Multilocus Phylogeny and Characterization of Five Undescribed Aquatic Carnivorous Fungi (Orbiliomycetes)
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Collection
2.2. Fungal Isolation
2.3. Morphological Observation
2.4. Collection of DNA Molecular Data
2.5. Phylogenetic Analysis
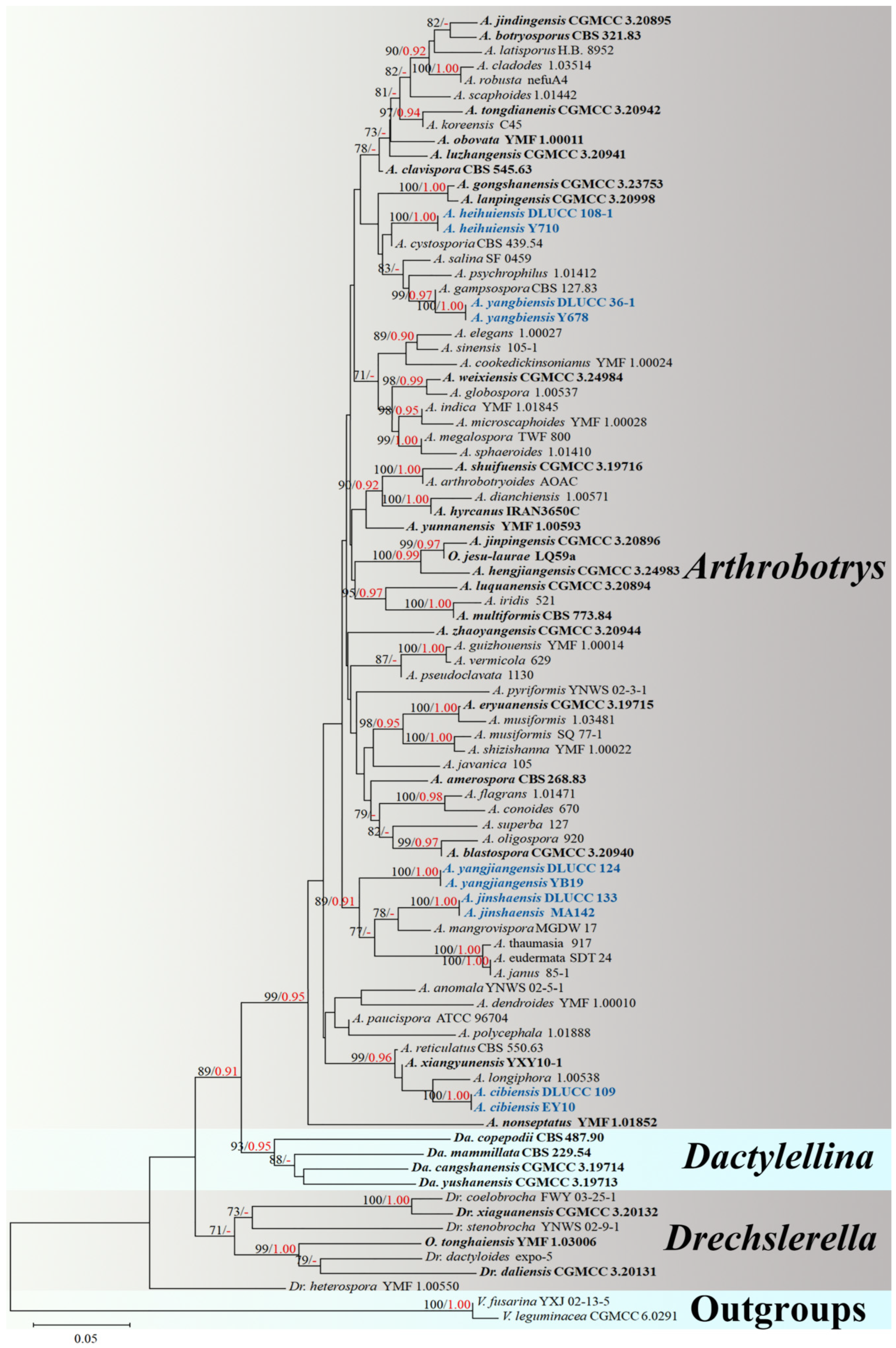
3. Results
3.1. Phylogenetic Analysis
3.2. Taxonomy
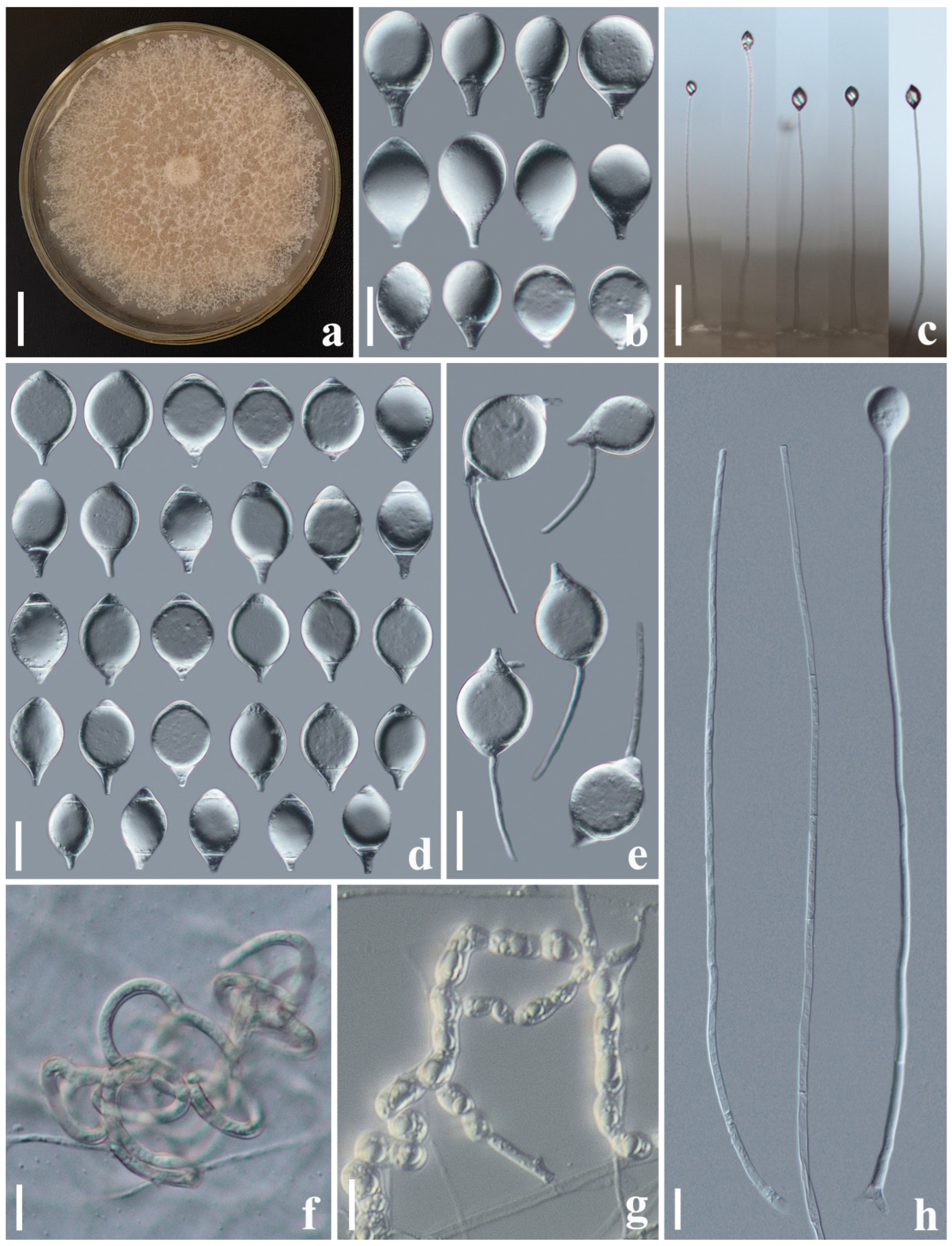
3.2.1. Arthrobotrys cibiensis F. Zhang, S. Boonmee, and X.Y. Yang sp. nov. (Figure 2)
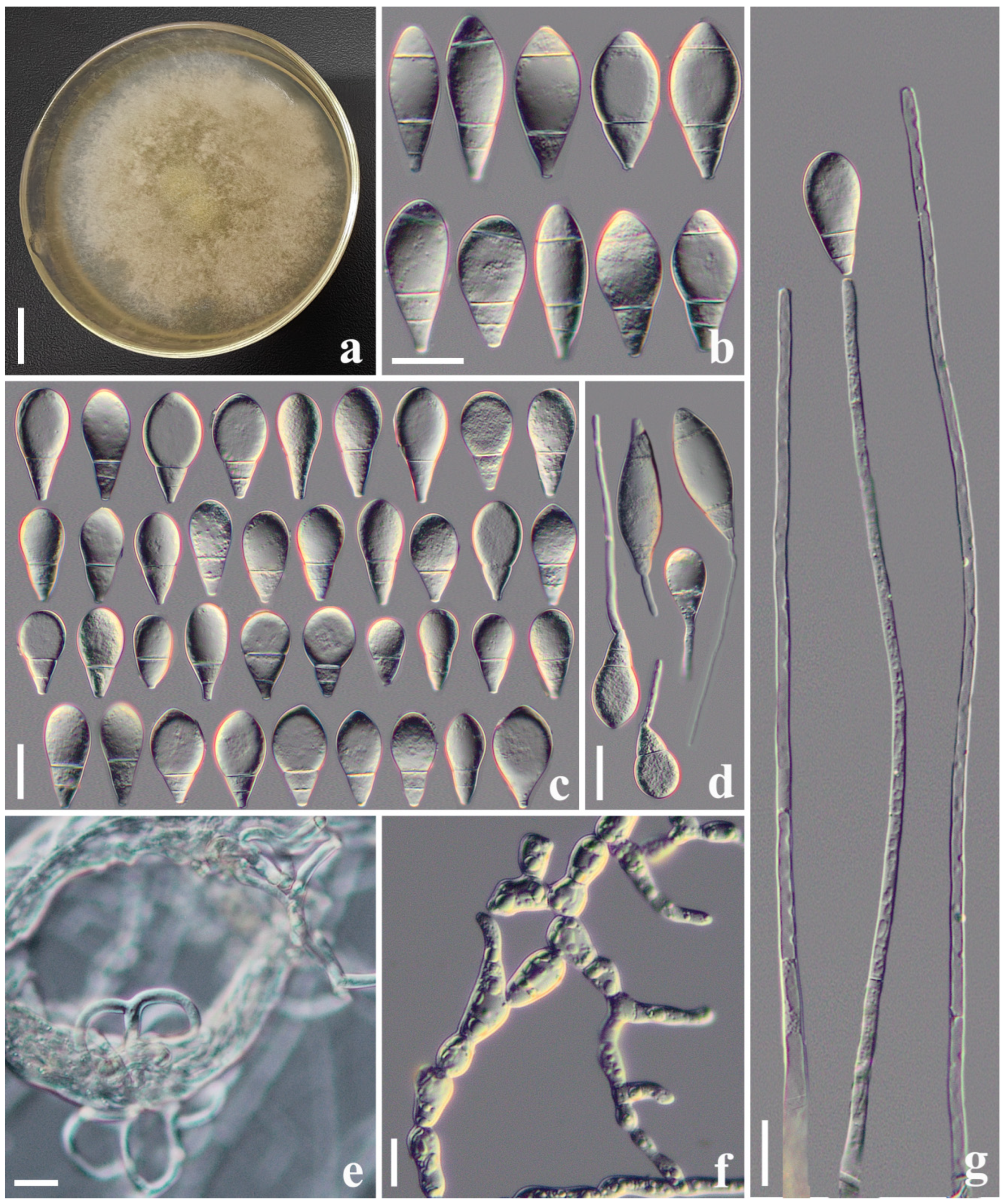
3.2.2. Arthrobotrys heihuiensis F. Zhang, S. Boonmee, and X.Y. Yang sp. nov. (Figure 3)

3.2.3. Arthrobotrys jinshaensis F. Zhang, S. Boonmee and X.Y. Yang sp. nov. (Figure 4)
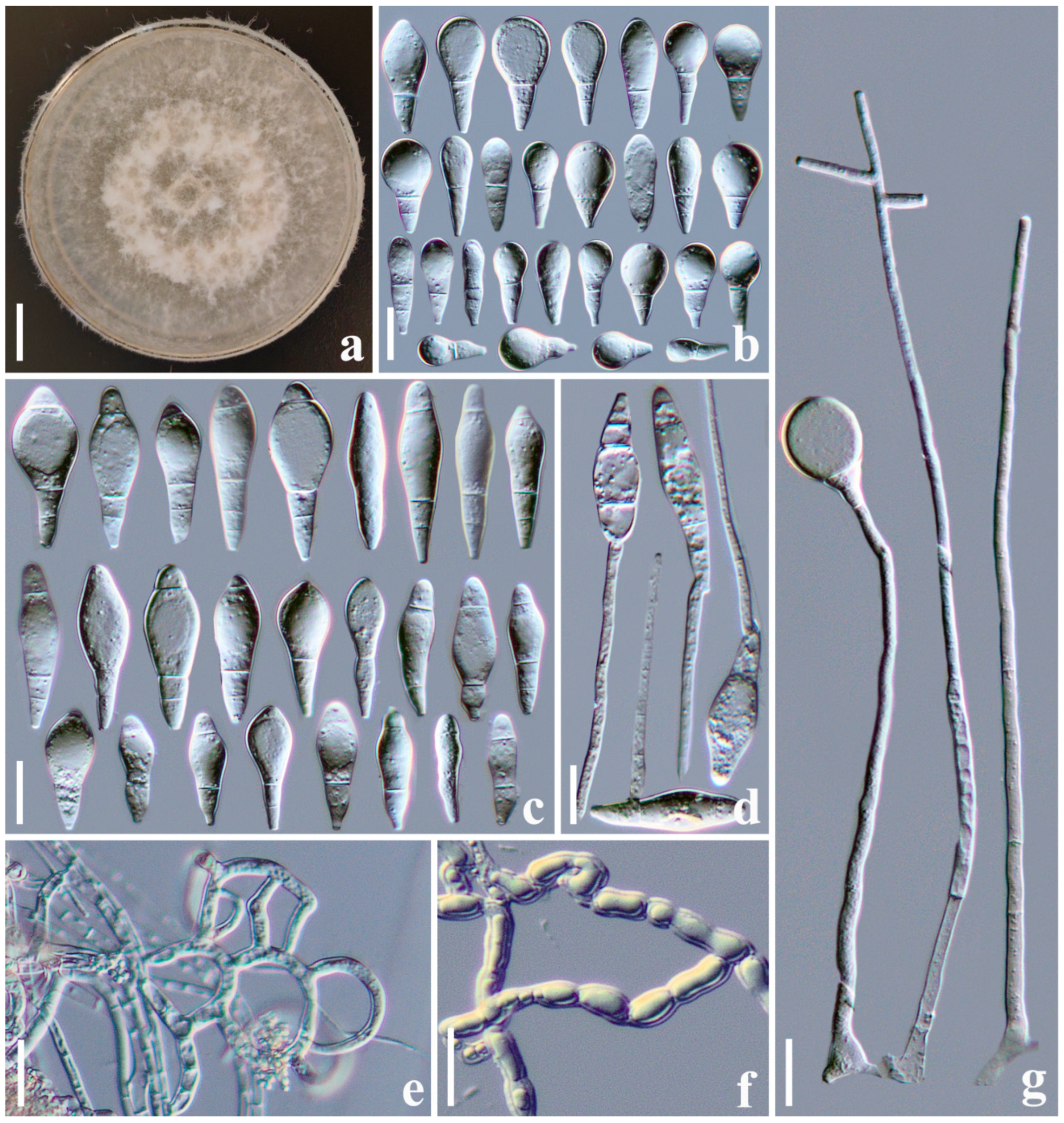
3.2.4. Arthrobotrys yangbiensis F. Zhang, S. Boonmee, and X.Y. Yang sp. nov. (Figure 5)
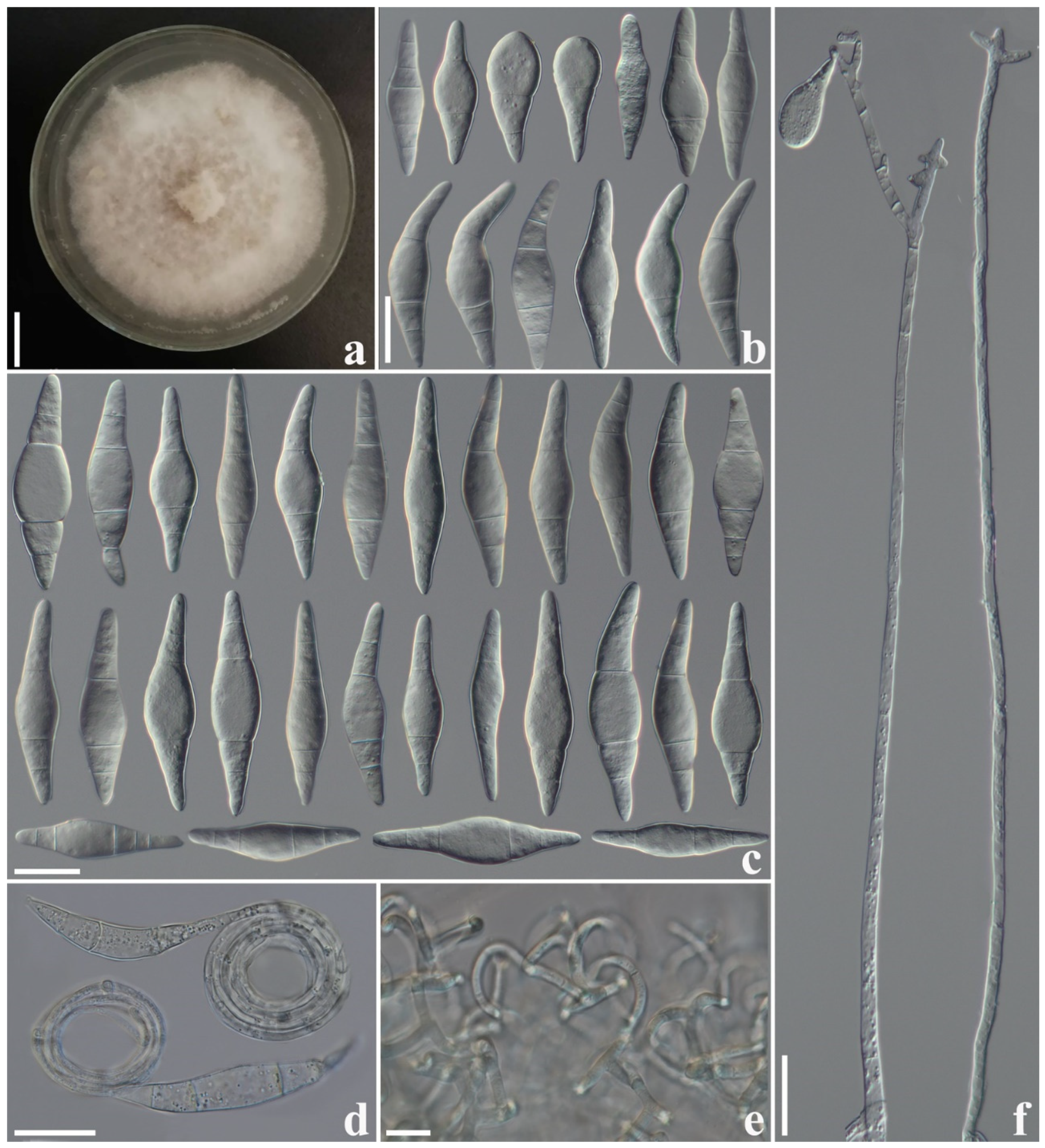
3.2.5. Arthrobotrys yangjiangensis F. Zhang, S. Boonmee, and X.Y. Yang sp. nov. (Figure 6)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barron, G.L. The Nematode-Destroying Fungi; Canadian Biological Publications Ltd.: Guelph, ON, Canada, 1977; p. 45. [Google Scholar]
- Swe, A.; Li, J.; Zhang, K.Q.; Pointing, S.B.; Jeewon, R.; Hyde, K.D. Nematode-trapping fungi. Curr. Res. Environ. Appl. Mycol. 2011, 1, 1–26. [Google Scholar]
