The Metabolic Regulation of Amino Acid Synthesis Counteracts Reactive Nitrogen Stress via Aspergillus nidulans Cross-Pathway Control
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains, Culture, and Media
2.2. Screening the RNS Tolerance Genes
2.3. Determination of Amino Acids and Cell Weight
2.4. Gene Disruption
2.5. Aspergillus nidulans Transformation
2.6. Quantitative (q)PCR
2.7. Sequencing mRNA
2.8. Informatics Analysis
2.9. Enzyme Activity
3. Results
3.1. Proline and Arginine Are Essential for RNS Tolerance
3.2. Cross-Pathway Control Mechanism Confers RNS Tolerance
3.3. Transcription Responses of Amino Acid Biosynthesis to RNS
3.4. Global Transcription Was Altered by RNS and under Cross-Pathway Control
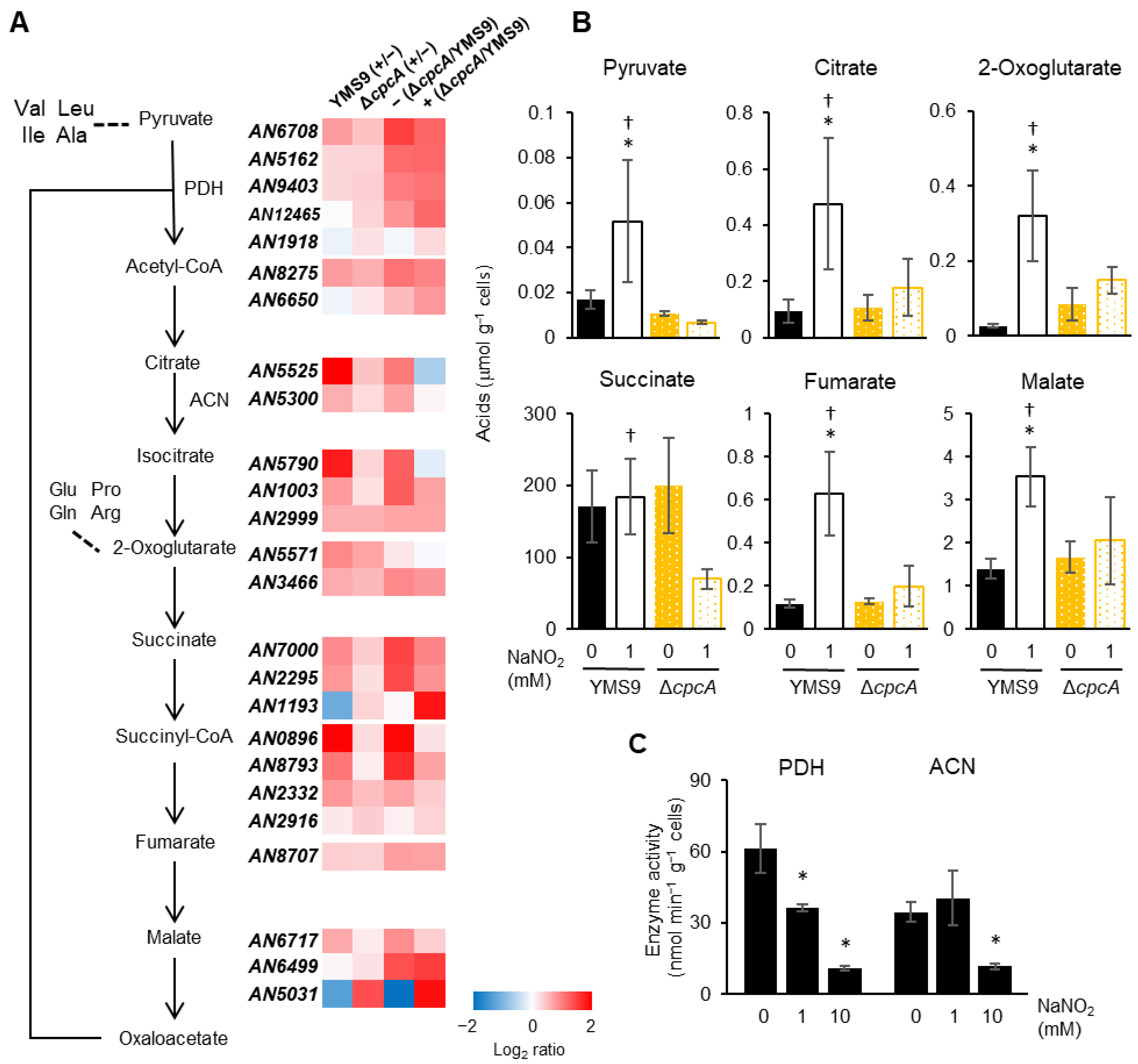
3.5. Levels of Carbon Metabolism and Amino Acid Precursors Were Decreased Due to RNS
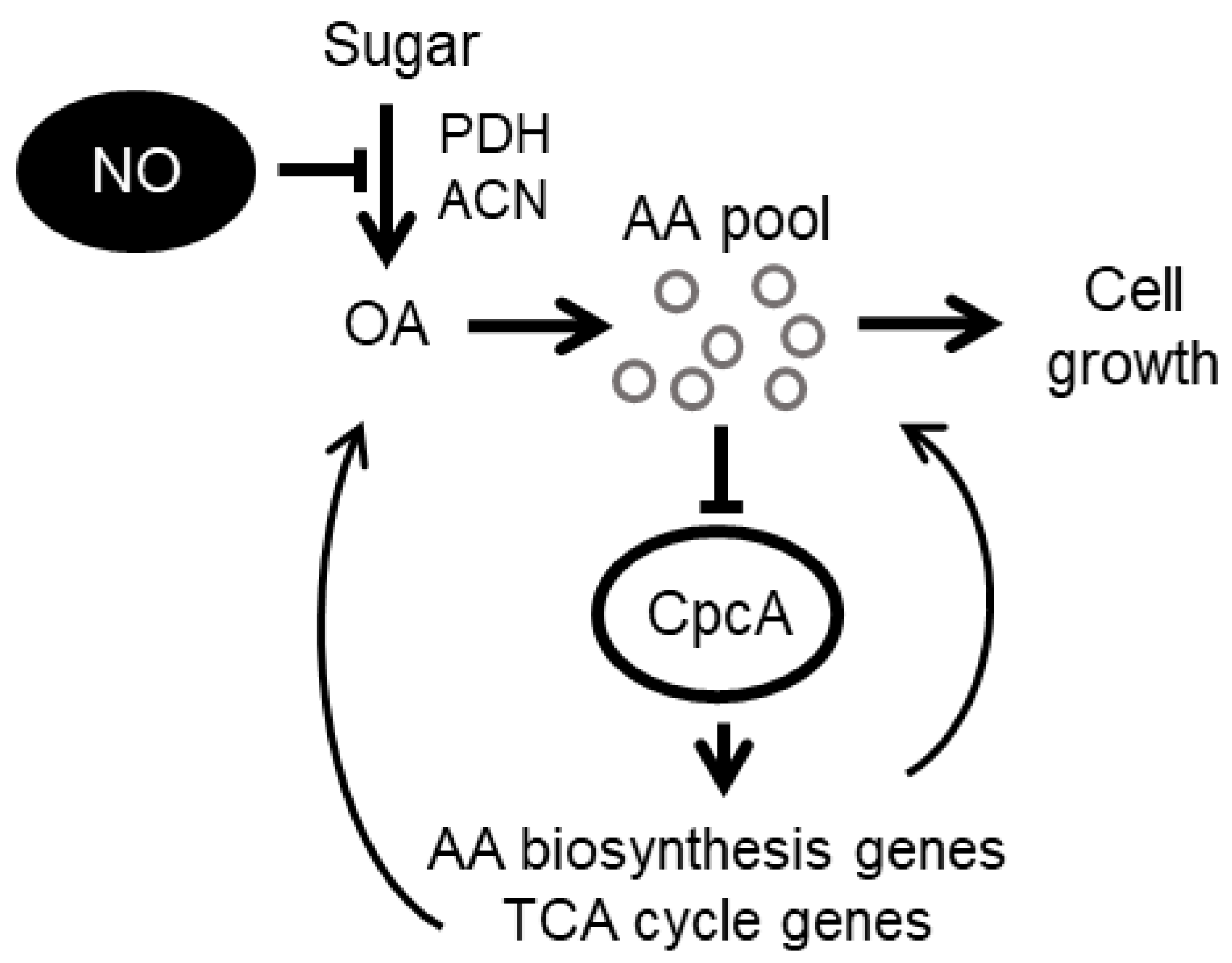
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robbins, R.A.; Grisham, M.B. Nitric Oxide. Int. J. Biochem. Cell Biol. 1997, 29, 857–860. [Google Scholar] [CrossRef] [PubMed]
- Forstermann, U.; Sessa, W.C. Nitric Oxide Synthases: Regulation and Function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Adak, S.; Sharma, M.; Meade, A.L.; Stuehr, D.J. A Conserved Flavin-Shielding Residue Regulates NO Synthase Electron Transfer and Nicotinamide Coenzyme Specificity. Proc. Natl. Acad. Sci. USA 2002, 99, 13516–13521. [Google Scholar] [CrossRef] [PubMed]
- Sudhamsu, J.; Crane, B.R. Bacterial Nitric Oxide Synthases: What Are They Good For? Trends Microbiol. 2009, 17, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Franco-Cano, A.; Marcos, A.T.; Strauss, J.; Cánovas, D. Evidence for an Arginine-Dependent Route for the Synthesis of NO in the Model Filamentous Fungus Aspergillus nidulans. Environ. Microbiol. 2021, 23, 6924–6939. [Google Scholar] [CrossRef] [PubMed]
