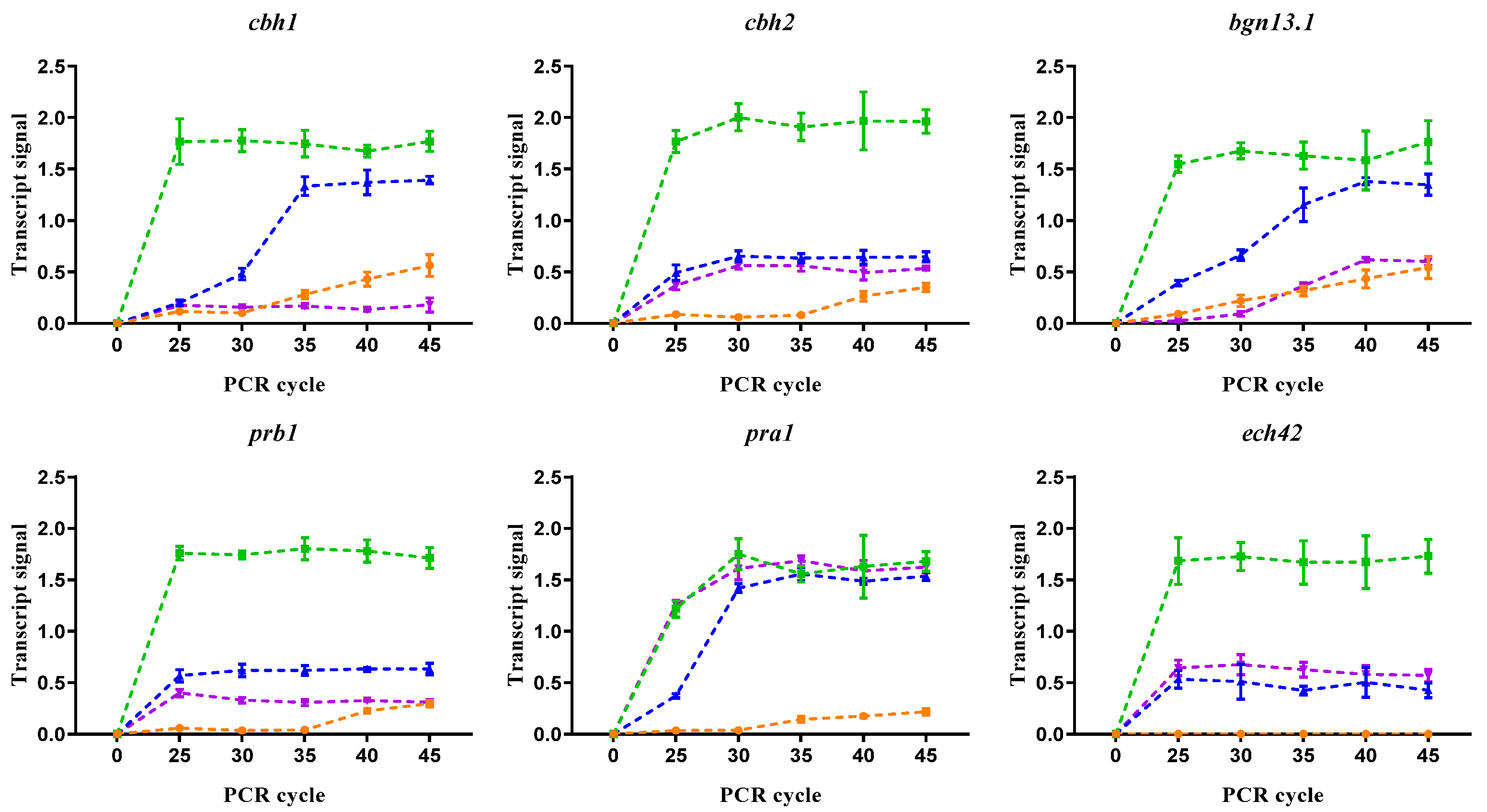
3.4. Primary Nitrogen Sources Promoted Mycoparasitic-Related Gene Expression
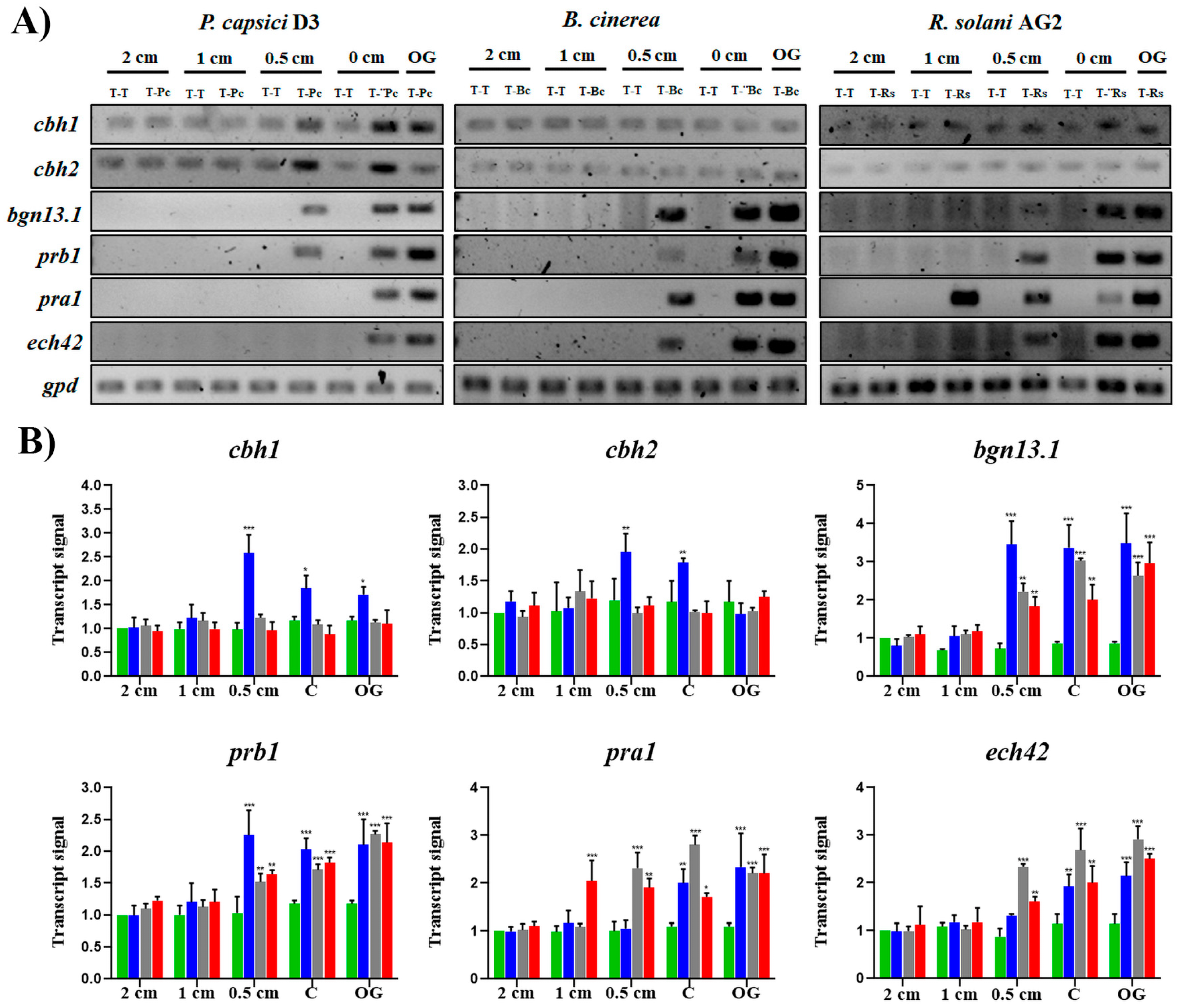
3.5. Trichoderma Atroviride Can Distinguish Its Prey
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mukherjee, P.K.; Mendoza-Mendoza, A.; Zeilinger, S.; Horwitz, B.A. Mycoparasitism as a Mechanism of Trichoderma-Mediated Suppression of Plant Diseases. Fungal Biol. Rev. 2022, 39, 15–33. [Google Scholar] [CrossRef]
- Karlsson, M.; Atanasova, L.; Jensen, D.F.; Zeilinger, S. Necrotrophic Mycoparasites and Their Genomes. Microbiol. Spectr. 2017, 5, 1–21. [Google Scholar] [CrossRef]
- Atanasova, L.; Crom, S.L.; Gruber, S.; Coulpier, F.; Seidl-Seiboth, V.; Kubicek, C.P.; Druzhinina, I.S. Comparative Transcriptomics Reveals Different Strategies of Trichoderma mycoparasitism. BMC Genom. 2013, 14, 121. [Google Scholar] [CrossRef]
- Singh, N.; Singh, A.; Dahiya, P. Plant Growth-Promoting Endophytic Fungi from Different Habitats and Their Potential Applications in Agriculture. In Recent Trends in Mycological Research: Volume 1: Agricultural and Medical Perspective; Yadav, A.N., Ed.; Fungal Biology; Springer International Publishing: Cham, Switzerland, 2021; pp. 69–87. ISBN 978-3-030-60659-6. [Google Scholar]
- Alfiky, A.; Weisskopf, L. Deciphering Trichoderma–Plant–Pathogen Interactions for Better Development of Biocontrol Applications. J. Fungi 2021, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Kappel, L.; Münsterkötter, M.; Sipos, G.; Rodriguez, C.E.; Gruber, S. Chitin and Chitosan Remodeling Defines Vegetative Development and Trichoderma Biocontrol. PLoS Pathog. 2020, 16, e1008320. [Google Scholar] [CrossRef]
- Brunner, K.; Peterbauer, C.K.; Mach, R.L.; Lorito, M.; Zeilinger, S.; Kubicek, C.P. The Nag1 N-Acetylglucosaminidase of Trichoderma atroviride Is Essential for Chitinase Induction by Chitin and of Major Relevance to Biocontrol. Curr. Genet. 2003, 43, 289–295. [Google Scholar] [CrossRef]
- Sharma, V.; Salwan, R.; Sharma, P.N.; Kanwar, S.S. Elucidation of Biocontrol Mechanisms of Trichoderma harzianum against Different Plant Fungal Pathogens: Universal yet Host Specific Response. Int. J. Biol. Macromol. 2017, 95, 72–79. [Google Scholar] [CrossRef]
- Guzmán-Guzmán, P.; Alemán-Duarte, M.I.; Delaye, L.; Herrera-Estrella, A.; Olmedo-Monfil, V. Identification of Effector-like Proteins in Trichoderma spp. and Role of a Hydrophobin in the Plant-Fungus Interaction and Mycoparasitism. BMC Genet. 2017, 18, 16. [Google Scholar] [CrossRef]