- Zhang, K.Q.; Mo, M.H. Flora Fungorum Sinicorum (Vol. 33): Arthrobotrys et Gengra Cetera Cognata; Science Press: Beijing, China, 2006. [Google Scholar]
- Zhang, K.Q.; Hyde, K.D. Nematode-Trapping Fungi; Springer Science & Business: Berlin, Germany, 2014. [Google Scholar] [CrossRef]
- Jiang, X.; Xiang, M.; Liu, X. Nematode-trapping fungi. Microbiol. Spectr. 2017, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.S.; El-Deriny, M.M.; Ibrahim, D.S.S.; Zakaria, H.; Ahmed, Y. Suppression of root-knot nematode Meloidogyne incognita on tomato plants using the nematode trapping fungus Arthrobotrys oligospora Fresenius. J. Appl. Microbiol. 2021, 131, 2402–2415. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, E.; An, Z.; Liu, X.Z. Evolution of nematode-trapping cells of predatory fungi of the Orbiliaceae based on evidence from rRNA-encoding DNA and multiprotein sequences. Proc. Natl. Acad. Sci. USA 2007, 104, 8379–8384. [Google Scholar] [CrossRef] [PubMed]
- Ahrén, D.; Ursing, B.M.; Tunlid, A. Phylogeny of nematode-trapping fungi based on 18S rDNA sequences. FEMS Microbiol. Lett. 1998, 158, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Mo, M.H.; Chen, W.M.; Yang, H.R.; Zhang, K.Q. Diversity and metal tolerance of nematode-trapping fungi in Pb-polluted soils. J. Microbiol. 