- Marcos, A.T.; Ramos, M.S.; Marcos, J.F.; Carmona, L.; Strauss, J.; Cánovas, D. Nitric Oxide Synthesis by Nitrate Reductase Is Regulated during Development in Aspergillus. Mol. Microbiol. 2016, 99, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Takaya, N. Response to Hypoxia, Reduction of Electron Acceptors, and Subsequent Survival by Filamentous Fungi. Biosci. Biotechnol. Biochem. 2009, 73, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The Nitrate–Nitrite–Nitric Oxide Pathway in Physiology and Therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef]
- Zhou, S.; Narukami, T.; Nameki, M.; Ozawa, T.; Kamimura, Y.; Hoshino, T.; Takaya, N. Heme-Biosynthetic Porphobilinogen Deaminase Protects Aspergillus nidulans from Nitrosative Stress. Appl. Environ. Microbiol. 2012, 78, 103–109. [Google Scholar] [CrossRef]
- Frey, A.D.; Farrés, J.; Bollinger, C.J.T.; Kallio, P.T. Bacterial Hemoglobins and Flavohemoglobins for Alleviation of Nitrosative Stress in Escherichia coli. Appl. Env. Microbiol. 2002, 68, 4835–4840. [Google Scholar] [CrossRef]
- Bonamore, A.; Boffi, A. Flavohemoglobin: Structure and Reactivity. IUBMB Life 2008, 60, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Forrester, M.T.; Foster, M.W. Protection from Nitrosative Stress: A Central Role for Microbial Flavohemoglobin. Free Radic. Biol. Med. 2012, 52, 1620–1633. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Narukami, T.; Masuo, S.; Shimizu, M.; Fujita, T.; Doi, Y.; Kamimura, Y.; Takaya, N. NO-Inducible Nitrosothionein Mediates NO Removal in Tandem with Thioredoxin. Nat. Chem. Biol. 2013, 9, 657–663. [Google Scholar] [CrossRef]
- Hinnebusch, A.G. Evidence for Translational Regulation of the Activator of General Amino Acid Control in Yeast. Proc. Natl. Acad. Sci. USA 1984, 81, 6442–6446. [Google Scholar] [CrossRef] [PubMed]
- Carsiotis, M.; Jones, R.F. Cross-Pathway Regulation: Tryptophan-Mediated Control of Histidine and Arginine Biosynthetic Enzymes in Neurospora crassa. J. Bacteriol. 1974, 119, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Paluh, J.L.; Plamann, M.; Krüger, D.; Barthelmess, I.B.; Yanofsky, C.; Perkins, D.D. Determination of the Inactivating Alterations in Two Mutant Alleles of the Neurospora crassa Cross-Pathway Control Gene cpc-1. Genetics 1990, 124, 599–606. [Google Scholar] [CrossRef]