- Reithner, B.; Ibarra-Laclette, E.; Mach, R.L.; Herrera-Estrella, A. Identification of Mycoparasitism-Related Genes in Trichoderma atroviride. Appl. Environ. Microbiol. 2011, 77, 4361–4370. [Google Scholar] [CrossRef]
- Adnan, M.; Zheng, W.; Islam, W.; Arif, M.; Abubakar, Y.S.; Wang, Z.; Lu, G. Carbon Catabolite Repression in Filamentous Fungi. Int. J. Mol. Sci. 2017, 19, 48. [Google Scholar] [CrossRef]
- Fayyad-Kazan, M.; Feller, A.; Bodo, E.; Boeckstaens, M.; Marini, A.M.; Dubois, E.; Georis, I. Yeast Nitrogen Catabolite Repression Is Sustained by Signals Distinct from Glutamine and Glutamate Reservoirs. Mol. Microbiol. 2016, 99, 360–379. [Google Scholar] [CrossRef]
- Han, L.; Liu, K.; Ma, W.; Jiang, Y.; Hou, S.; Tan, Y.; Yuan, Q.; Niu, K.; Fang, X. Redesigning Transcription Factor Cre1 for Alleviating Carbon Catabolite Repression in Trichoderma reesei. Synth. Syst. Biotechnol. 2020, 5, 230–235. [Google Scholar] [CrossRef]
- Pang, A.-P.; Zhang, F.; Hu, X.; Luo, Y.; Wang, H.; Durrani, S.; Wu, F.-G.; Li, B.-Z.; Zhou, Z.; Lu, Z.; et al. Glutamine Involvement in Nitrogen Regulation of Cellulase Production in Fungi. Biotechnol. Biofuels 2021, 14, 199. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Qian, Y.; Zhang, J.; Wang, Y.; Li, X.; Zhang, W.; Wang, L.; Liu, H.; Zhong, Y. Extracellular Protease Production Regulated by Nitrogen and Carbon Sources in Trichoderma reesei. J. Basic Microbiol. 2021, 61, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Suarez, B.; Rey, M.; Castillo, P.; Monte, E.; Llobell, A. Isolation and Characterization of PRA1, a Trypsin-like Protease from the Biocontrol Agent Trichoderma harzianum CECT 2413 Displaying Nematicidal Activity. Appl. Microbiol. Biotechnol. 2004, 65, 46–55. [Google Scholar] [CrossRef]
- Olmedo-Monfil, V.; Mendoza-Mendoza, A.; Gómez, I.; Cortés, C.; Herrera-Estrella, A. Multiple Environmental Signals Determine the Transcriptional Activation of the Mycoparasitism Related Gene Prb1 in Trichoderma atroviride. Mol. Genet. Genom. 2002, 267, 703–712. [Google Scholar] [CrossRef]
- Cortés, C.; Gutierrez, A.; Olmedo, V.; Inbar, J.; Chet, I.; Herrera-Estrella, A. The Expression of Genes Involved in Parasitism by Trichoderma harzianum Is Triggered by a Diffusible Factor. Mol. Gen. Genet. 1998, 260, 218–225. [Google Scholar] [CrossRef]
- Pons-Hernández, J.L.; Guerrero-Aguilar, B.Z.; González-Chavira, M.M.; González-Pérez, E.; Villalobos-Reyes, S.; Muñoz-Sánchez, C.I.; Pons-Hernández, J.L.; Guerrero-Aguilar, B.Z.; González-Chavira, M.M.; González-Pérez, E.; et al. Phenotypic Variability of Phytophthora capsici Isolates in Guanajuato. Rev. Mex. Cienc. Agrícolas 2020, 11, 1891–1901. [Google Scholar] [CrossRef]