2008, 46, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Pandey, S.K.; Chattopadhyay, A. Biodiversity and periodical/seasonal distribution of nematode trapping fungi from different habitats. J. Pure Appl. Microbiol. 2014, 9, 767–776. [Google Scholar]
- Zhang, Y.; Li, S.; Li, H.; Wang, R.; Zhang, K.Q.; Xu, J. Fungi–nematode interactions: Diversity, ecology, and biocontrol prospects in agriculture. J. Fungi 2020, 6, 206. [Google Scholar] [CrossRef]
- Liu, S.; Su, H.; Su, X.; Zhang, F.; Liao, G.; Yang, X. Arthrobotrys xiangyunensis, a novel nematode-trapping taxon from a hot-spring in Yunnan Province, China. Phytotaxa 2014, 174, 89–96. [Google Scholar] [CrossRef]
- Hao, Y.; Mo, M.; Su, H.; Zhang, K.Q. Ecology of aquatic nematode-trapping hyphomycetes in southwestern China. Aquat. Microb. Ecol. 2005, 40, 175–181. [Google Scholar] [CrossRef]
- Swe, A.; Jeewon, R.; Pointing, S.B.; Hyde, K.D. Diversity and abundance of nematode-trapping fungi from decaying litter in terrestrial, freshwater and mangrove habitats. Biodivers. Conserv. 2009, 18, 1695–1714. [Google Scholar] [CrossRef]
- Zhang, F.; Boonmee, S.; Bhat, J.D.; Xiao, W.; Yang, X.Y. New Arthrobotrys nematode-trapping species (Orbiliaceae) from terrestrial soils and freshwater sediments in China. J. Fungi 2022, 8, 671. [Google Scholar] [CrossRef] [PubMed]