- Hoffmann, B.; Valerius, O.; Andermann, M.; Braus, G.H. Transcriptional Autoregulation and Inhibition of mRNA Translation of Amino Acid Regulator Gene cpcA of Filamentous Fungus Aspergillus nidulans. Mol. Biol. Cell 2001, 12, 2846–2857. [Google Scholar] [CrossRef][Green Version]
- Strittmatter, A.W.; Irniger, S.; Braus, G.H. Induction of jlbA mRNA Synthesis for a Putative bZIP Protein of Aspergillus nidulans by Amino Acid Starvation. Curr. Genet. 2001, 39, 327–334. [Google Scholar] [CrossRef]
- Busch, S.; Bode, H.B.; Brakhage, A.A.; Braus, G.H. Impact of the Cross-Pathway Control on the Regulation of Lysine and Penicillin Biosynthesis in Aspergillus nidulans. Curr. Genet. 2003, 42, 209–219. [Google Scholar] [CrossRef]
- Nayak, T.; Szewczyk, E.; Oakley, C.E.; Osmani, A.; Ukil, L.; Murray, S.L.; Hynes, M.J.; Osmani, S.A.; Oakley, B.R. A Versatile and Efficient Gene-Targeting System for Aspergillus nidulans. Genetics 2006, 172, 1557–1566. [Google Scholar] [CrossRef]
- Hill, T.; Kafer, E. Improved Protocols for Aspergillus Minimal Medium: Trace Element and Minimal Medium Salt Stock Solutions. Fungal Genet. Rep. 2001, 48, 20–21. [Google Scholar] [CrossRef]
- Osherov, N.; May, G. Conidial Germination in Aspergillus nidulans Requires RAS Signaling and Protein Synthesis. Genetics 2000, 155, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Gomi, K.; Iimura, Y.; Hara, S. Integrative Transformation of Aspergillus oryzae with a Plasmid Containing the Aspergillus nidulans argB Gene. Agric. Biol. Chem. 1987, 51, 2549–2555. [Google Scholar] [CrossRef]
- Sugasawa, T.; Komine, R.; Manevich, L.; Tamai, S.; Takekoshi, K.; Kanki, Y. Gene Expression Profile Provides Novel Insights of Fasting-Refeeding Response in Zebrafish Skeletal Muscle. Nutrients 2022, 14, 2239. [Google Scholar] [CrossRef]
