Abstract
The therapeutic effectiveness of current neurodegenerative disease treatments is still under debate because of problems with bioavailability and a range of side effects. Fungi, which are increasingly recognized as sources of natural antioxidants and acetylcholinesterase (AChE) enzyme inhibitors, may thus serve as potent neuroprotective agents. Previous studies have associated the anti-AChE and antioxidant activities of fungi mostly with polysaccharides and phenolic compounds, while other secondary metabolites such as polyamines (PAs) have been neglected. This study aimed to investigate eight edible and medicinal fungi from Serbia, marking the initial investigation into the neuroprotective capabilities of Postia caesia, Clitocybe odora, Clitopilus prunulus, and Morchella elata. Neuroprotective activity was examined using the Ellman assay, while the antioxidant capacity was tested by conducting DPPH, NO, ABTS, and FRAP tests. PA levels were determined by high-performance liquid chromatography (HPLC) coupled with fluorescent detection. Ganoderma applanatum and Lepista nuda exhibited the most robust anti-AChE (98.05 ± 0.83% and 99.94 ± 3.10%, respectively) and antioxidant activities, attributed to the synergistic effects of the total protein, total phenolic, and PA levels. Furthermore, P. caesia displayed significant AChE inhibition (88.21 ± 4.76%), primarily linked to the elevated spermidine (SPD) (62.98 ± 3.19 mg/kg d.w.) and putrescine (PUT) levels (55.87 ± 3.16 mg/kg d.w.). Our results highlight the need for thorough research to comprehend the intricate relationships between distinct fungus species and AChE inhibition. However, it is important to recognize that more research is required to identify the precise substances causing the reported inhibitory effects.
Keywords:
polyamines; G. applanatum; L. nuda; antioxidant; phenolics; synergism; anti-acetylcholinesterase 1. Introduction
Neurodegenerative diseases (NDDs) represent a diverse group of pathological conditions characterized by the progressive and irreversible degeneration of nervous tissue, primarily affecting the elderly. Despite the clinical heterogeneity of neurological diseases, such as Alzheimer’s (AD) and Parkison’s, elevated levels of oxidative stress have been consistently identified as a prominent hallmark in their pathogenesis [1,2,3]. A demographic projection that the global population aged over 60 will surpass 2 billion within the next 30 years underscores the challenge posed by the absence of effective treatments for NDDs, as the increasing elderly population intensifies the socioeconomic impact of these conditions [2]. When it comes to AD, an association of the degeneration of cholinergic and dopaminergic neurons with low levels of acetylcholine led to the creation of the “cholinergic hypothesis”, where inhibitors of the acetylcholinesterase (AChE) enzyme have been used to raise the level of acetylcholine [4]. However, despite animal models consistently showing increased brain acetylcholine and improved cognitive deficits in AD, the clinical effectiveness of AChE inhibitors, including natural alkaloids huperzine A and galantamine, remains a subject of debate due to challenges related to bioavailability and various side effects [3,4,5].
As a result, fungi have gained recognition as sources of non-alkaloid agents that inhibit the AChE enzyme, and they are also acknowledged for being organisms that naturally contain antioxidants [3]. Nevertheless, edible mushrooms are considered nutritionally valuable since they are low in calories (with low fat content) and rich in minerals (especially potassium), essential amino acids, vitamins (including provitamin D2 and vitamin B12), and fiber [6]. Additionally, mushrooms contain various natural compounds with diverse positive health effects and medicinal benefits, including positive impacts on brain health and cognition, weight management, oral health, constipation, and diabetes [7]. Many of the immunomodulatory effects associated with mushrooms are linked to polysaccharides, specifically β-glucans or polysaccharide–protein complexes [3], while a portion of their therapeutic properties is related to their ability to inhibit oxidative stress [7] which is attributed to the presence of phenolics [3,8,9,10] and bioactive amines [6,11,12].
Polyamines (PAs) represent a ubiquitous category of small, positively charged polycations within the broader spectrum of biogenic amines [13,14]. They play a crucial role in various physiological processes, including but not limited to cell growth, gene regulation, autophagy, differentiation, proliferation, the modulation of ion channels, the regulation of inflammation, oxidative stress management, and immune responses [1,13,15]. Triamine spermidine (SPD), tetraamine spermine (SPM), and diamine precursor putrescine (PUT) are the most common natural amines found extensively in all cells, but variations in PA concentrations have been observed in fruits, vegetables, and animal-derived food products [2]. The levels of individual PA levels depend on the coordination of biosynthesis, degradation, uptake, and excretion reactions, since they are highly interconvertible, and deviations from the narrow concentration range can lead to significant physiological and metabolic disturbances [14]. A substantial decrease in PA levels can impede cell proliferation and migration, while conversely, an excess of polyamines leads to apoptosis and cell transformation [13]. PA degradation is a prooxidative process, since it leads to the generation of reactive aldehydes and reactive oxygen species (ROS) which contributes to increased oxidative stress, partly attributed to the reduction in PA levels, while, on the other hand, PAs are potent antioxidants and ROS scavengers [14].
Nevertheless, the decrease in PA levels with age has been a long-recognized phenomenon, which leads to the age-related impairment of cognitive and other behavioral reactions [1]. This observation prompts the hypothesis that preserving SPD levels during the aging process may contribute to an extension of longevity and that PA levels can be increased through supplementation in food or water [16,17].
Examples of food and beverages rich in PAs include rice bran, green pepper, broccoli, soybeans, mushrooms, oranges, and green tea, which opens avenues for exploring the potential correlation between PA intake and the aging process, providing a practical approach to studying their effects on longevity and antioxidant activity as well [1,15,16,17]. Studies have demonstrated that SPM, SPD, and PUT effectively act as free radical scavengers, bind to toxic aldehydes, and showcase chelating abilities toward metal ions, while SPM and SPD exhibit the capacity to modify the activity of antioxidant enzymes through their chaperone-like activity and through the alternation of gene expression, contributing to the modulation of oxidative stress-triggering mechanisms [1,11,18,19]. Given these critical roles, there is currently a special emphasis on antioxidant PAs and phenolic compounds as well as polyamines conjugated with phenolics such as phenolamides in nutrition science and, preferably, those secondary metabolites that can prevent harmful environmental and dietary effects, while amidst the myriad sources of these intriguing molecules, edible and medicinal fungi emerge as particularly promising reservoirs.
The advantageous effects of fungal polyphenols are mainly connected to their antioxidant attributes, whereas carbohydrates obtained from fungi exhibit immunomodulatory activity, along with various other biological activities. Nevertheless, emerging studies indicate that these compounds might also contribute to promoting neuroprotection [8,20,21,22]. Generally, previous findings have emphasized the potential of phenolic acids from fungi—particularly caffeic, cinnamic, and p-hydroxybenzoic acids—as effective antioxidants and inhibitors of AChE [3,8,22,23], while the effects of other secondary metabolites (e.g., polyamines, terpenes, etc.) on the mentioned activities are largely neglected.
There are limited data on the PA contents in edible mushrooms, with an emphasis on cultivated mushrooms such as Agaricus bisporus, Flammulina velutipes, Lentinula edodes, Grifola frondosa, Hericium erinaceus, Pleurotus ostreatus, Volvariella volvacea, etc. [6,24]. The predominant PAs found in fungi are PUT, SPD, and SPM; however, SPM is not universally present in all fungi [14].
Since the prevalence of neurodegenerative disorders continues to rise globally, the identification and characterization of novel neuroprotective agents from natural sources holds significant promise for the development of innovative therapeutic strategies.
This study is focused on exploring the occurrence and quantification of main polyamines and its correlation to the antioxidant and neuroprotective activities of eight edible and medicinal fungi from Serbia. Hence, this pioneering report is aimed at unravelling the complex interaction between PAs, phenolics, neuroprotection, and antioxidant activity in autochthonous wild fungal species (Lepista nuda, Clitocybe odora, Clitopilus prunulus, Cyclocybe aegerita, Lepista nuda, Morchella elata, Ganoderma applanatum, and Ganoderma resinaceum), shedding light on their potential role in mitigating neurodegenerative processes.
2. Materials and Methods
2.1. Fungal Material
Fruiting bodies of eight selected fungal species were collected at full maturity from different regions of Serbia and were identified based on their microscopic and macroscopic characteristics by Dr. Eleonora Čapelja. They were stored and deposited at the ProFungi Laboratory, Department of Biology and Ecology, University of Novi Sad (Serbia), under the numbers presented in Table 1.

Table 1.
Analyzed fungal species, localities, sampling dates, and voucher numbers.
The fruiting bodies of the selected fungal species were sliced into small fragments and subsequently subjected to hot-air drying in an oven (Universal oven UF55—Memmert GmbH + Co. KG, Schwabach, Germany) at 45 °C for 24 h prior to additional analysis. All samples were kept in a light-protected storage within paper bags.