- Tarigan, W.E.; Munir, E.; Hastuti, L.D.S.; Hartanto, A. Occurrence of nematophagous fungi in freshwater samples of Toba Lake, North Sumatra, Indonesia. J. Phys. Conf. Ser. 2020, 1462, 012052. [Google Scholar] [CrossRef]
- Hao, Y.E.; Luo, J.; Zhang, K.Q. A new aquatic nematode trapping hyphomycete. Mycotaxon 2004, 89, 235–239. [Google Scholar]
- González-Solís, D.; Jiménez-García, M.I. Parasitic nematodes of freshwater fishes from two Nicaraguan crater lakes. Comp. Parasitol. 2006, 73, 188–192. [Google Scholar] [CrossRef]
- Tahseen, Q. Nematodes in aquatic environments: Adaptations and survival strategies. Biodivers. J. 2012, 3, 13–40. [Google Scholar]
- Staniland, L.N. A modification of the Baermann funnel technique for the collection of nematodes from plant material. J. Helminthol. 1954, 28, 115–117. [Google Scholar] [CrossRef]
- Zhang, F.; Boonmee, S.; Yang, Y.Q.; Zhou, F.P.; Xiao, W.; Yang, X.Y. Arthrobotrys blastospora sp. nov. (Orbiliomycetes): A Living Fossil Displaying Morphological Traits of Mesozoic Carnivorous Fungi. J. Fungi 2023, 9, 451. [Google Scholar] [CrossRef]
- Su, H.Y.; Li, M.; Liu, X.X. Improvement of the slide making method of nematode trapping fungi. J. Dali Univ. 2006, 6, 25–26+30. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. A Guide Methods Appl. 1990, 18, 315–322. [Google Scholar] [CrossRef]