- Tian, J.; Bryk, R.; Shi, S.; Erdjument-Bromage, H.; Tempst, P.; Nathan, C. Mycobacterium tuberculosis Appears to Lack α-Ketoglutarate Dehydrogenase and Encodes Pyruvate Dehydrogenase in Widely Separated Genes. Mol. Microbiol. 2005, 57, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Doi, Y.; Takaya, N. A Novel A3 Group Aconitase Tolerates Oxidation and Nitric Oxide. J. Biol. Chem. 2015, 290, 1412–1421. [Google Scholar] [CrossRef] [PubMed]
- Cove, D.J. Genetic Studies of Nitrate Assimilation in Aspergillus nidulans. Biol. Rev. 1979, 54, 291–327. [Google Scholar] [CrossRef] [PubMed]
- Tazebay, U.H.; Sophianopoulou, V.; Scazzocchio, C.; Diallinas, G. The Gene Encoding the Major Proline Transporter of Aspergillus nidulans Is Upregulated during Conidiospore Germination and in Response to Proline Induction and Amino Acid Starvation. Mol. Microbiol. 1997, 24, 105–117. [Google Scholar] [CrossRef]
- Goc, A.; Wěgleńiski, P. Regulatory Region of the Aspergillus nidulans argB Gene. Curr. Genet. 1988, 14, 425–429. [Google Scholar] [CrossRef]
- Hyduke, D.R.; Jarboe, L.R.; Tran, L.M.; Chou, K.J.Y.; Liao, J.C. Integrated Network Analysis Identifies Nitric Oxide Response Networks and Dihydroxyacid Dehydratase as a Crucial Target in Escherichia coli. Proc. Natl. Acad. Sci. USA 2007, 104, 8484–8489. [Google Scholar] [CrossRef]
- Duan, X.; Yang, J.; Ren, B.; Tan, G.; Ding, H. Reactivity of Nitric Oxide with the [4Fe–4S] Cluster of Dihydroxyacid Dehydratase from Escherichia coli. Biochem. J. 2009, 417, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Velayudhan, J.; Karlinsey, J.E.; Frawley, E.R.; Becker, L.A.; Nartea, M.; Fang, F.C. Distinct Roles of the Salmonella enterica Serovar Typhimurium CyaY and YggX Proteins in the Biosynthesis and Repair of Iron-Sulfur Clusters. Infect. Immun. 2014, 82, 1390–1401. [Google Scholar] [CrossRef] [PubMed]
- Zaman, Z.; Bowman, S.B.; Kornfeld, G.D.; Brown, A.J.; Dawes, I.W. Transcription Factor GCN4 for Control of Amino Acid Biosynthesis Also Regulates the Expression of the Gene for Lipoamide Dehydrogenase. Biochem. J. 1999, 340, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Biswas, P.; Ghosh, S. Global Transcriptomic Profiling of Schizosaccharomyces pombe in Response to Nitrosative Stress. Gene 2015, 558, 241–253. [Google Scholar] [CrossRef]