2.2. Polyamine Analysis
Fungal tissues, approximately 30 mg of lyophilized material (dry weight—d.w.), underwent extraction using a solution of 4% perchloric acid (v/v) in a volume ten times that of the sample. The resultant homogenate was cooled on ice for 1 h and then centrifuged at 15,000× g for 30 min. Following this, supernatant samples, along with standard solutions containing PUT, SPD, and SPM, were subjected to dansylation treatment as per the method outlined by Scaramagli et al. [25]. The resulting dansylated derivatives were extracted using toluene and were dried and reconstituted in acetonitrile. The separation and quantification of polyamines (PAs) were conducted using high-performance liquid chromatography (HPLC) coupled with fluorescent detection (Nexera XR, Shimadzu, Kyoto, Japan) employing a reverse-phase C18 column (Spherisorb ODS, 2.5-μm particle diameter, 4.6 × 250 mm, Waters, Wexford, Ireland). The following five steps of the programmed acetonitrile:water (v/v) gradient were applied: 60 to 70% of acetonitrile in 5.5 min, 70 to 80% in 1.5 min, 80 to 100% in 2 min, 100 to 100% in 2 min, 100 to 70% in 2 min, and 70 to 60% in 2 min at a flow rate of 1.5 mL/min. Eluted compounds were identified using a prominence fluorescence detector RF-20A (365 nm excitation, 510 nm emission). A post-run program Lab Solutions was used to integrate the areas of the peaks originated from dansylated polyamines. The results of PA quantification were expressed as mg per kg of dry weight (mg/kg d.w.).
2.3. Carbon and Nitrogen Elemental Analysis
Tiny tin capsules, each containing approximately 25 mg of each fungal sample in powdered form, were incinerated in the combustion box of an elemental analyzer (model Elementar Vario EL III, Hanau, Germany) for approximately 2 min at 900 °C. During this process, carbon and nitrogen from the samples were transformed into CO2 and N2, respectively. Analytical columns were employed to separate the resulting gasses, and their quantification relative to the standard was achieved through variations in the thermal conductivity detector (TCD). Acetanilide (with a known C content of 71.09% and an N content of 10.36%) served as the standard, and the percentages of carbon and nitrogen in the samples were automatically computed according to the method of Karthikeyan and Kumaravel [26]. Furthermore, the total protein content (TP) was determined by calculating it from the N content.
2.4. Measurement of Total Phenolic and Total Flavonoid Contents and Assessment of Antioxidant Capacity
2.4.1. Extract Preparation
All experiments were conducted using 80% methanol (MeOH) extracts of fungal species. To prepare the extracts, 50 mg of powdered samples from dried basidioms were macerated with 2 mL of 80% MeOH using a magnetic stirrer (MS-3000 High speed magnetic stirrer, Biosan, Riga, Latvia) for 20 min. The mixture was then subjected to centrifugation at 4 °C for 20 min (Microcentrifuge 5424 R, Eppendorf, Germany). The resulting extracts were further analyzed, and the filtrate was stored at +4 °C, reaching a final concentration of 25 mg/mL.
2.4.2. The Total Phenolic Content
The total phenolic content (TPC) was determined using the Folin–Ciocalteu (FC) reagent following the method outlined by Singleton et al. [27]. In brief, 30 µL of the MeOH extract or a standard (gallic acid, 0.625–80.0 µg/mL) was combined with 150 µL of a 0.1 M FC reagent, followed by a 10 min incubation. Subsequently, 120 µL of 0.7 M sodium carbonate was added, and absorbance was measured after 2 h at 720 nm (Spectrophotometer—MultiScan Go, ThermoScientific, Waltham, MA, USA). All tests were conducted in triplicate, and the TPC was expressed as milligrams of gallic acid equivalents per gram of dry weight (mg GAE/g d.w.).
2.4.3. The Total Flavonoid Content
The total flavonoid content (TFC) was carried out through a colorimetric method, as described by Chang et al. [28]. In summary, 30 µL of the MeOH extract or a standard (quercetin, 0.625–80.0 µg/mL) was mixed with 6 µL of 10% aluminum chloride, 6 µL of 1 M sodium acetate, 90 µL of methanol, and 170 µL of distilled water. Absorbance was measured after 30 min at 415 nm. The tests were performed in triplicate, and the results were expressed as milligrams of quercetin equivalents per gram of dry weight (mg QE/g d.w.).
2.4.4. Antioxidant Capacity
- DPPH assay: The ability of the extracts to neutralize the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical was assessed following the procedure outlined by Espín et al. [29] with slight modifications. In summary, 10 µL of the sample was combined with 100 µL of a 90 µM DPPH solution in MeOH and 190 µL of MeOH. The absorbance was recorded following a 30 min incubation period in a dark place at 515 nm.
- ABTS assay: This assay involves spectrophotometric monitoring of the conversion of the blue-green colored cation radical ABTS•+ into its neutral, colorless form and was conducted following the method described by Arnao et al. [30]. ABTS•+ was generated by directly reacting a 7 mM ABTS solution with 2.45 mM of K2S2O8. Subsequently, 10 µL of the fungal extracts was added to 290 µL of an ABTS solution and was mixed. The absorbance of the sample was read at 734 nm after 5 min of incubation at room temperature.
- NO assay: The inhibition of the nitric oxide radical (NO•) was evaluated using the Griess diazotization process, as outlined in the methodology developed by Green et al. [31]. The reaction mixture consisted of 15 µL of the extract, 250 µL of 10 mmol/L of sodium nitroprusside and 250 µL of a phosphate buffer (pH 7.4). After incubation for 90 min at room temperature under constant light, 500 µL of a Griess reagent (a combination of a 0.2% solution of N-(1-naphthyl)-ethylenediamine dihydrochloride and a 2% solution of sulfanilamide in 4% phosphoric acid) was added. The degree of inhibition was gauged by quantifying the absorbance of the resultant chromophore at 546 nm.
- FRAP assay: This assay was carried out according to Benzie and Strain [32]. The freshly prepared FRAP (Ferric Reducing Antioxidant Power) reagent comprises a solution containing 10 mmol/L of TPTZ in 40 mmol/L of HCl, 0.02 mmol/L of FeCl3 × 6H2O, and an acetate buffer (pH 3.6) in a ratio of 10:1:1. Briefly, 10 µL of each extract was combined with 225 µL of the FRAP reagent and 22.5 µL of distilled water (dH2O). Absorbance was measured after 6 min at 593 nm.
The radical scavenging capacity (RSC) against DPPH, ABTS, and NO radicals, as well as the reduction potential in the FRAP assay, were evaluated by establishing a standard curve of Trolox. The outcomes were quantified and presented as millimoles of Trolox equivalents per gram of dry fungal material, either fresh or dry weight (mmol TE/g d.w.), depending on the particular extract utilized in the assay.
2.5. Neuroprotective Activity
The neuroprotective activity of the extracts was evaluated by assessing their ability to inhibit the AChE enzyme using Ellman’s method [33]. In brief, 20 µL of AChE (0.5 U/mL) was combined with 110 µL of a 20 mM Tris-HCl buffer at pH 8, along with 10 µL of the extract. In the blank sample, AChE was substituted with the 20 mM Tris-HCl buffer at pH 7.5, and in the control, the 20 mM Tris-HCl buffer at pH 8 was added instead of the sample. The 96-well plate was then incubated for 15 min at 37 °C with continuous shaking. Following incubation, 40 µL of a 3 mM solution of 5,5′-dithio-bis(2-nitrobenzoic acid) and 20 µL of 15 mM acetylthiocholine iodide were added to the plate. The absorbance at 412 nm with a total of 15 measurements was taken at 1 min intervals. The percentage (%) of enzyme inhibition was calculated using the equation detailed in Mišković et al. [3]. The reference inhibition time was set at 10 min or 600 s, respectively.
2.6. Statistical Analysis
This study employed a variety of statistical methods, encompassing descriptive statistics, a one-way Analysis of Variance (ANOVA) with Tukey post hoc tests, a Principal Component Analysis (PCA), dendrogram hierarchical clustering, as well as a Pearson correlation analysis. In the context of one-way ANOVA, the research investigated the differentiation among analyzed fungi species, assessing the statistical significance of these distinctions using the Fisher (F) test and presenting the results as “p-values”. All statistical data processing was conducted using the R programming environment. Descriptive statistics, two-way ANOVA, and t-tests were executed with the “rstatix” R package [34]. Dendrogram clustering utilized the “dendextend” R package [35], while diverse data visualizations were generated with the “ggplot2” R package [36].
3. Results and Discussion
3.1. Mycochemical Analysis
The mycochemical analysis of eight fungal species included the assessment of PA content, TP, TPC, and TFC. For the majority of the analyzed species, with the exception of the Ganoderma species, there is only a limited amount of available data, making it challenging to compare results due to differences in the methodological approach.
3.1.1. Polyamine Content
The results of the HPLC analysis of the selected PAs—PUT, SPD, and SPM—in eight fungal species are presented in Figure 1 and represent the first report of PA content in these species.