- O’Donnell, K.; Kistler, H.C.; Cigelnik, E.; Ploetz, R.C. Multiple evolutionary origins of the fungus causing Panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA 1998, 95, 2044–2049. [Google Scholar] [CrossRef]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerse II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Swindell, S.R.; Plasterer, T.N. Seqman. In Sequence Data Analysis Guidebook; Swindell, S.R., Ed.; Springer: Totowa, NJ, USA, 1997; pp. 75–89. [Google Scholar]
- Masigol, H.; Rezakhani, F.; Pourmoghaddam, M.J.; Khodaparast, S.A.; Grossart, H.P. The Introduction of Two New Species of Aquatic Fungi from Anzali Lagoon, Northern Iran. Diversity 2022, 14, 889. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Posada, D. jModelTest: Phylogenetic Model Averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree v1. 3.1. 2010. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 16 March 2012).
- Peach, M. Aquatic predacious fungi. Trans. Br. Mycol. Soc. 1950, 33, 148–153. [Google Scholar] [CrossRef]
- Peach, M. Aquatic predacious fungi. II. Trans. Br. Mycol. Soc. 1952, 35, 19–23. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiao, M.; Weber, E.; Baral, H.O.; Hagedorn, G.; Yu, Z.F. Arthrobotrys scaphoides from China and Europe with a phylogenetic analysis including the type strain. Mycotaxon 2010, 111, 291. [Google Scholar] [CrossRef]