- Horan, S.; Bourges, I.; Meunier, B. Transcriptional Response to Nitrosative Stress in Saccharomyces cerevisiae. Yeast 2006, 23, 519–535. [Google Scholar] [CrossRef]
- Hromatka, B.S.; Noble, S.M.; Johnson, A.D. Transcriptional Response of Candida albicans to Nitric Oxide and the Role of the YHB1 Gene in Nitrosative Stress and Virulence. Mol. Biol. Cell 2005, 16, 4814–4826. [Google Scholar] [CrossRef]
- Sarver, A.; DeRisi, J. Fzf1p Regulates an Inducible Response to Nitrosative Stress in Saccharomyces cerevisiae. Mol. Biol. Cell 2005, 16, 4781–4791. [Google Scholar] [CrossRef]
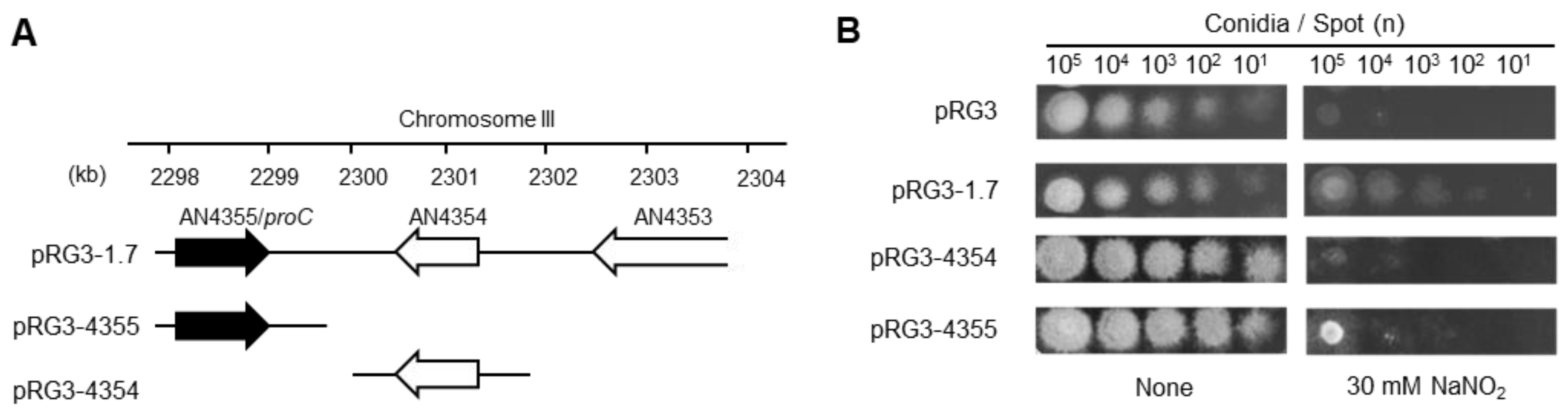
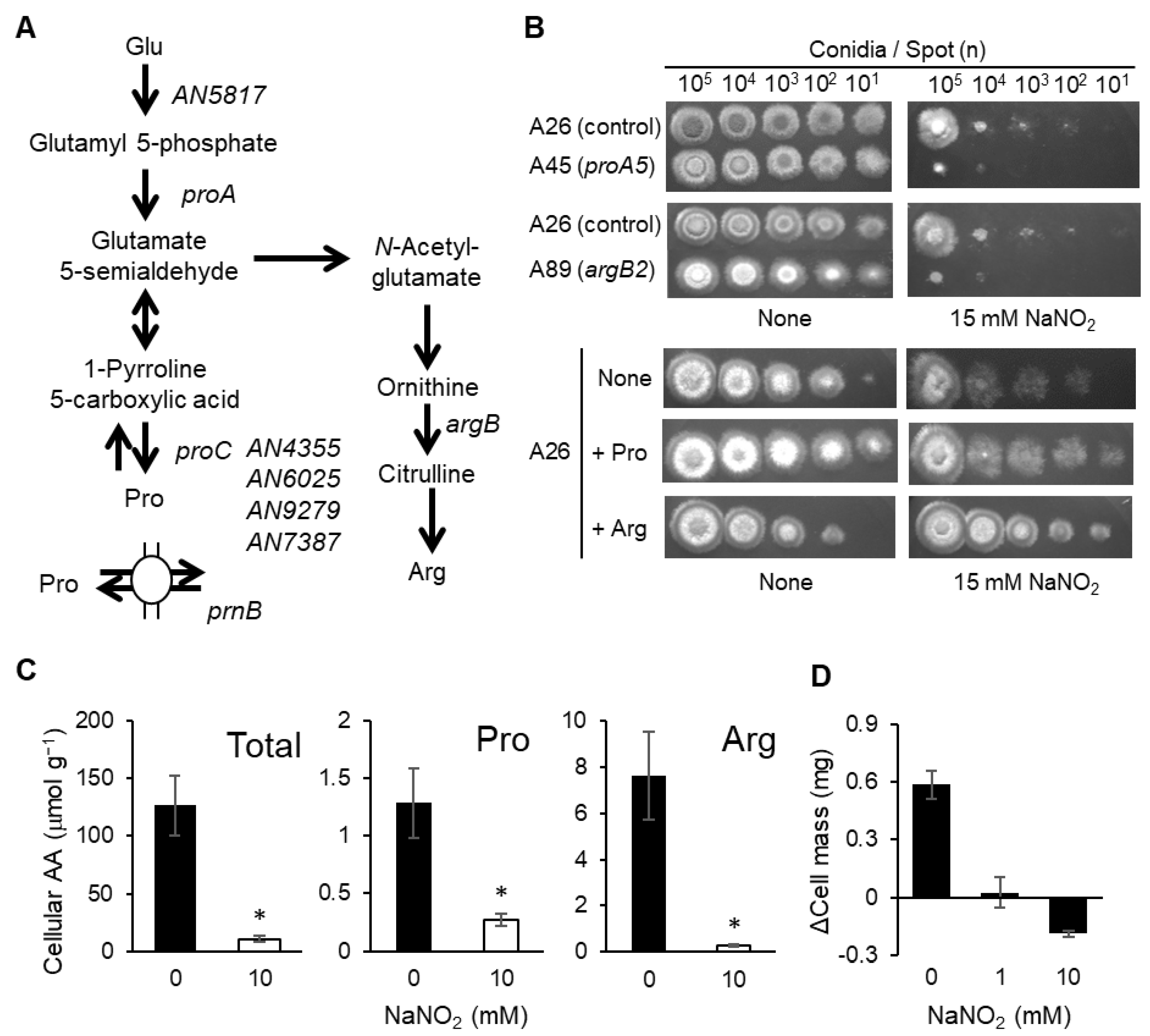
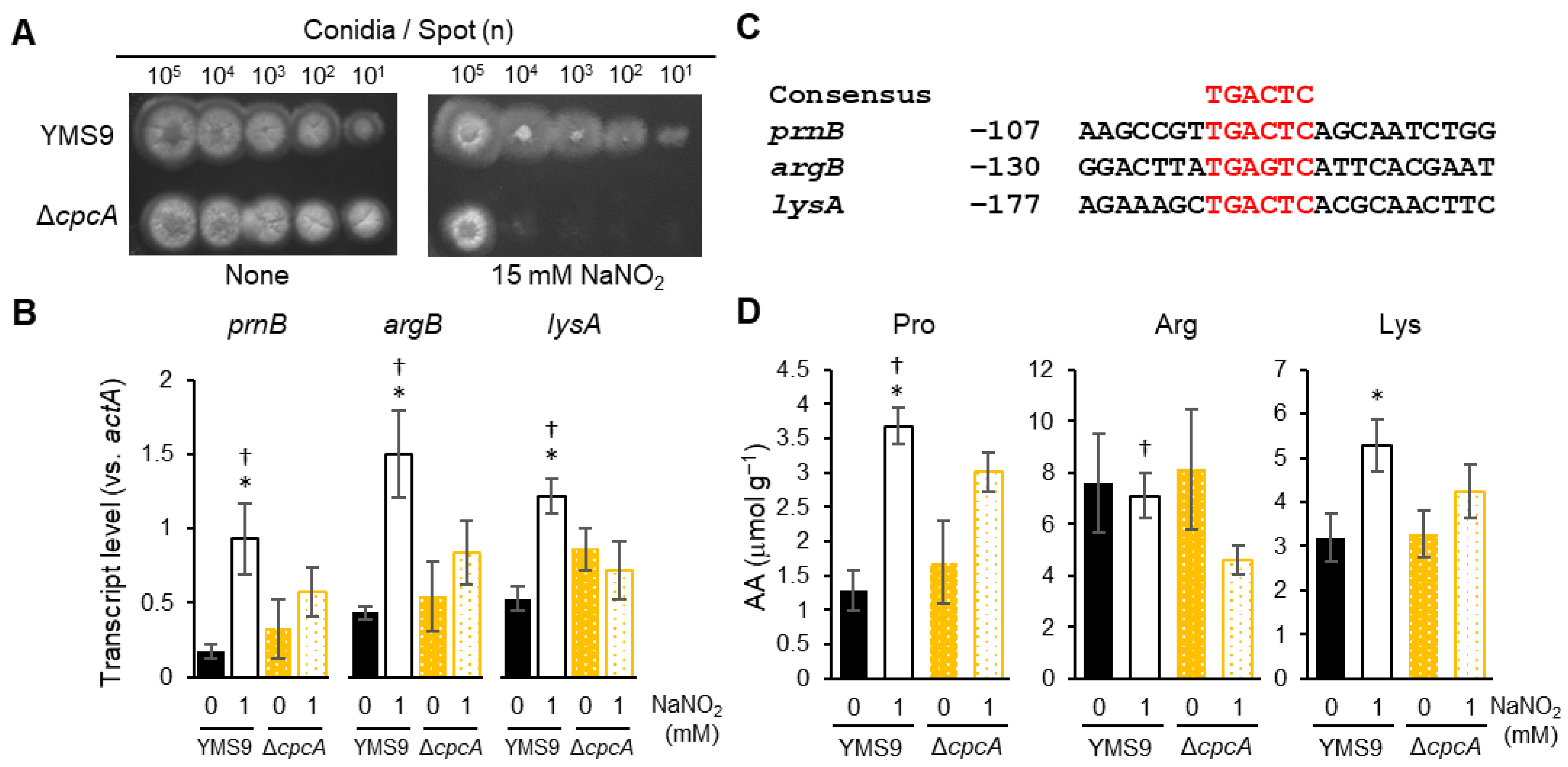
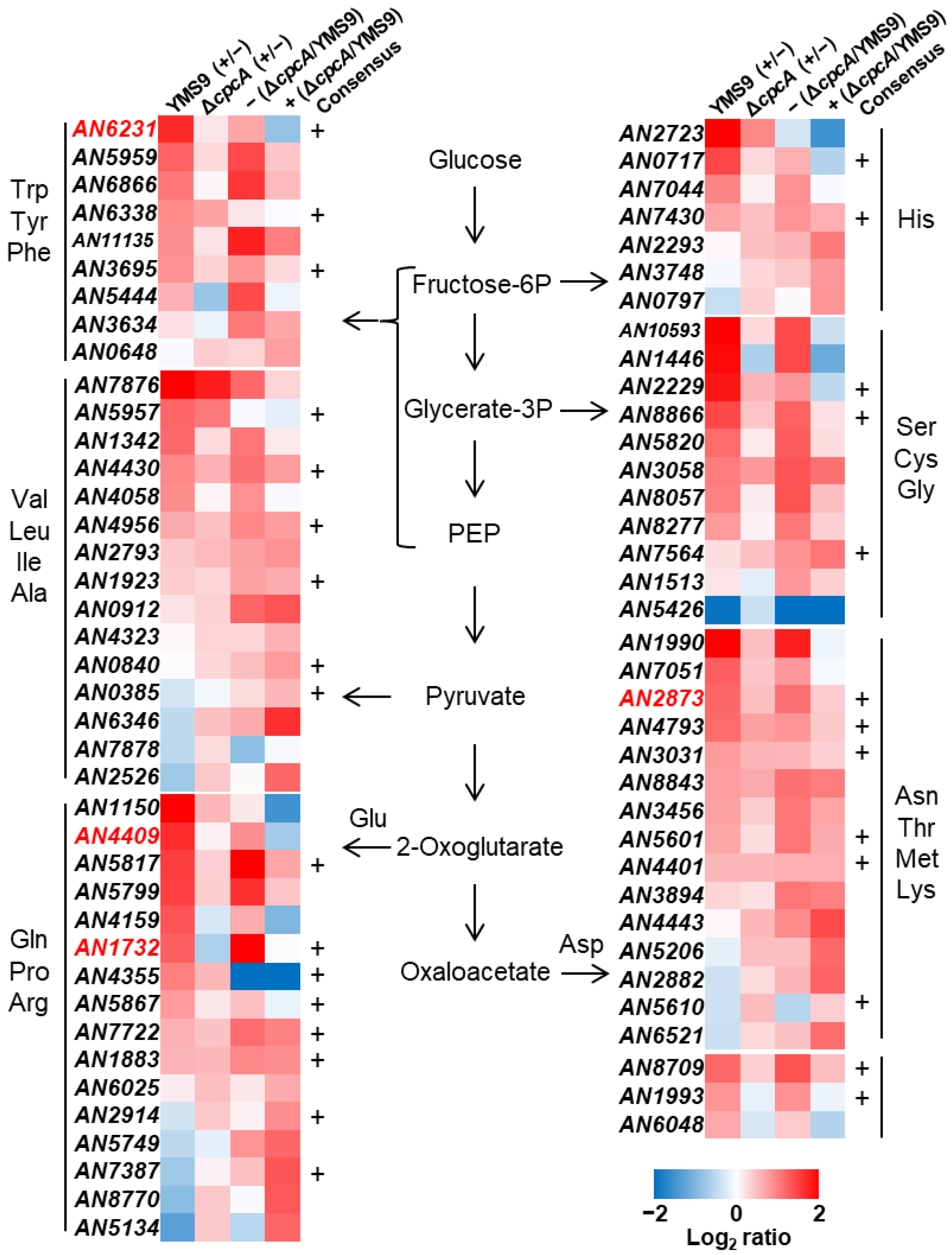
| Strain | Genotype | Source |
|---|---|---|
| YMS9 | yA2; pyrG89; pyroA4 (progeny of a meiotic cross between ABPU1 and A952) | Reference [9] |
| pRG3 | yA2; pyrG89; pyroA4; pRG3-AMA1 | This study |
| pRG3-1.7 | yA2; pyrG89; pyroA4; pRG3-AMA1.7 | This study |
| pRG3-4354 | yA2; pyrG89; pyroA4; pRG3-AMA1.7_AN4354 | This study |
| pRG3-4355 | yA2; pyrG89; pyroA4; pRG3-AMA1.7_AN4355 | This study |
| TN02A3 | pyrG89; argB2; nkuA::argB; pyroA4 | FGSC |
| ΔAN4355 | pyrG89; argB2; nkuA::argB; pyroA4; ΔAN4355::pyrG | This study |
| ΔAN6025 | pyrG89; argB2; nkuA::argB; pyroA4; ΔAN6025::pyrG | This study |
| ΔAN7387 | pyrG89; argB2; nkuA::argB; pyroA4; ΔAN7387::pyrG | This study |
| ΔAN9279 | pyrG89; argB2; nkuA::argB; pyroA4; ΔAN9279::pyrG | This study |
| A26 | biA1 | FGSC |
| A45 | biA1; proA5 | FGSC |
| A89 | biA1; argB2 | FGSC |
| ΔcpcA | yA2; pyrG89; pyroA4; ΔcpcA::pyrG | This study |
| GO ID | GO Term | n | p |
|---|---|---|---|
| With and without NO2− (pH 5.5); YMS9, log2 > 3 | |||
| GO:0055085 | Transmembrane transport | 33 | 0.001 |
| GO:0044282 | Small-molecule catabolic process | 11 | 0.04 |
| GO:0006855 | Xenobiotic transmembrane transport | 6 | <0.001 |