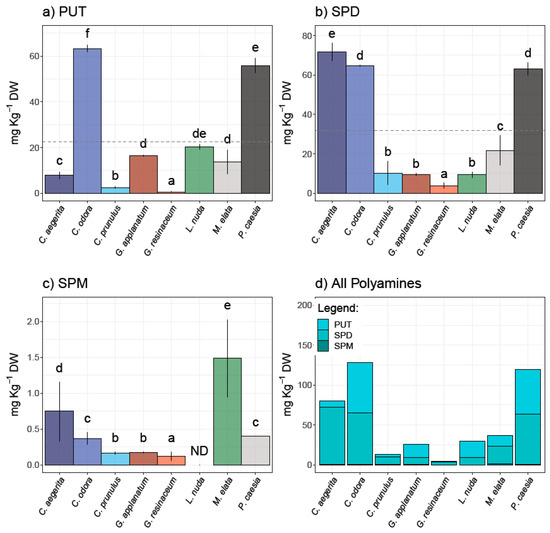
Figure 1.
Polyamine content in analyzed fungal species—(a) putrescine (PUT), (b) spermidine (SPD), and (c) spermine (SPM)—as well as (d) total PAs. Different small letters indicate significant differences among different species; Tukey’s significant difference (HSD) post hoc test (p ≤ 0.05). Data represent the mean ± standard deviation (SD).
PUT and SPD were quantified in all analyzed samples, while SPM was not detected in L. nuda. It is important to note that the predominant PA was SPD in a range from 3.86 to 71.58 mg/kg d.w. (Figure 1). The highest contents of PUT and SPD were observed in C. odora (63.30 ± 1.60 and 64.64 ± 0.29 mg/kg d.w., respectively) and P. caesia (55.87 ± 3.16 and 62.98 ± 3.19 mg/kg d.w., respectively), while C. aegerita contained the highest amount of SPD (71.58 ± 6.47 mg/kg d.w.). On the other hand, SPM was predominant in M. elata (1.49 ± 0.14 mg/kg d.w.), followed by C. aegerita (0.75 ± 0.42 mg/kg d.w.). The highest content of total polyamines in the inspected fungi was recorded in the following order: C. odora > P. caesia > C. aegerita > M. elata > L. nuda > G. applanatum > C. prunulus > G. resinaceum. In contrast to plants, the significance of PA content in fungi is still not fully recognized, although it is known that PAs affect fungal growth and development through the entire fungal life cycle including the sporulation, growth, development, and modulation of host interactions [14]. To the best of our knowledge, PA content was not determined for species investigated in this study. However, the quantification of PAs is mostly conducted in cultivated species, including Agaricus bisporus, Lentinula edodes, and Pleurotus spp. For example, Dadakova et al. [24] summarized the PA content in thirteen cultivated mushrooms, where the most prevalent PA was SPD (22.2–195.0 mg/kg wet weight and 157–522 mg/kg d.w., respectively), followed by PUT (0.9–90.6 mg/kg w.w. and 3.8–59.4 mg/kg d.w.), while SPM was not detected in all species, which is in accordance with the results of this study. A decade later, Reis et al. [6] quantified only SPD in fresh, cooked, and canned mushroom A. bisporus (6.4–8.5 mg/100 g). On the other hand, there is scarce information regarding the PA content in wild-growing mushrooms [12,24]. Dadakova et al. [24] analyzed the content of six biogenic amines, including three Pas in seventeen wild-growing edible mushrooms from Poland, where Boletus erythropus contained the highest amount of SPD (2219 mg/kg d.w.). For instance, wild-growing mushrooms in Turkey have been documented as significant sources of SPD, while in Xerocomus badius, X. chrysentereon, and Suillus variegatus, PUT was the most prevailing amine [12,24].
3.1.2. Total Protein Content
Table 2, among others, presents the results of TP, while the results of the CHN analysis are provided in Table S1. The TP content, calculated based on nitrogen (N) content, was the highest in L. nuda, C. prunulus, and C. odora (47.40, 46.70, and 39.70%, respectively). The TP content in C. odora was twice as high as that found in C. odora from Portugal (TP = 17.33 ± 1.37%) according to Vaz et al. [37], which was also the case with G. resinaceum from Serbia as well where TP was 4.7% [38]. A similar trend was noted in C. prunulus, C. aegerita, and L. nuda, where the species from other studies exhibited more than a 50% lower TP content (18.13 ± 0.37%, 6.68 ± 0.26%, and 8.11–12.18%, respectively) [39,40,41,42], while the TP content of L. nuda from West Macedonia and Epirus was in accordance with our results [43].

Table 2.
Mean values with standard deviation and Tukey post hoc test classification of antioxidant profile and total flavonoid (TFC), total protein (TP), and total phenolic content (TPC) among analyzed fungal species.
3.1.3. Total Phenolic and Total Flavonoid Contents
C. prunulus and G. applanatum exhibited the highest TPC values (49.02 ± 0.59 and 45.75 ± 5.36 mg GAE/g d.w., respectively), followed by M. elata, L. nuda, and C. aegerita (Table 2). G. applanatum also displayed the highest TFC content (7.02 ± 0.89 mg QE/g d.w.). In contrast, P. caesia had the lowest TPC, with no detectable TFC. The TPC of commercial and wild samples from pine and oak forests of L. nuda MeOH extracts from Portugal was significantly lower compared to the results of this study (15.98–20.54 mg GAE/g d.w.) [40]. The obtained TPC in a C. aegerita extract was also greater compared to other samples from Serbia, where EtOH (17.36 ± 0.88 mg GAE/g d.w.) and MeOH (13.80 ± 0.21 mg GAE/g d.w.) extracts were analyzed [41,44]. The most impressive difference in TPC was observed in M. elata, since the MeOH extract from Australia exhibited 86 times lower content (0.46 ± 0.01 mg GAE/g d.w.) [45]. Furthermore, it is noteworthy that the TFC of the C. aegerita MeOH extract from other parts of Serbia [44] was quantified at a very low level (0.73 ± 0.37 mg QE/g d.w.), contrary to the findings in this study. Conversely, the TPC and TFC in MeOH extracts of C. odora from Turkey [46] were significantly higher (TP: 82.646 ± 1.623 mg/g; TFC: 117.753 ± 3.491 mg/g) compared to the EtOH extract from Serbia (38.112 ± 0.251 mg/g) [47]. On the other hand, the TPC and TFC content of the Ganoderma species are in accordance with the analyzed MeOH extracts from Turkey [48], while compared to our previously published data, EtOH extracts had a higher content of phenolics and flavonoids [8,9,49]. All of this further indicates that the mycochemical composition of fruiting bodies is significantly influenced by their growth habitat and the type of extraction solvent polarity [3,40]. The mycelia of G. applanatum from Poland contained a significantly lower TPC in contrast to G. resinaceum, where the TPC was in accordance with our results [22]. It is important to highlight that this study represents the first report of TFC content in C. prunulus, L. nuda, and M. elata.
3.2. Antioxidant Potential
All tested fungal species exhibited antioxidant activity, as shown in Table 2. The most notable variations in the strength of antioxidant activity among the analyzed species were observed in the ABTS, FRAP, and DPPH assays. Conversely, no differences were detected in the NO assay. However, it is important to note that the activity of extracts in this assay showed a significant correlation with both primary and secondary metabolites (Figure 2).
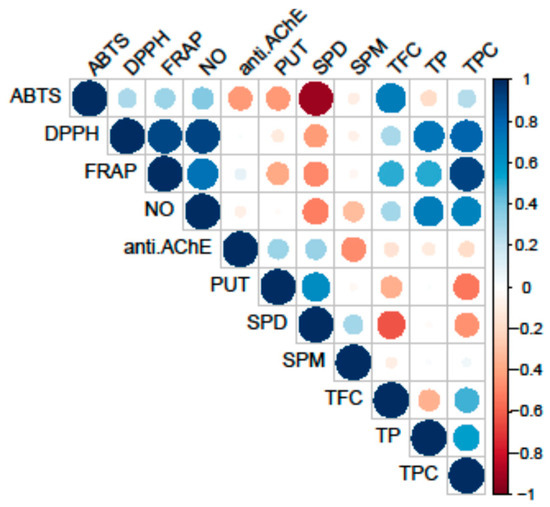
Figure 2.
Pearson’s coefficient of the correlation matrix of the examined parameters in eight selected fungal species collected on the territory of the Republic of Serbia. Blue squares represent a highly significant correlation of inspected parameters, while red squares present low interactions, assessed according to the corresponding Pearson’s coefficient. The following are the abbreviations of the examined parameters: ABTS—radical scavenger capacity against 2,2′-azinobis(3-ethylbenzothiozoline)-6-sulfonic acid, ABTS•+; DPPH—radical scavenger capacity against 2,2-diphenyl-1-picrylhydrazyl radical, DPPH•; FRAP—ferric-reducing antioxidant power; NO—radical scavenger capacity against NO radical; anti.AChE—anti-acetylcholinesterase activity; PUT—putrescine; SPD—spermidine; SPM—spermine; TFC—total flavonoid content; TP—total protein content; TPC—total phenolic content.
Conversely, PAs did not correlate with the analyzed antioxidant activity. The highest correlation was observed between the reduction potential (FRAP assay) and TPC, while the scavenging potential for the DPPH and NO radicals demonstrated significant correlations both with the TPC and TP.