- Swe, A.; Jeewon, R.; Pointing, S.B.; Hyde, K.D. Taxonomy and molecular phylogeny of Arthrobotrys mangrovispora, a new marine nematode-trapping fungal species. Bot. Mar. 2008, 51, 331–338. [Google Scholar] [CrossRef]
- Drechsler, C. Some clampless hyphomycetes predacious on nematodes and rhizopods. Sydowi Annal. Mycol. Ser. II 1962, 15, 9–24. [Google Scholar]
- Li, S.D.; Miao, Z.Q.; Zhang, Y.H.; Liu, X.Z. Monacrosporium janus sp. nov., a new nematode-trapping hyphomycete parasitizing sclerotia and hyphae of Sclerotinia sclerotiorum. Mycol. Res. 2003, 107, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Drechsler, C. Some hyphomycetes that prey on free-living terricolous nematodes. Mycologia 1937, 29, 447–552. [Google Scholar] [CrossRef]
- Drechsler, C. Several species of Dactylella and Dactylaria that capture free-living nematodes. Mycologia 1950, 42, 1–79. [Google Scholar] [CrossRef]
- Deng, W.; Zhang, F.; Li, Y.P.; Zhang, X.; Fornacca, D.; Yang, X.Y.; Wen, X. Uncovering the biogeographic pattern of the widespread nematode-trapping fungi Arthrobotrys oligospora: Watershed is the key. Front. Microbiol. 2023, 14, 1152751. [Google Scholar] [CrossRef]