| GO:0042908 | Xenobiotic transport | 6 | 0.002 |
| GO:2001057 | Reactive nitrogen species metabolic process | 5 | <0.001 |
| GO:0043648 | Dicarboxylic acid metabolic process | 5 | 0.03 |
| GO:1901606 | Alpha-amino acid catabolic process | 5 | 0.04 |
| GO:0042128 | Nitrate assimilation | 4 | <0.001 |
| GO:0042126 | Nitrate metabolic process | 4 | <0.001 |
| GO:0071941 | Nitrogen cycle metabolic process | 4 | <0.001 |
| GO:0009410 | Response to xenobiotic stimuli | 3 | 0.007 |
| GO:0006536 | Glutamate metabolic process | 3 | 0.01 |
| GO:0009065 | Glutamine family amino acid catabolic process | 3 | 0.01 |
| GO:0006083 | Acetate metabolic process | 3 | 0.02 |
| GO:0015074 | DNA integration | 3 | 0.02 |
| GO:0015706 | Nitrate transport | 2 | <0.001 |
| GO:0046209 | Nitric oxide metabolic process | 2 | 0.002 |
| GO:0015707 | Nitrite transport | 2 | 0.002 |
| GO:0045807 | Positive regulation of endocytosis | 2 | 0.009 |
| GO:0045041 | Protein import into mitochondrial intermembrane spaces | 2 | 0.02 |
| GO:0071466 | Cellular response to xenobiotic stimulus | 2 | 0.02 |
| GO:0015740 | C4-dicarboxylate transport | 2 | 0.02 |
| GO:0032196 | Transposition | 2 | 0.02 |
| GO:0033609 | Oxalate metabolic process | 2 | 0.02 |
| GO:0006538 | Glutamate catabolic process | 2 | 0.02 |
| GO:0043649 | Dicarboxylic acid catabolic process | 2 | 0.03 |
| GO:0030100 | Regulation of endocytosis | 2 | 0.03 |
| GO:0043942 | Negative regulation of sexual sporulation resulting in the formation of a cellular spore | 2 | 0.05 |
| GO ID | GO Term | n | p |
|---|---|---|---|
| YMS9 vs. ΔcpcA without NO2− (pH 5.5); log2 > 2 | |||
| GO:0009058 | Biosynthetic process | 21 | 0.040 |
| GO:0044550 | Secondary metabolite biosynthetic process | 17 | <0.001 |
| GO:0019748 | Secondary metabolic process | 17 | <0.001 |
| GO:0030639 | Polyketide biosynthetic process | 4 | <0.001 |
| GO:0030638 | Polyketide metabolic process | 4 | <0.001 |
| GO:0006091 | Generation of precursor metabolites and energy | 4 | 0.044 |
| GO:0019646 | Aerobic electron transport chain | 3 | 0.002 |