- Sevillano-Serrano, J.; Larsen, J.; Rojas-Rojas, F.U.; Vega-Arreguín, J.C. Increasing Virulence and Decreasing Fungicide Sensitivity in Phytophthora capsici after Continuous Metalaxyl-Chlorothalonil Exposure. J. Plant Pathol. 2024. [Google Scholar] [CrossRef]
- Velazquez-Robledo, R.; Contreras-Cornejo, H.A.; Macias-Rodriguez, L.; Hernandez-Morales, A.; Aguirre, J.; Casas-Flores, S.; Lopez-Bucio, J.; Herrera-Estrella, A. Role of the 4-Phosphopantetheinyl Transferase of Trichoderma virens in Secondary Metabolism and Induction of Plant Defense Responses. Mol. Plant Microbe Interact. 2011, 24, 1459–1471. [Google Scholar] [CrossRef]
- Cummings, M.P. PAUP* (Phylogenetic Analysis Using Parsimony (and Other Methods)). In Dictionary of Bioinformatics and Computational Biology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; ISBN 978-0-471-65012-6. [Google Scholar]
- Stamatakis, A. RAxML-VI-HPC: Maximum Likelihood-Based Phylogenetic Analyses with Thousands of Taxa and Mixed Models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Samuels, G.J.; Ismaiel, A. Trichoderma evansii and T. lieckfeldtiae: Two New T. hamatum-like Species. Mycologia 2009, 101, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Kubicek, C.P.; Herrera-Estrella, A.; Seidl-Seiboth, V.; Martinez, D.A.; Druzhinina, I.S.; Thon, M.; Zeilinger, S.; Casas-Flores, S.; Horwitz, B.A.; Mukherjee, P.K.; et al. Comparative Genome Sequence Analysis Underscores Mycoparasitism as the Ancestral Life Style of Trichoderma. Genome Biol. 2011, 12, R40. [Google Scholar] [CrossRef]
- Kullnig, C.; Mach, R.L.; Lorito, M.; Kubicek, C.P. Enzyme Diffusion from Trichoderma atroviride (=T. harzianum P1) to Rhizoctonia solani Is a Prerequisite for Triggering of Trichoderma Ech42 Gene Expression before Mycoparasitic Contact. Appl. Environ. Microbiol. 2000, 66, 2232–2234. [Google Scholar] [CrossRef]
- Hoyos-Carvajal, L.; Orduz, S.; Bissett, J. Genetic and Metabolic Biodiversity of Trichoderma from Colombia and Adjacent Neotropic Regions. Fungal Genet. Biol. 2009, 46, 615–631. [Google Scholar] [CrossRef]
- Samuels, G.J.; Ismaiel, A.; Bon, M.-C.; De Respinis, S.; Petrini, O. Trichoderma asperellum Sensu Lato Consists of Two Cryptic Species. Mycologia 2010, 102, 944–966. [Google Scholar] [CrossRef]
- Druzhinina, I.S.; Kopchinskiy, A.G.; Komoń, M.; Bissett, J.; Szakacs, G.; Kubicek, C.P. An Oligonucleotide Barcode for Species Identification in Trichoderma and Hypocrea. Fungal Genet. Biol. 2005, 42, 813–828. [Google Scholar] [CrossRef]