- de Jesús-Navarrete, A.; Armenteros, M.; Espositos, A.V.; Rocha-Olivares, A. Size spectra, biomass, and trophic groups of free-living marine nematodes along a water-depth gradient in the northwestern Gulf of Mexico. Mar. Ecol. 2022, 43, e12723. [Google Scholar] [CrossRef]
- Ptatscheck, C.; Traunspurger, W. The ability to get everywhere: Dispersal modes of free-living, aquatic nematodes. Hydrobiologia 2020, 847, 3519–3547. [Google Scholar] [CrossRef]
- Hsueh, Y.P.; Mahanti, P.; Schroeder, F.C.; Sternberg, P.W. Nematode-trapping fungi eavesdrop on nematode pheromones. Curr. Biol. 2013, 23, 83–86. [Google Scholar] [CrossRef]
- Kuo, T.H.; Yang, C.T.; Chang, H.Y.; Hsueh, Y.P.; Hsu, C.C. Nematode-trapping fungi produce diverse metabolites during predator–prey interaction. Metabolites 2020, 10, 117. [Google Scholar] [CrossRef] [PubMed]
- Tunlid, A.; Ahrén, D. Molecular mechanisms of the interaction between nematode-trapping fungi and nematodes: Lessons from genomics. In Biological Control of Plant-Parasitic Nematodes: Building Coherence between Microbial Ecology and Molecular Mechanisms; Springer: Dordrecht, The Netherlands, 2011; pp. 145–169. [Google Scholar] [CrossRef]
| Sample Source | Sampling Location | Sampling Date | Number of Samples |
|---|---|---|---|
| Cibi Lake | 26°9′7.14″ N, 99°56’32.72″ E | 4 June 2013 | 25 |