| GO:0042775 | Mitochondrial ATP-synthesis-coupled electron transport | 3 | 0.002 |
| GO:0042773 | ATP-synthesis-coupled electron transport | 3 | 0.002 |
| GO:0006119 | Oxidative phosphorylation | 3 | 0.002 |
| GO:0022904 | Respiratory electron transport chain | 3 | 0.003 |
| GO:0022900 | Electron transport chain | 3 | 0.004 |
| GO:1901606 | Alpha-amino acid catabolic process | 3 | 0.01 |
| GO:0009060 | Aerobic respiration | 3 | 0.02 |
| GO:0009063 | Cellular amino acid catabolic process | 3 | 0.02 |
| GO:0045333 | Cellular respiration | 3 | 0.02 |
| GO:0046034 | ATP metabolic process | 3 | 0.02 |
| GO:0015980 | Energy derivation via the oxidation of organic compounds | 3 | 0.04 |
| GO:0006123 | Mitochondrial electron transport, cytochrome c to oxygen | 2 | 0.001 |
| GO:0009081 | Branched-chain amino acid metabolic process | 2 | 0.02 |
| YMS9 vs. ΔcpcA with NO2− (pH 5.5); log2 > 2 | |||
| GO:0055114 | Obsolete oxidation-reduction process | 52 | <0.001 |
| GO:0055085 | Transmembrane transport | 47 | <0.001 |
| GO:0019748 | Secondary metabolic process | 35 | 0.002 |
| GO:0044550 | Secondary metabolite biosynthetic process | 33 | 0.003 |
| GO:0042908 | Xenobiotic transport | 11 | <0.001 |
| GO:0006855 | Xenobiotic transmembrane transport | 9 | <0.001 |
| GO:0008645 | Hexose transmembrane transport | 6 | 0.008 |
| GO:0015749 | Monosaccharide transmembrane transport | 6 | 0.008 |
| GO:0034219 | Carbohydrate transmembrane transport | 6 | 0.009 |
| GO:0008643 | Carbohydrate transport | 6 | 0.02 |
| GO:0046323 | Glucose import | 5 | 0.02 |
| GO:1904659 | Glucose transmembrane transport | 5 | 0.02 |
| GO:0009410 | Response to xenobiotic stimuli | 4 | 0.001 |
| GO:0015698 | Inorganic anion transport | 4 | 0.02 |
| GO:0009636 | Response to toxic substances | 4 | 0.04 |
| GO:0006577 | Amino acid betaine metabolic process | 3 | 0.004 |
| GO:0043386 | Mycotoxin biosynthetic process | 3 | 0.02 |
| GO:0043385 | Mycotoxin metabolic process | 3 | 0.03 |
| GO:0006578 | Amino acid betaine biosynthetic process | 2 | 0.02 |