- Holmes, K.A.; Schroers, H.-J.; Thomas, S.E.; Evans, H.C.; Samuels, G.J. Taxonomy and Biocontrol Potential of a New Species of Trichoderma from the Amazon Basin of South America. Mycol. Prog. Progress. 2004, 3, 199–210. [Google Scholar] [CrossRef]
- Samuels, G.J.; Dodd, S.L.; Lu, B.-S.; Petrini, O.; Schroers, H.-J.; Druzhinina, I.S. The Trichoderma koningii Aggregate Species. Stud. Mycol. 2006, 56, 67–133. [Google Scholar] [CrossRef]
- Rodrigues, A.; Mueller, U.G.; Ishak, H.D.; Bacci, M.; Pagnocca, F.C. Ecology of Microfungal Communities in Gardens of Fungus-Growing Ants (Hymenoptera: Formicidae): A Year-Long Survey of Three Species of Attine Ants in Central Texas. FEMS Microbiol. Ecol. 2011, 78, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Jaklitsch, W.M.; Samuels, G.J.; Dodd, S.L.; Lu, B.-S.; Druzhinina, I.S. Hypocrea rufa/Trichoderma viride: A Reassessment, and Description of Five Closely Related Species with and without Warted Conidia. Stud. Mycol. 2006, 56, 135–177. [Google Scholar] [CrossRef] [PubMed]
- Li Destri Nicosia, M.G.; Mosca, S.; Mercurio, R.; Schena, L. Dieback of Pinus Nigra Seedlings Caused by a Strain of Trichoderma viride. Plant Dis. 2015, 99, 44–49. [Google Scholar] [CrossRef]
- Bissett, J.; Gams, W.; Jaklitsch, W.; Samuels, G.J. Accepted Trichoderma Names in the Year 2015. IMA Fungus 2015, 6, 263–295. [Google Scholar] [CrossRef]
- Vu, D.; Groenewald, M.; de Vries, M.; Gehrmann, T.; Stielow, B.; Eberhardt, U.; Al-Hatmi, A.; Groenewald, J.Z.; Cardinali, G.; Houbraken, J.; et al. Large-Scale Generation and Analysis of Filamentous Fungal DNA Barcodes Boosts Coverage for Kingdom Fungi and Reveals Thresholds for Fungal Species and Higher Taxon Delimitation. Stud. Mycol. 2019, 92, 135–154. [Google Scholar] [CrossRef]
- Samuels, G.J. Trichoderma: Systematics, the Sexual State, and Ecology. Phytopathology 2006, 96, 195–206. [Google Scholar] [CrossRef]
- Druzhinina, I.S.; Kubicek, C.P.; Komoń-Zelazowska, M.; Mulaw, T.B.; Bissett, J. The Trichoderma harzianum Demon: Complex Speciation History Resulting in Coexistence of Hypothetical Biological Species, Recent Agamospecies and Numerous Relict Lineages. BMC Evol. Biol. 2010, 10, 94. [Google Scholar] [CrossRef]
- Druzhinina, I.S.; Komoń-Zelazowska, M.; Atanasova, L.; Seidl, V.; Kubicek, C.P. Evolution and Ecophysiology of the Industrial Producer Hypocrea jecorina (Anamorph Trichoderma reesei) and a New Sympatric Agamospecies Related to It. PLoS ONE 2010, 5, e9191. [Google Scholar] [CrossRef][Green Version]
- Degenkolb, T.; Dieckmann, R.; Nielsen, K.F.; Gräfenhan, T.; Theis, C.; Zafari, D.; Chaverri, P.; Ismaiel, A.; Brückner, H.; von Döhren, H.; et al. The Trichoderma brevicompactum Clade: A Separate Lineage with New Species, New Peptaibiotics, and Mycotoxins. Mycol. Prog. 2008, 7, 177–219. [Google Scholar] [CrossRef]