The observed antioxidant activities among the tested fungal species reveal notable variations, with G. applanatum and L. nuda emerging as the most potent contributors to antioxidative potential. This outcome is attributed to their high scavenging ability and impressive reduction potential, as outlined in Table 2. The strongest ability to capture the DPPH radical was observed in the following order: L. nuda > C. odora > C. prunulus > M. elata > G. applanatum (Table 2). G. applanatum, G. resinaceum, M. elata, and L. nuda showed the highest neutralization of the ABTS radical (70.42 ± 2.60, 69.70 ± 2.54, 68.88 ± 1.13, and 60.93 ± 4.72 mg TE/g d.w., respectively), while the most potent reduction potential was demonstrated by G. applanatum and L. nuda (19.24 ± 1.77 and 18.32 ± 2.89 mg TE/g d.w., respectively). In contrast, the lowest reduction potential, together with the DPPH and ABTS scavenging ability, was observed in P. caesia and C. aegerita (Table 2). Also, it is important to note that G. resinaceum showed low antioxidant activity, with the exception of the ABTS radical neutralization (69.70 ± 2.54 mg TE/g d.w.).
Notably, the antioxidant activities of the Ganoderma species observed in our study align with several previous investigations. For instance, Zengin et al. [48] reported a higher DPPH and FRAP potential in MeOH extracts of G. applanatum and G. resinaceum from Turkey, attributing this to its rich content of phenolic compounds, while the ABTS scavenging ability was weaker compared to our results (14.85 ± 1.31 and 41.32 ± 0.39 mg TE/g extract, respectively). The same trend is observed when comparing the results of this study to the antioxidant study of these two Ganoderma species from Poland, where MeOH was also used as the extraction solvent [22]. Similarly, the robust antioxidative performance of G. applanatum from India and Kenya, especially in DPPH radical scavenging and reduction potential, has been corroborated by studies conducted by Rajoriya et al. [50] and Siangu et al. [51]. On the contrary, the cultivated mycelia of G. applanatum extracted with EtOH had a weaker DPPH activity (20.35% at the concentration of 20 mg/mL), compared to the scavenging ability observed here [52]. Nevertheless, in our previous investigations, EtOH extracts obtained from the Ganoderma species native to Serbia exhibited a markedly greater antioxidant capacity [8,9,49]. A similar pattern was noted in G. applanatum sourced from China, wherein EtOH extracts demonstrated notable antioxidant activity, achieving 91.76% DPPH inhibition (IC50 = 0.05642 mg/mL) and 100% ABTS activity (IC50 = 0.01962 mg/mL) [53]. This variance could be ascribed to differences in solvent polarity and variations in the experimental protocols employed, since it is well known that solvent polarity affects phenolic content and, consequently, impacts antioxidant activity [54].
Our study reveals the significant antioxidant potential in L. nuda MeOH extracts, but the lack of extensive comparative data on L. nuda’s antioxidant activity makes it challenging to contextualize our findings within the broader scientific landscape. Pinto et al. [40] compared the antioxidant activity of the commercial samples of a fruiting body and the in vitro cultured mycelia of L. nuda with wild samples of a fruiting body. Their results revealed that the iMMN culture medium allowed for the highest antioxidant potential, followed by the commercial fruiting bodies, while the wild types showed a lower activity and TPC [40]. When compared to our results, it can be observed that the DPPH activity of the wild type of this fungal species from Portugal was higher (EC50 = 15.48 ± 0.23 mg/mL) [40], while the EtOH extract of L. nuda from Turkey showed a lower DPPH activity with a range of 2.79% to 50.20% inhibition [55]. The observed antioxidant activity suggests that L. nuda may hold promise as a natural source of antioxidants, but caution is warranted in drawing definitive conclusions without a more extensive comparative framework.
C. odora demonstrated a pronounced neutralization of the DPPH radicals, showcasing notable antioxidant activity, comparable to the results of the MeOH extracts from Turkey, where a high DPPH scavenging ability was noticed (73.38 ± 1.60% at the concentration of 2 mg/mL) [46]. Moreover, Vaz et al. [37] performed two different extraction methods to obtain extracts of C. odora from Portugal with high molecular weight compounds, such as polysaccharides, and low molecular weight compounds, such as phenolic compounds. Their results demonstrated a twice-as-high DPPH activity of a water-soluble polysaccharide extract (EC50 = 3.56 ± 0.13 mg/mL) compared to the tested EtOH extract (EC50 = 6.77 ± 0.05 mg/mL), which was in accordance with the extraction yield, since EtOH fractions were lower compared to water soluble ones [37]. In contrast, the EtOH extract from a wild sample gathered in the Niš region of Serbia displayed comparatively lower antioxidant activity, likely attributed to the reduced levels of antioxidant components present in the extract [47]. This further indicates the importance of fungal geographical origin and the polarity of the solvent for the extraction yield of bioactive compounds and, consequently, the investigated bioactivity.
C. prunulus and M. elata also demonstrated notable antioxidant activity, and, to the best of our knowledge, this is the first report on the antioxidant activity of these species from Serbia and the Balkan region in general. In the literature data, there is only one available study of C. prunulus from Portugal, where a moderate DPPH scavenging ability was observed, owing to the lower content of TPC, while the highest level of ascorbic acid was detected [39]. However, compared to our results, a sample from Portugal exhibited higher DPPH activity and reduction potential with EC50 values of 1.75 ± 0.13 mg/mL and 3.36 ± 0.03 mg/mL, respectively [39]. Also, the ethyl acetate and MeOH extracts of M. elata from India showed a higher DPPH, NO, and ABTS neutralization compared to the results of this study [56], while Kalyoncu et al. [57] reported a lower antioxidant activity of M. elata from Turkey, with 59.22% of DPPH inhibition. The reduction potential of this species collected in Australia was also significantly higher (63.0 ± 0.3 mmol Fe[II]-E/g extract) [45], supporting the notion that habitat ecology, the extraction procedure, and solvent polarity may affect antioxidant activity.
Conversely, the comparatively lower antioxidant activity observed in the C. aegerita in our study is consistent with the findings of Karaman et al. [44], where a low DPPH activity and reduction potential were detected (45.3 ± 0.35 mg TE/g d.w. and 10.74 ± 0.09 mg TE/g d.w., respectively), while Petrović et al. [41] reported a higher DPPH activity of an MeOH extract (EC50 = 7.23 ± 0.18 mg/mL). Also, higher DPPH and NO scavenging abilities were demonstrated for the EtOH extract of C. aegerita from India at the same concentration tested—20 mg/mL (85.63 ± 0.12% and 82.02 ± 0.12%, respectively) [58]. Concerning the P. caesia extract, the notably low antioxidant activity may be linked to its very low TPC. It is crucial to underscore the pioneering nature of this research, as there is currently no existing literature data regarding the antioxidant activity of this particular species. Hence, the investigation into the antioxidant activity of P. caesia has provided valuable insights, although the scarcity of comparable literature on this specific mushroom presents both a challenge and an opportunity for further research.
All these observations align with the concept that the antioxidant capabilities can vary significantly across distinct fungal taxa.
3.3. AChE Inhibitory Potential
Among the eight different fungal extracts, only three did not exhibit anti-AChE activity (Figure 3).
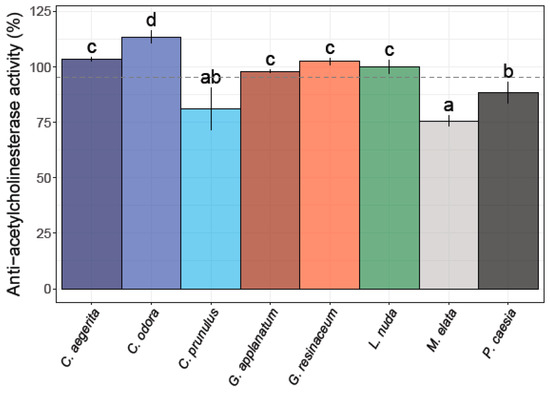
Figure 3.
Anti-acetylcholinesterase activity of the eight analyzed fungal species. Different small letters indicate significant differences among different species; Tukey’s significant difference (HSD) post hoc test (p ≤ 0.05). Data represent the mean ± standard deviation (SD).
The strongest potency for the inhibition of the AChE enzyme was observed for L. nuda (99.94 ± 3.10%), G. applanatum (98.05 ± 0.83%), and P. caesia (88.21 ± 4.76%), which is higher compared to the percent of inhibition of the positive control (donepezil) (87.44%). M. elata and C. prunulus exhibited a moderate level of anti-AChE activity, whereas C. odora, C. aegerita, and G. resinaceum demonstrated an inhibition exceeding 100% across the tested concentration range (Figure 3).