| GO:0006528 | Asparagine metabolic process | 2 | 0.03 |
| GO:0033609 | Oxalate metabolic process | 2 | 0.03 |
| GO:0071466 | Cellular response to xenobiotic stimuli | 2 | 0.03 |
| GO:2001308 | Gliotoxin metabolic process | 2 | 0.04 |
| GO:0015802 | Basic amino acid transport | 2 | 0.04 |
| GO:2001310 | Gliotoxin biosynthetic process | 2 | 0.04 |
| GO:0006817 | Phosphate ion transport | 2 | 0.04 |
| GO:1900554 | Asperfuranone biosynthetic process | 2 | 0.04 |
| GO:1902644 | Tertiary alcohol metabolic process | 2 | 0.04 |
| GO:1902645 | Tertiary alcohol biosynthetic process | 2 | 0.04 |
| GO:1900552 | Asperfuranone metabolic process | 2 | 0.04 |
| GO:0042727 | Favin-containing compound biosynthetic process | 2 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amahisa, M.; Tsukagoshi, M.; Kadooka, C.; Masuo, S.; Takeshita, N.; Doi, Y.; Takagi, H.; Takaya, N. The Metabolic Regulation of Amino Acid Synthesis Counteracts Reactive Nitrogen Stress via Aspergillus nidulans Cross-Pathway Control. J. Fungi 2024, 10, 58. https://doi.org/10.3390/jof10010058
Amahisa M, Tsukagoshi M, Kadooka C, Masuo S, Takeshita N, Doi Y, Takagi H, Takaya N. The Metabolic Regulation of Amino Acid Synthesis Counteracts Reactive Nitrogen Stress via Aspergillus nidulans Cross-Pathway Control. Journal of Fungi. 2024; 10(1):58. https://doi.org/10.3390/jof10010058
Chicago/Turabian StyleAmahisa, Madoka, Madoka Tsukagoshi, Chihiro Kadooka, Shunsuke Masuo, Norio Takeshita, Yuki Doi, Hiroshi Takagi, and Naoki Takaya. 2024. "The Metabolic Regulation of Amino Acid Synthesis Counteracts Reactive Nitrogen Stress via Aspergillus nidulans Cross-Pathway Control" Journal of Fungi 10, no. 1: 58. https://doi.org/10.3390/jof10010058
APA StyleAmahisa, M., Tsukagoshi, M., Kadooka, C., Masuo, S., Takeshita, N., Doi, Y., Takagi, H., & Takaya, N. (2024). The Metabolic Regulation of Amino Acid Synthesis Counteracts Reactive Nitrogen Stress via Aspergillus nidulans Cross-Pathway Control. Journal of Fungi, 10(1), 58. https://doi.org/10.3390/jof10010058