- Druzhinina, I.S.; Chenthamara, K.; Zhang, J.; Atanasova, L.; Yang, D.; Miao, Y.; Rahimi, M.J.; Grujic, M.; Cai, F.; Pourmehdi, S.; et al. Massive Lateral Transfer of Genes Encoding Plant Cell Wall-Degrading Enzymes to the Mycoparasitic Fungus Trichoderma from Its Plant-Associated Hosts. PLoS Genet. 2018, 14, e1007322. [Google Scholar] [CrossRef]
- Esquivel-Naranjo, E.U.; Herrera-Estrella, A. Enhanced Responsiveness and Sensitivity to Blue Light by Blr-2 Overexpression in Trichoderma atroviride. Microbiology 2007, 153, 3909–3922. [Google Scholar] [CrossRef][Green Version]
- Kibbe, W.A. OligoCalc: An Online Oligonucleotide Properties Calculator. Nucleic Acids Res. 2007, 35, W43–W46. [Google Scholar] [CrossRef] [PubMed]
- Calcáneo-Hernández, G.; Landeros-Jaime, F.; Cervantes-Chávez, J.A.; Mendoza-Mendoza, A.; Esquivel-Naranjo, E.U. Osmotic Stress Responses, Cell Wall Integrity, and Conidiation Are Regulated by a Histidine Kinase Sensor in Trichoderma atroviride. J Fungi 2023, 9, 939. [Google Scholar] [CrossRef] [PubMed]
- Van Den, T.; Biermann, C.J.; Marlett, J.A. Simple Sugars, Oligosaccharides and Starch Concentrations in Raw and Cooked Sweet Potato. J. Agric. Food Chem. 1986, 34, 421–425. [Google Scholar] [CrossRef]
- Hughes, B.P. The Amino-Acid Composition of Potato Protein and of Cooked Potato. Br. J. Nutr. 1958, 12, 188–195. [Google Scholar] [CrossRef]
- Ilmén, M.; Saloheimo, A.; Onnela, M.L.; Penttilä, M.E. Regulation of Cellulase Gene Expression in the Filamentous Fungus Trichoderma reesei. Appl. Environ. Microbiol. 1997, 63, 1298–1306. [Google Scholar] [CrossRef]
- Marcello, C.M.; Steindorff, A.S.; da Silva, S.P.; do Nascimento Silva, R.; Mendes Bataus, L.A.; Ulhoa, C.J. Expression Analysis of the Exo-β-1,3-Glucanase from the Mycoparasitic Fungus Trichoderma asperellum. Microbiol. Res. 2010, 165, 75–81. [Google Scholar] [CrossRef]
- Nitsche, B.M.; Jørgensen, T.R.; Akeroyd, M.; Meyer, V.; Ram, A.F. The Carbon Starvation Response of Aspergillus niger during Submerged Cultivation: Insights from the Transcriptome and Secretome. BMC Genom. 2012, 13, 380. [Google Scholar] [CrossRef]
- Ait-Lahsen, H.; Soler, A.; Rey, M.; de la Cruz, J.; Monte, E.; Llobell, A. An Antifungal Exo-α-1,3-Glucanase (AGN13.1) from the Biocontrol Fungus Trichoderma harzianum. Appl. Environ. Microbiol. 2001, 67, 5833–5839. [Google Scholar] [CrossRef]