The observed high anti-AChE activity of L. nuda, G. applanatum, and P. caesia suggests that these species may contain bioactive compounds capable of interfering with the AchE enzyme, which is implicated in neurodegenerative disorders such as Alzheimer’s disease. Moreover, various species of Ganoderma, especially G. lucidum, have been reported to possess neuroprotective properties and exhibit cholinesterase inhibitory activity [8,22,59]. Tel-Cayan et al. [60] documented anti-AChE activity in four distinct types of extracts from G. adspersum sourced from Turkey, with the MeOH extract demonstrating an inhibition rate of 41.34 ± 3.79, which was more than two times lower in comparison with this study for G. applanatum. Moreover, Kozarski et al. [38] reported that the hot-water extract of G. resinaceum at a concentration of 1 mg/mL achieved 81.6 ± 6.5% anti-AChE activity, which was significantly higher than the results from our study. At the same time, Rašeta et al. [8] observed comparable anti-AChE effects in water extracts from four distinct autochthonous Ganoderma species from Serbia (G. applanatum, G. lucidum, G. pfeifferi, and G. resinaceum). The reported activity fell within the 1.04–1.05 mg GALAE/g extract range. In a subsequent study conducted two years later, Sułkowska-Ziaja et al. [22] documented comparable activity in MeOH extracts derived from the mycelial cultures of four specific Ganoderma species, G. adspersum, G. applanatum, G. carnosum, G. lucidum, G. pfeifferi, and G. resinaceum, with reported values in the range of 1.19 to 1.22 mg GALAE/g extract. However, extracts from G. applanatum and G. resinaceum did not exhibit any discernible activity.
Additionally, Akata and colleagues [61] documented anti-AchE effects in various fungal species, including Agaricus campestris, Coprinus comatus, Leucoagaricus leucothites, Lycoperdon utriforme, Macrolepiota mastoidea, and Macrolepiota procera, sourced from diverse regions in Turkey. They observed that the activity was notably lower (ranging from 0.83 to 0.97 mg GALAE/g extract) compared to our previously reported study involving Ganoderma species using the same experimental procedure [8].
The concentration of phenolic compounds from Ganoderma and other fungal species was attributed to AChE inhibition by many authors [62,63,64,65,66]. Hence, differences in the inhibition of AChE observed in our study between the two Ganoderma species may be linked to variations in their secondary metabolite profiles, since the G. applanatum extract demonstrated twice the TPC compared to the extract of G. resinaceum, which did not exhibit anti-AChE activity at the tested concentration. Noteworthy is that G. applanatum contained a higher TP compared to the extract of G. resinaceum, which may have had an effect on the obtained activity. In our previously published study, it was observed that G. applanatum EtOH extracts exhibited a fivefold higher TPC compared to the corresponding extract from G. resinaceum (265.38 ± 0.81 and 50.87 ± 0.29 mg GAE/g d.w., respectively). Interestingly, despite this difference in TPC, both extracts demonstrated equivalent levels of activity [8].
For instance, polysaccharides isolated from both the fruiting bodies and primordia of G. lucidum demonstrated neuroprotective properties [21,54,59,67]. In addition, based on the study conducted by Liu et al. [68], it is known that polysaccharides from C. aegerita significantly prolong the lifespan of Drosophila melanogaster as a model organism and alleviate the oxidative stress induced by H2O2. Interestingly, G. applanatum, which exhibited significant AChE inhibition, also demonstrated a higher concentration of PUT and SPD compared to G. resinaceum. The presence of the elevated levels of PUT and SPD in G. applanatum may also contribute to its pronounced AChE inhibitory effects, since these PAs have been implicated in neuroprotective mechanisms and may interact with AChE, potentially altering its catalytic activity due to its positive charge and chaperone-like activity [15,69]. This is supported by the results of the correlation analysis, which showed a high positive correlation between AChE inhibition and levels of PUT and SPD (Figure 3). Conversely, G. resinaceum, which did not show a notable AChE inhibition in our study, displayed a distinct PA profile. The lower levels of specific PAs in G. resinaceum may suggest a reduced impact on AChE activity. This correlation raises intriguing questions about the connection between PAs and AChE inhibition in the Ganoderma species and suggests a possible synergistic effect.
In this study, the investigation into AChE inhibition among other investigated fungal species marks a novel exploration in the field. To the best of our knowledge, prior to this research, the AChE activity in L. nuda, P. caesia, C. aegerita, C. odora, M. elata, and C. prunulus has remained largely unexplored. Our findings reveal a previously unrecognized potential for AChE modulation within these fungal species.
Polysaccharides isolated from L. nuda showed antioxidant and immuno-modulatory activities [70], while the substantial anti-AChE activity observed in the L. nuda extract may be attributed to a synergistic influence of both secondary and primary metabolites. This is supported by the presence of a high TPC, moderate levels of PUT and SPD, and a high TP content quantified in this extract. On the other hand, a high inhibition of the AChE enzyme by the extract of P. caesia coincides with an elevation in specific PAs, including PUT and SPD, while the TPC and TP were among the lowest. This supports the indications that PAs may play a crucial role as neuroprotective agents [69,71,72,73].
The anticipated inhibitory activity against the target enzyme was not expressed in C. odora and C. aegerita, despite the high PA levels. This suggests the possibility that the assessed concentration might fall below the effective threshold required for the manifestation of the desired biological activity and that the selected concentration may not have been optimal for eliciting the anticipated responses. Hence, a broader concentration range or alternative formulations may be necessary to uncover the latent pharmacological potential of these fungal species.
The variations in anti-AChE activity across different mushroom species, as evidenced by the contrast within this study, underscore the importance of species-specific considerations. Differences in phytochemical profiles, environmental factors, and genetic variations among mushrooms may influence their bioactivity [3]. This emphasizes the significance of comprehensive studies to understand the complex effects of individual fungal species on AChE inhibition. Furthermore, the connection between PAs and neuroprotective activity has not been completely understood, although it has been suggested that it is closely linked to PA machinery. Especially, the SPM and SPD accumulated in glia brain cells, due to their multiple positive charges, affect many neuronal and glial receptors, channels, and transporters and, therefore, affect many neurologic diseases including global amnesia, depression, stress, anxiety, autism, glioblastoma multiforme, glaucoma, migraines, neuropathic pain, sleeplessness, and drug addiction [74]. Furthermore, PAs are prone to the cause-forming of protein aggregation and to accelerating the rate of fibrillization in lysozymes, which can lead to neuropathic and non-neuropathic amyloidosis, which are associated with Alzheimer’s and Parkinson’s disease as well as with aging [75]. Intriguingly, it has been documented that PAs improve social memory formation, synoptic plasticity, behavior, and learning but also increase the production of Pas, as has been reported for Alzheimer’s disease, although biphasic behavior and ambiguous effects PAs to AChE activity has been established [76]. Although PAs are generally proposed to support cholinergic activity, according to Kossorotow et al. [77], at a low micromolar concentration range, SPM and SPD may either stimulate or inhibit AChE activity depending on the amounts of acetylcholine, whereby at low acetylcholine amounts, an inhibitory effect of PAs on AChE prevails. Moreover, it has been documented that PAs are powerful regulators of Alzheimer’s disease through dietary interventions and microbiota manipulations, such as probiotic supplementation, e.g., Bifidobacterium animalis subsp. lactis LKM512 [78]. This supplementation of gut probiotics resulted in an increase in PA levels in the intestines and alleviated inflammation in the colon [79], while on the other hand, it affected the brain’s memory and mice’s social behavior during AD [78]. A potential avenue for future research in this area could involve elucidating the effects of a PA-abundant fungi diet on mice’s PA metabolism and the development of AD.
Our study sheds light on this uncharted territory, as we demonstrate, for the first time, that PAs in these fungal strains may exhibit neuroprotective properties. This discovery challenges the current understanding of the role of PAs in fungal biology and underscores the need for future investigations into the potential therapeutic applications of fungal PAs in neuroprotection. However, it is crucial to acknowledge the limitations of our study and the need for further investigations to elucidate the specific compounds responsible for the observed inhibitory effects. The identification of these compounds could provide insights into the underlying mechanisms and could contribute to the development of targeted therapies for neurodegenerative conditions. Moreover, in vivo studies are crucial to validate our in vitro findings and to assess the potential bioavailability and pharmacokinetics of active compounds from edible fungi.
3.4. PCA Analysis
The PCA was employed to discern patterns and relationships among the quantified compounds (TP, TPC, TF, and PAs) and the antioxidant and anti-AChE activity of all investigated fungal species (Figure 4).
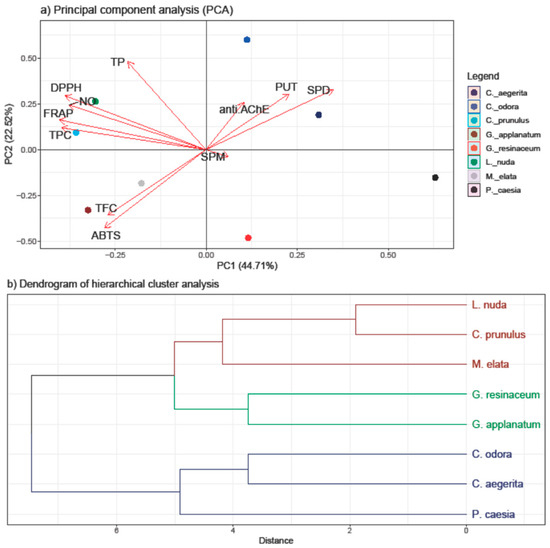
Figure 4.