| Heihui River | 25°37′4.13″ N, 100°1′52.06″ E | 6 April 2018 | 10 |
| Jinsha River | 27°8′50.56″ N, 99°49′39.43″ E | 9 July 2014 | 10 |
| Yangbi River | 25°42′37.94″ N, 99°54′52.15″ E | 4 April 2018 | 10 |
| Yangjiang River | 25°45′52.11″ N, 99°54′46.43″ E | 14 May 2018 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Yang, Y.-Q.; Zhou, F.-P.; Xiao, W.; Boonmee, S.; Yang, X.-Y. Multilocus Phylogeny and Characterization of Five Undescribed Aquatic Carnivorous Fungi (Orbiliomycetes). J. Fungi 2024, 10, 81. https://doi.org/10.3390/jof10010081
Zhang F, Yang Y-Q, Zhou F-P, Xiao W, Boonmee S, Yang X-Y. Multilocus Phylogeny and Characterization of Five Undescribed Aquatic Carnivorous Fungi (Orbiliomycetes). Journal of Fungi. 2024; 10(1):81. https://doi.org/10.3390/jof10010081
Chicago/Turabian StyleZhang, Fa, Yao-Quan Yang, Fa-Ping Zhou, Wen Xiao, Saranyaphat Boonmee, and Xiao-Yan Yang. 2024. "Multilocus Phylogeny and Characterization of Five Undescribed Aquatic Carnivorous Fungi (Orbiliomycetes)" Journal of Fungi 10, no. 1: 81. https://doi.org/10.3390/jof10010081
APA StyleZhang, F., Yang, Y.-Q., Zhou, F.-P., Xiao, W., Boonmee, S., & Yang, X.-Y. (2024). Multilocus Phylogeny and Characterization of Five Undescribed Aquatic Carnivorous Fungi (Orbiliomycetes). Journal of Fungi, 10(1), 81. https://doi.org/10.3390/jof10010081