- Geremia, R.A.; Goldman, G.H.; Jacobs, D.; Ardiles, W.; Vila, S.B.; Van Montagu, M.; Herrera-Estrella, A. Molecular Characterization of the Proteinase-Encoding Gene, Prb1, Related to Mycoparasitism by Trichoderma harzianum. Mol. Microbiol. 1993, 8, 603–613. [Google Scholar] [CrossRef]
- Oszust, K.; Cybulska, J.; Frąc, M. How Do Trichoderma Genus Fungi Win a Nutritional Competition Battle against Soft Fruit Pathogens? A Report on Niche Overlap Nutritional Potentiates. Int. J. Mol. Sci. 2020, 21, 4235. [Google Scholar] [CrossRef] [PubMed]
- Segreto, R.; Bazafkan, H.; Millinger, J.; Schenk, M.; Atanasova, L.; Doppler, M.; Büschl, C.; Boeckstaens, M.; Soto Diaz, S.; Schreiner, U.; et al. The TOR Kinase Pathway Is Relevant for Nitrogen Signaling and Antagonism of the Mycoparasite Trichoderma atroviride. PLoS ONE 2021, 16, e0262180. [Google Scholar] [CrossRef]
- Mélida, H.; Sandoval-Sierra, J.V.; Diéguez-Uribeondo, J.; Bulone, V. Analyses of Extracellular Carbohydrates in Oomycetes Unveil the Existence of Three Different Cell Wall Types. Eukaryot. Cell 2013, 12, 194–203. [Google Scholar] [CrossRef]
- Meijer, H.J.G.; Mancuso, F.M.; Espadas, G.; Seidl, M.F.; Chiva, C.; Govers, F.; Sabidó, E. Profiling the Secretome and Extracellular Proteome of the Potato Late Blight Pathogen Phytophthora infestans. Mol. Cell Proteom. 2014, 13, 2101–2113. [Google Scholar] [CrossRef]
- Bonnet, P.; Bourdon, E.; Ponchet, M.; Blein, J.-P.; Ricci, P. Acquired Resistance Triggered by Elicitins in Tobacco and Other Plants. Eur. J. Plant Pathol. 1996, 102, 181–192. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, M.; Wang, Y.; Wang, L.; Liu, H.; Shi, M.; Zhong, Y. Constitutive Overexpression of Cellobiohydrolase 2 in Trichoderma reesei Reveals Its Ability to Initiate Cellulose Degradation. Eng. Microbiol. 2023, 3, 100059. [Google Scholar] [CrossRef]
- Ramada, M.H.S.; Steindorff, A.S.; Bloch, C.; Ulhoa, C.J. Secretome Analysis of the Mycoparasitic Fungus Trichoderma harzianum ALL 42 Cultivated in Different Media Supplemented with Fusarium solani Cell Wall or Glucose. Proteomics 2016, 16, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Hinkel, L.; Ospina-Giraldo, M.D. Structural Characterization of a Putative Chitin Synthase Gene in Phytophthora spp. and Analysis of Its Transcriptional Activity during Pathogenesis on Potato and Soybean Plants. Curr. Genet. 2017, 63, 909–921. [Google Scholar] [CrossRef]
- Cheng, W.; Lin, M.; Qiu, M.; Kong, L.; Xu, Y.; Li, Y.; Wang, Y.; Ye, W.; Dong, S.; He, S.; et al. Chitin Synthase Is Involved in Vegetative Growth, Asexual Reproduction and Pathogenesis of Phytophthora capsici and Phytophthora sojae. Environ. Microbiol. 2019, 21, 4537–4547. [Google Scholar] [CrossRef]