PCA analysis of all examined parameters in the eight selected fungal species collected on the territory of the Republic of Serbia (a) and the hierarchical clustering of the eight analyzed fungal species (b). The following are the abbreviations of the examined parameters: ABTS—radical scavenger capacity against 2,2′-azinobis(3-ethylbenzothiozoline)-6-sulfonic acid, ABTS•+; DPPH—radical scavenger capacity against 2,2-diphenyl-1-picrylhydrazyl radical, DPPH•; FRAP—ferric reducing antioxidant power; NO—radical scavenger capacity against NO radical; anti.AChE—anti-acetylcholinesterase activity; PUT—putrescine; SPD—spermidine; SPM—spermine; TFC—total flavonoid content; TP—total protein content; TPC—total phenolic content; Tukey’s honestly significant difference (HSD) post hoc test (p ≤ 0.05). Data represent the mean ± standard deviation (SD).
The first two principal components, PC1 and PC2, accounted for 67.23% of the total variance, indicating a substantial representation of the dataset. The results of the PCA highlight the inherent variability in our dataset and offer insights into the key factors influencing the observed trends. A stronger separation was conducted in the horizontal plane of the PC2, with a TP and TPC loading in the I quadrant and PAs in the II quadrant.
Significantly, both PUT and SPD demonstrated a high positive loading on both PCs, along with anti-AChE, indicating their notable involvement in this activity—a correlation that is further supported by the correlation analysis results. Additionally, these variables separate C. aegerita, C. odora, and P. caesia from the other fungal species, suggesting that PAs play a pivotal role in influencing the observed AChE-inhibitory activity of the selected fungi. This is supported with the results of PA quantification, since the highest levels of SPD and PUT were detected in these species. While C. aegerita and C. odora did not demonstrate AchE-enzyme inhibition, the levels of PUT and SPD in P. caesia likely exerted a significant influence on this activity. This is evident as P. caesia exhibited one of the most robust anti-AChE activities among the tested fungal species. When it comes to the SPM levels, the right angle with the anti-AChE vector indicates that there is no correlation between these two variables.
Conversely, TP and TPC displayed a negative loading on PC2 together with variables of antioxidant assays, indicating its inverse relationship with the anti-AChE activity and PA levels. Within the first quadrant, TPC showed a notably strong positive correlation with the reduction potential (FRAP assay) and a slightly weaker positive correlation with the NO and DPPH scavenging ability. Conversely, DPPH exhibited a strong correlation with TP, followed by the NO and FRAP activity. These correlations align with those observed in the heatmap from the correlation analysis (Figure 2). In the negative sector of both PC1 and PC2, TFC and ABTS were present, indicating a robust positive correlation between these variables, as confirmed by the correlation analysis as well. Nevertheless, the clustering of the Ganoderma species and M. elata in the negative part of PC is in accordance with the results of this study, since these species had the strongest ABTS scavenging ability and the highest levels of TFC. On the other hand, the clustering of C. prunulus and L. nuda are characterized with a high TPC and TP and a strong DPPH, NO, and FRAP activity and lower PA levels, which is in accordance with the results obtained.
The clustering observed in the score plot hints at potential subgroups which exhibit different levels of tested activities and active compounds, but that grouping is not based on the tropism nor the taxonomic placement of the investigated species, except for the closely related Ganoderma species. Hence, we assume that different environmental factors and species-specific characteristics affect variations in the detected compounds and activities, prompting a further investigation into the biological factors contributing to these distinct patterns.
4. Conclusions
In conclusion, the findings presented in this study highlight the neuroprotective and antioxidant properties exhibited by PAs and phenolic compounds extracted from selected edible and medicinal fungi. Furthermore, this investigation represents the first documentation of PA levels in the inspected fungi species. It also signifies the initial exploration of the neuroprotective capabilities of P. caesia, C. odora, C. prunulus, and M. elata, along with the pioneering examination of the antioxidant potential of the P. caesia extract. The species with the most potent anti-AchE and antioxidant activities were G. applanatum and L. nuda, which was attributed to the synergistic effects of TP, TPC, and PAs. Additionally, P. caesia demonstrated significant AChE inhibition, primarily associated with elevated SPD and PUT levels, while the significant antioxidant potential of C. odora was related to the synergism of primary (TP) and secondary metabolites (TPC). Besides the experimental results, a correlation analysis and PCA also confirmed the connection of PA levels in the selected fungal species with AChE inhibition, while TPC was closely correlated with the antioxidant activities estimated by the DPPH, ABTS, and NO assays.
Moreover, the different fungal species investigated in this study revealed intriguing variations in the composition and efficacy of PAs and phenolic compounds and emphasize the potential for harnessing fungal resources in the development of therapeutic strategies for neurological disorders as well as through dietary interventions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof10010021/s1, Table S1: Results of CHN analysis.
Author Contributions
Conceptualization, M.R. (Milena Rašeta), M.K. (Marko Kebert), M.R. (Milana Rakić) and E.Č.; methodology, M.R. (Milena Rašeta), M.K. (Marko Kebert) and J.M.; validation, M.R. (Milena Rašeta), M.K. (Marko Kebert) and J.M.; formal analysis, M.R. (Milena Rašeta), M.K. (Marko Kebert), J.M., M.R. (Milana Rakić) and E.Č.; investigation, M.R. (Milena Rašeta), M.K. (Marko Kebert), J.M., M.R. (Milana Rakić) and E.Č.; resources, M.R. (Milena Rašeta), M.K. (Marko Kebert), J.M., M.R. (Milana Rakić) and E.Č.; data curation, M.R. (Milena Rašeta), M.K. (Marko Kebert), J.M. and S.K.; writing—original draft preparation, M.R. (Milena Rašeta), M.K. (Marko Kebert), J.M., M.R. (Milana Rakić), S.K., E.Č. and M.K. (Maja Karaman); writing—review and editing, M.R. (Milena Rašeta), M.K. (Marko Kebert), J.M., M.R. (Milana Rakić), E.Č. and M.K. (Maja Karaman); visualization, M.R. (Milena Rašeta) and M.K. (Marko Kebert); supervision, M.R. (Milena Rašeta) and M.K. (Marko Kebert). All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge the financial support of the Ministry of Science, Technological Development, and Innovation of the Republic of Serbia (Grant No. 451-03-47/2023-01/200125 and 451-03-47/2023-01/200197), as well as the joint project of National Park Tara with the Faculty of Sciences, University of Novi Sad—ProFungi Laboratory (contracts no. 47/2021, 43/2022, and 41/2023).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Makletsova, M.G.; Syatkin, S.P.; Poleshchuk, V.V.; Urazgildeeva, G.R.; Chigaleychik, L.A.; Sungrapova, C.Y.; Illarioshkin, S.N. Polyamines in Parkinson’s disease: Their role in oxidative stress induction and protein aggregation. J. Neurol. Res. 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Bacci, A.; Runfola, M.; Sestito, S.; Rapposelli, S. Beyond antioxidant effects: Nature-based templates unveil new strategies for neurodegenerative diseases. Antioxidants 2021, 10, 367. [Google Scholar] [CrossRef] [PubMed]