- Bellincampi, D.; Cervone, F.; Lionetti, V. Plant Cell Wall Dynamics and Wall-Related Susceptibility in Plant–Pathogen Interactions. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef]
- Neves Monteiro, V.; Stecca Steindorff, A.; Dos Reis Almeida, F.B.; Cardoso Lopes, F.A.; Ulhoa, C.J.; Roberto Félix, C.; Silva, R.N. Trichoderma reesei Mycoparasitism against Pythium ultimum Is Coordinated by G-Alpha Protein GNA1 Signaling. J. Microb. Biochem. Technol. 2015, 7, 1–7. [Google Scholar] [CrossRef]
- La Spada, F.; Stracquadanio, C.; Riolo, M.; Pane, A.; Cacciola, S.O. Trichoderma Counteracts the Challenge of Phytophthora nicotianae Infections on Tomato by Modulating Plant Defense Mechanisms and the Expression of Crinkler, Necrosis-Inducing Phytophthora Protein 1, and Cellulose-Binding Elicitor Lectin Pathogenic Effectors. Front. Plant Sci. 2020, 11, 583539. [Google Scholar] [CrossRef]
- LoBuglio, K.F.; Pitt, J.I.; Taylor, J.W. Phylogenetic Analysis of Two Ribosomal Dna Regions Indicates Multiple Independent Losses of a Sexual Talaromyces State Among Asexual Penicillium Species in Subgenus Biverticillium. Mycologia 1993, 85, 592–604. [Google Scholar] [CrossRef]
- Nagy, V.; Seidl, V.; Szakacs, G.; Komoń-Zelazowska, M.; Kubicek, C.P.; Druzhinina, I.S. Application of DNA Bar Codes for Screening of Industrially Important Fungi: The Haplotype of Trichoderma harzianum Sensu Stricto Indicates Superior Chitinase Formation. Appl. Environ. Microbiol. 2007, 73, 7048–7058. [Google Scholar] [CrossRef]
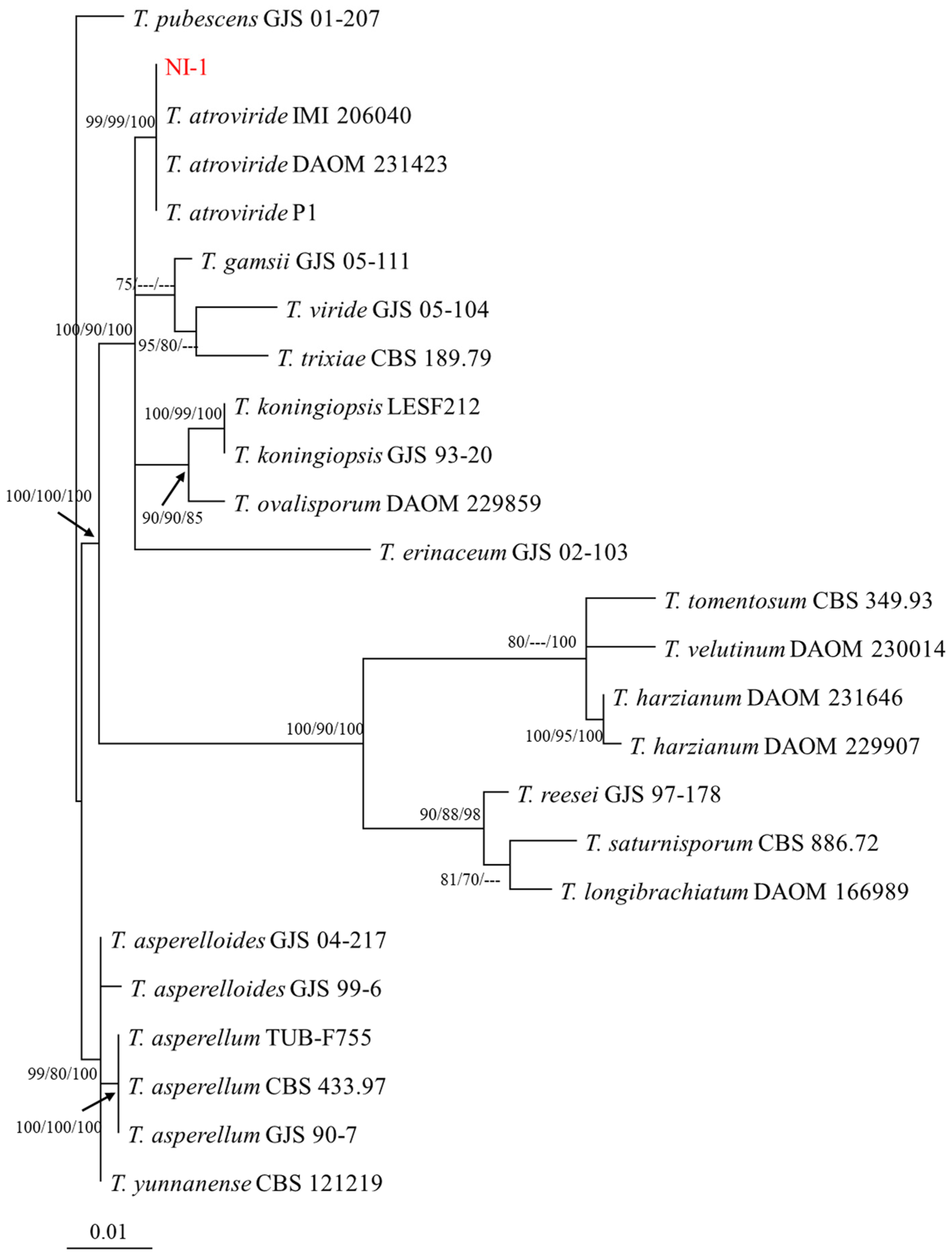
| Species | Strain | Source | Country | ITS | Tef-1α | Reference |
|---|---|---|---|---|---|---|
| T. pubescens | GJS 01-207 | Bark | Cameroon | EU856280 | EU856304 | [25] |
| T. atroviride | NI-1 | Soil | Mexico | PQ162586 | PQ335171 | This work |
| T. atroviride | IMI 206040 | Soil | Sweden | AF278795 | 300828 1 | [26] |
| T. atroviride | P1 | Soil | UK | OQ360634 | EF581849 | [27] |
| T. atroviride | DAOM 231423 | Soil | Mexico | EU280111 | EU280002 | [28] |
| T. asperelloides | GJS 04-217 | Theobroma cacao | Peru | DQ381957 | DQ381958 | [29] |
| T. asperelloides | GJS 99-6 | Wood | USA | DQ315464 | GU198240 | [29] |
| T. asperellum | TUB-F755 | Soil | Mexico | AY857217 | AY857273 | [30] |
| T. asperellum | CBS 433.97 | Sclerotia | USA | AY380912 | AY376058 | [31] |
| T. asperellum | GJS 90-7 | Soil | Vietnam | GU198317 | EU338333 | [29] |
| T. yunnanense | CBS 121219 | Soil | China | GU198302 | GU198243 | [29] |
| T. gamsii | GJS 05-111 | Ricinus comunis | Italy | DQ841730 | DQ841722 | [32] |
| T. koningiopsis | LESF212 | Cyphomyrmex wheeleri nest | USA | HQ608031 | KT278985 | [33] |
| T. koningiopsis | GJS 93-20 | Branch | Cuba | DQ313140 | DQ284966 | [32] |
| T. viride | GJS 05-104 | Peat | Italy | DQ841741 | DQ841727 | [34] |
| T. trixiae | CBS 189.79 | Wood | Italy | KJ482546 | KJ482552 | [35] |
| T. ovalisporum | DAOM 229859 | Soil | Panama | EU280118 | EU280003 | [28] |
| T. erinaceum | GJS 02-103 | Wood | Sri Lanka | KR873100 | KR873098 | [36] |
| T. tomentosum | CBS 349.93 | ND 2 | Canada | MH862417 | AF401024 | [37] |
| T. velutinum | DAOM 230014 | Soil | Nepal | DQ083010 | AY937446 | [38] |
| T. harzianum | DAOM 231646 | Soil | South Africa | AY605723 | AY605766 | [39] |
| T. harzianum | DAOM 229907 | Soil | USA | AY605734 | AY605777 | [39] |
| T. reesei | GJS 97-178 | Theobroma sp. | French Guiana | AJ004964 | GQ354348 | [40] |
| T. saturnisporum | CBS 886.72 | Triticum sp. | South Africa | X93974 | AY937414 | [38] |
| T. longibrachiatum | DAOM 166989 | Soil | Canada | EU330961 | EU338335 | [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Sánchez, V.J.; Sánchez-López, K.L.; Esquivel Méndez, J.J.; Sánchez-Hernández, D.; Cervantes-Chávez, J.A.; Landeros-Jaime, F.; Mendoza-Mendoza, A.; Vega-Arreguín, J.C.; Esquivel-Naranjo, E.U. Carbon and Nitrogen Sources Influence Parasitic Responsiveness in Trichoderma atroviride NI-1. J. Fungi 2024, 10, 671. https://doi.org/10.3390/jof10100671
García-Sánchez VJ, Sánchez-López KL, Esquivel Méndez JJ, Sánchez-Hernández D, Cervantes-Chávez JA, Landeros-Jaime F, Mendoza-Mendoza A, Vega-Arreguín JC, Esquivel-Naranjo EU. Carbon and Nitrogen Sources Influence Parasitic Responsiveness in Trichoderma atroviride NI-1. Journal of Fungi. 2024; 10(10):671. https://doi.org/10.3390/jof10100671
Chicago/Turabian StyleGarcía-Sánchez, Víctor Javier, Karina Lizbeth Sánchez-López, Juana Jazmín Esquivel Méndez, Daniel Sánchez-Hernández, José Antonio Cervantes-Chávez, Fidel Landeros-Jaime, Artemio Mendoza-Mendoza, Julio Cesar Vega-Arreguín, and Edgardo Ulises Esquivel-Naranjo. 2024. "Carbon and Nitrogen Sources Influence Parasitic Responsiveness in Trichoderma atroviride NI-1" Journal of Fungi 10, no. 10: 671. https://doi.org/10.3390/jof10100671
APA StyleGarcía-Sánchez, V. J., Sánchez-López, K. L., Esquivel Méndez, J. J., Sánchez-Hernández, D., Cervantes-Chávez, J. A., Landeros-Jaime, F., Mendoza-Mendoza, A., Vega-Arreguín, J. C., & Esquivel-Naranjo, E. U. (2024). Carbon and Nitrogen Sources Influence Parasitic Responsiveness in Trichoderma atroviride NI-1. Journal of Fungi, 10(10), 671. https://doi.org/10.3390/jof10100671