- Mišković, J.; Karaman, M.; Rašeta, M.; Krsmanović, N.; Berežni, S.; Jakovljević, D.; Piattoni, F.; Zambonelli, A.; Gargano, M.L.; Venturella, G. Comparison of two Schizophyllum commune strains in production of acetylcholinesterase inhibitors and antioxidants from submerged cultivation. J. Fungi 2021, 7, 115. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, I.R.; Rockwood, K.; Martin, E.; Darvesh, S. Cholinesterase inhibition in Alzheimer’s disease: Is specificity the answer? J. Alzheimers Dis. 2014, 42, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Ademosun, A.O.; Ogunsuyi, O.B.; Oyedola, E.T.; Olasehinde, T.A.; Oyeleye, S.I. In vitro anticholinesterase, antimonoamine oxidase and antioxidant properties of alkaloid extracts from kola nuts (Cola acuminata and Cola nitida). J. Complement. Integr. Med. 2018, 16, 20160155. [Google Scholar] [CrossRef] [PubMed]
- Reis, G.C.; Guidi, L.R.; Fernandes, C.; Godoy, H.T.; Glória, M.B. UPLC-UV method for the quantification of free amino acids, bioactive amines, and ammonia in fresh, cooked, and canned mushrooms. Food Anal. Methods 2020, 13, 1613–1626. [Google Scholar] [CrossRef]
- Pop, R.M.; Puia, I.C.; Puia, A.; Chedea, V.S.; Leopold, N.; Bocsan, I.C.; Buzoianu, A.D. Characterization of Trametes versicolor: Medicinal mushroom with important health benefits. Not. Bot. Horti Agrobot. Cluj Napoca 2018, 46, 343–349. [Google Scholar] [CrossRef]
- Rašeta, M.; Popović, M.; Beara, I.; Šibul, F.; Zengin, G.; Krstić, S.; Karaman, M. Anti-inflammatory, antioxidant and enzyme inhibition activities in correlation with mycochemical profile of selected indigenous Ganoderma spp. from Balkan region (Serbia). Chem. Biodivers. 2020, 18, e2000828. [Google Scholar] [CrossRef]
- Rašeta, M.; Popović, M.; Čapo, I.; Stilinović, N.; Vukmirović, S.; Milošević, B.; Karaman, M. Antidiabetic effect of two different Ganoderma species tested in alloxan diabetic rats. RSC Adv. 2020, 10, 10382–10393. [Google Scholar] [CrossRef]
- Krsmanović, N.; Rašeta, M.; Mišković, J.; Bekvalac, K.; Bogavac, M.; Karaman, M.; Isikhuemhen, O.S. Effects of UV stress in promoting antioxidant activities in fungal species Trametes versicolor (L.) Lloyd and Flammulina velutipes (Curtis) Singer. Antioxidants 2023, 12, 302. [Google Scholar] [CrossRef]
- Toro-Funes, N.; Bosch-Fusté, J.; Veciana-Nogués, M.T.; Izquierdo-Pulido, M.; Vidal-Carou, M.C. In vitro antioxidant activity of dietary polyamines. Food Res. Int. 2013, 51, 141–147. [Google Scholar] [CrossRef]
- Reis, G.C.; Custódio, F.B.; Botelho, B.G.; Guidi, L.R.; Gloria, M.B.A. Investigation of biologically active amines in some selected edible mushrooms. J. Food Compost. Anal. 2020, 86, 103375. [Google Scholar] [CrossRef]
- Bae, D.H.; Lane, D.J.; Jansson, P.J.; Richardson, D.R. The old and new biochemistry of polyamines. Biochim. Biophys. Acta Gen. Subj. BBA Gen. Subj. 2018, 1862, 2053–2068. [Google Scholar] [CrossRef] [PubMed]
- Rocha, R.O.; Wilson, R.A. Essential, deadly, enigmatic: Polyamine metabolism and roles in fungal cells. Fungal Biol. Rev. 2019, 33, 47–57. [Google Scholar] [CrossRef]
- Vrijsen, S.; Houdou, M.; Cascalho, A.; Eggermont, J.; Vangheluwe, P. Polyamines in Parkinson’s disease: Balancing between neurotoxicity and neuroprotection. Annu. Rev. Biochem. 2023, 92, 435–464. [Google Scholar] [CrossRef]
- Đorđievski, S.; Vukašinović, E.L.; Čelić, T.V.; Pihler, I.; Kebert, M.; Kojić, D.; Purać, J. Spermidine dietary supplementation and polyamines level in reference to survival and lifespan of honey bees. Sci. Rep. 2023, 13, 4329. [Google Scholar] [CrossRef] [PubMed]
- Minois, N. Molecular basis of the ‘anti-aging’ effect of spermidine and other natural polyamines-a mini-review. Gerontology 2014, 60, 319–326. [Google Scholar] [CrossRef]
- Peng, D.; Wang, X.; Li, Z.; Zhang, Y.; Peng, Y.; Li, Y.; He, X.; Zhang, X.; Ma, X.; Huang, L.; et al. NO is involved in spermidine-induced drought tolerance in white clover via activation of antioxidant enzymes and genes. Protoplasma 2016, 253, 1243–1254. [Google Scholar] [CrossRef]
- Park, S.J.; Kwak, M.K.; Kang, S.O. Schiff bases of putrescine with methylglyoxal protect from cellular damage caused by accumulation of methylglyoxal and reactive oxygen species in Dictyostelium discoideum. Int. J. Biochem. Cell Biol. 2017, 86, 54–66. [Google Scholar] [CrossRef]
- Jiang, Y.; Gao, H.; Turdu, G. Traditional Chinese medicinal herbs as potential AChE inhibitors for anti-Alzheimer’s disease: A review. Bioorg. Chem. 2017, 75, 50–61. [Google Scholar] [CrossRef]
- Sun, X.Z.; Liao, Y.; Li, W.; Guo, L.M. Neuroprotective effects of Ganoderma lucidum polysaccharides against oxidative stress-induced neuronal apoptosis. Neural Regen. Res. 2017, 12, 953–958. [Google Scholar]
- Sułkowska-Ziaja, K.; Zengin, G.; Gunia-Krzyżak, A.; Popiół, J.; Szewczyk, A.; Jaszek, M.; Rogalski, J.; Muszyńska, B. Bioactivity and mycochemical profile of extracts from mycelial cultures of Ganoderma spp. Molecules 2022, 27, 275. [Google Scholar] [CrossRef] [PubMed]
- Sułkowska-Ziaja, K.; Balik, M.; Szczepkowski, A.; Trepa, M.; Zengin, G.; Kała, K.; Muszyńska, B. A review of chemical composition and bioactivity studies of the most promising species of Ganoderma spp. Diversity 2023, 15, 882. [Google Scholar] [CrossRef]
- Dadáková, E.; Pelikánová, T.; Kalač, P. Content of biogenic amines and polyamines in some species of European wild-growing edible mushrooms. Eur. Food Res. Technol. 2009, 230, 163–171. [Google Scholar] [CrossRef]
- Scaramagli, S.; Biondi, S.; Capitani, F.; Gerola, P.; Altamura, M.M.; Torrigiani, P. Polyamine conjugate levels and ethylene biosynthesis: Inverse relationship with vegetative bud formation in tobacco Thin layers. Physiol. Plant. 1999, 105, 366–375. [Google Scholar] [CrossRef]
- Karthikeyan, R.; Kumaravel, S. Study on phenolic content, antioxidant activity and CHNS elemental analysis of Amorphophallus sylvaticus. Int. J. Agric. Life Sci. 2016, 2, 12–17. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Espín, J.C.; Soler-Rivas, C.; Wichers, H.J. Characterization of the total free radical scavenger capacity of vegetable oils and oil fractions using 2,2-diphenyl-1-picrylhydrazyl radical. J. Agric. Food Chem. 2000, 48, 648–656. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Green, L.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid and concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [PubMed]
- Ellman, G.L.; Courtney, K.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Kassambra, A. rstatix: Pipe-Friendly Framework for Basic Statistical Tests. R Package Version 0.6.0. 2020. Available online: https://CRAN.R-project.org/package=rstatix (accessed on 20 December 2023).
- Galili, T. dendextend: An R package for visualizing, adjusting and comparing trees of hierarchical clustering. Bioinformatics 2015, 31, 3718–3720. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2. Wiley Interdiscip. Rev. Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Vaz, J.A.; Barros, L.; Martins, A.; Santos-Buelga, C.; Vasconcelos, M.H.; Ferreira, I.C. Chemical composition of wild edible mushrooms and antioxidant properties of their water soluble polysaccharidic and ethanolic fractions. Food Chem. 2011, 126, 610–616. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Vunduk, J.; Jakovljevic, D.; Jadranin, M.B.; Nikšić, M.P. Health impact of the commercially cultivated mushroom Agaricus bisporus and wild-growing mushroom Ganoderma resinaceum—A comparative overview. J. Serb. Chem. Soc. 2020, 85, 721–735. [Google Scholar] [CrossRef]
- Grangeia, C.; Heleno, S.A.; Barros, L.; Martins, A.; Ferreira, I.C. Effects of trophism on nutritional and nutraceutical potential of wild edible mushrooms. Food Res. Int. 2011, 44, 1029–1035. [Google Scholar] [CrossRef]
- Pinto, S.; Barros, L.; Sousa, M.J.; Ferreira, I.C. Chemical characterization and antioxidant properties of Lepista nuda fruiting bodies and mycelia obtained by in vitro culture: Effects of collection habitat and culture media. Food Res. Int. 2013, 51, 496–502. [Google Scholar] [CrossRef]
- Petrović, J.; Glamočlija, J.; Stojković, D.S.; Ćirić, A.; Barros, L.; Ferreira, I.C.; Soković, M.D. Nutritional value, chemical composition, antioxidant activity and enrichment of cream cheese with chestnut mushroom Agrocybe aegerita (Brig.) Sing. J. Food Sci. Technol. 2015, 52, 6711–6718. [Google Scholar] [CrossRef]
- Niego, A.G.; Rapior, S.; Thongklang, N.; Raspé, O.; Jaidee, W.; Lumyong, S.; Hyde, K.D. Macrofungi as a nutraceutical source: Promising bioactive compounds and market value. J. Fungi 2021, 7, 397. [Google Scholar] [CrossRef] [PubMed]
- Ouzouni, P.K.; Petridis, D.; Koller, W.D.; Riganakos, K.A. Nutritional value and metal content of wild edible mushrooms collected from West Macedonia and Epirus, Greece. Food Chem. 2009, 115, 1575–1580. [Google Scholar] [CrossRef]
- Karaman, M.; Stahl, M.; Vulić, J.; Vesić, M.; Čanadanović-Brunet, J. Wild-growing lignicolous mushroom species as sources of novel agents with antioxidative and antibacterial potentials. Int. J. Food Sci. Nutr. 2014, 65, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.P.; Suwandi, J.F.; Fuller, J.; Doronila, A.I.; Ng, K.S. Antioxidant capacity and mineral contents of edible wild Australian mushrooms. Food Sci. Technol. Int. 2012, 18, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Sevindik, M. Total phenolic, total flavonoid contents and antioxidant potential of the wild edible mushroom Clitocybe odora. KSU J. Agric. Nat. 2024, 27, 75–81. [Google Scholar] [CrossRef]
- Dimitrijević, M.V.; Jovanović, V.S.; Cvetkovic, J.S.; Mihajilov-Krstev, T.M.; Stojanović, G.S.; Mitić, V.D. Screening of antioxidant, antimicrobial and antiradical activities of twelve selected Serbian wild mushrooms. Anal. Methods 2015, 7, 4181–4191. [Google Scholar] [CrossRef]
- Zengin, G.; Sarikurkcu, C.; Gunes, E.; Uysal, A.; Ceylan, R.; Uysal, S.; Gungor, H.; Aktumsek, A. Two Ganoderma species: Profiling of phenolic compounds by HPLC-DAD, antioxidant, antimicrobial and inhibitory activities on key enzymes linked to diabetes mellitus, Alzheimer’s disease and skin disorders. Food Funct. 2015, 6, 2794–2802. [Google Scholar] [CrossRef]
- Rašeta, M.; Karaman, M.; Jakšić, M.; Šibul, F.; Kebert, M.; Novaković, A.; Popović, M. Mineral composition, antioxidant and cytotoxic biopotentials of wild-growing Ganoderma species (Serbia): G. lucidum (Curtis) P. Karst vs. G. applanatum (Pers.) Pat. Int. J. Food Sci. Technol. 2016, 51, 2583–2590. [Google Scholar] [CrossRef]
- Rajoriya, A.; Tripathy, S.S.; Gupta, N. In vitro antioxidant activity of selected Ganoderma species found in Odisha, India. Trop. Plant Res. 2015, 2, 72–77. [Google Scholar]
- Siangu, B.N.; Sauda, S.; John, M.K.; Njue, W. Antioxidant activity, total phenolic and flavonoid content of selected Kenyan medicinal plants, sea algae and medicinal wild mushrooms. Afr. J. Pure Appl. 2019, 13, 43–48. [Google Scholar]
- Ćilerdžić, J.; Stajić, M.; Vukojevic, J. Potential of submergedly cultivated mycelia of Ganoderma spp. as antioxidant and antimicrobial agents. Curr. Pharm. Biotechnol. 2016, 17, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, S.; Yin, F.; Chen, H.; Yang, D.; Liu, X.; Jin, Q.; Lv, X.; Mans, D.R.; Zhang, X.; et al. Antioxidative and cytoprotective effects of Ganoderma applanatum and Fomitopsis pinicola in PC12 adrenal phaeochromocytoma cells. Int. J. Med. Mushrooms 2022, 24, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Cör, D.; Botić, T.; Gregori, A.; Pohleven, F.; Knez, Ž. The effects of different solvents on bioactive metabolites and “in vitro” antioxidant and anti-acetylcholinesterase activity of Ganoderma lucidum fruiting body and primordia extracts. Maced. J. Chem. Chem. Eng. 2017, 36, 129–141. [Google Scholar] [CrossRef]
- Bal, C.; Sevindik, M.; Akgul, H.; Selamoglu, Z. Oxidative stress index and antioxidant capacity of Lepista nuda collected from Gaziantep/Turkey. Sigma J. Eng. Nat. Sci. 2019, 37, 1–5. [Google Scholar]
- Ramya, H.; Ravikumar, K.S.; Fathimathu, Z.; Janardhanan, K.K.; Ajith, T.A.; Shah, M.A.; Reshi, Z.A. Morel mushroom, Morchella from Kashmir Himalaya: A potential source of therapeutically useful bioactives that possess free radical scavenging, anti-inflammatory, and arthritic edema-inhibiting activities. Drug Chem. Toxicol. 2022, 45, 2014–2023. [Google Scholar] [CrossRef] [PubMed]
- Kalyoncu, F.; Oskay, M.; Sağlam, H.; Erdoğan, T.F.; Tamer, A.Ü. Antimicrobial and antioxidant activities of mycelia of 10 wild mushroom species. J. Med. Food 2010, 13, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Bains, A.; Chawla, P.; Inbaraj, B.S. Evaluation of in vitro antimicrobial, antioxidant, and anti-quorum sensing activity of edible mushroom (Agrocybe aegerita). Foods 2023, 12, 3562. [Google Scholar] [CrossRef]
- Cör, D.; Botić, T.; Knez, Ž.; Batista, U.; Gregori, A.; Pohleven, F.; Bončina, T. Two-stage extraction of antitumor, antioxidant and antiacetylcholinesterase compounds from Ganoderma lucidum fruiting body. J. Supercrit. Fluids 2014, 91, 53–60. [Google Scholar] [CrossRef]
- Tel-Çayan, G.; Öztürk, M.; Duru, M.E.; Rehman, M.U.; Adhikari, A.; Türkoğlu, A.; Choudhary, M.I. Phytochemical investigation, antioxidant and anticholinesterase activities of Ganoderma adspersum. Ind. Crops Prod. 2015, 76, 749–754. [Google Scholar] [CrossRef]
- Akata, I.; Zengin, G.; Picot, C.M.; Mahomoodally, M.F. Enzyme inhibitory and antioxidant properties of six mushroom species from the Agaricaceae family. S. Afr. J. Bot. 2019, 120, 95–99. [Google Scholar] [CrossRef]
- Dündar, A.; Okumuş, V.; Özdemir, S.; Çelik, K.S.; Boğa, M.S.; Ozcagli, E.; Özhan, G.; Yildiz, A. Antioxidant, antimicrobial, cytotoxic and anticholinesterase activities of seven mushroom species with their phenolic acid composition. J. Hortic. 2015, 2, 4. [Google Scholar] [CrossRef]
- Hasnat, M.A.; Pervin, M.; Lim, B.O. Acetylcholinesterase inhibition and in vitro and in vivo antioxidant activities of Ganoderma lucidum grown on germinated brown rice. Molecules 2013, 18, 6663–6678. [Google Scholar] [CrossRef] [PubMed]
- Karaman, M.; Tesanovic, K.; Novakovic, A.; Jakovljevic, D.; Janjusevic, L.; Sibul, F.; Pejin, B. Coprinus comatus filtrate extract, a novel neuroprotective agent of natural origin. Nat. Prod. Res. 2020, 34, 2346–2350. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.E.; Üstün, O. Determination of total phenol content, antioxidant activity and acetylcholinesterase inhibition in selected mushrooms from Turkey. J. Food Compost. Anal. 2011, 24, 386–390. [Google Scholar] [CrossRef]
- Zawadzka, A.; Kobus-Cisowska, J.; Szwajgier, D.; Szczepaniak, O.M.; Szulc, P.; Siwulski, M. Dual functional cholinesterase inhibitors and complexing of aluminum ions of five species of fungi family depended of drying conditions and extraction process—In vitro study. LWT 2021, 154, 112712. [Google Scholar] [CrossRef]
- Liu, X.; Yang, L.; Li, G.; Jiang, Y.; Zhang, G.; Ling, J. A novel promising neuroprotective agent: Ganoderma lucidum polysaccharide. Int. J. Biol. Macromol. 2022, 229, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, L.; Tong, A.; Zhen, H.; Han, D.; Yuan, H.; Li, F.; Wang, C.; Fan, G. Anti-aging effect of Agrocybe aegerita polysaccharide through regulation of oxidative stress and gut microbiota. Foods 2022, 11, 3783. [Google Scholar] [CrossRef]
- Makletsova, M.G.; Rikhireva, G.T.; Kirichenko, E.Y.; Trinitatsky, I.Y.; Vakulenko, M.Y.; Ermakov, A.M. The role of polyamines in the mechanisms of cognitive impairment. Neurochem. J. 2022, 16, 283–294. [Google Scholar] [CrossRef]
- Li, Y.; Guo, X.; Zhong, R.; Ye, C.; Chen, J. Structure characterization and biological activities evaluation of two hetero-polysaccharides from Lepista nuda: Cell antioxidant, anticancer and immune-modulatory activities. Int. J. Biol. Macromol. 2023, 244, 125204. [Google Scholar] [CrossRef]
- Dhara, M.; Matta, J.A.; Lei, M.; Knowland, D.; Yu, H.; Gu, S.; Bredt, D.S. Polyamine regulation of ion channel assembly and implications for nicotinic acetylcholine receptor pharmacology. Nat. Commun. 2020, 11, 2799. [Google Scholar] [CrossRef]
- Sagar, N.A.; Tarafdar, S.; Agarwal, S.; Tarafdar, A.; Sharma, S. Polyamines: Functions, metabolism, and role in human disease management. Med. Sci. 2021, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Hamon, L.; Savarin, P.; Pastré, D. Polyamine signal through gap junctions: A key regulator of proliferation and gap-junction organization in mammalian tissues? BioEssays 2016, 38, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Skatchkov, S.N.; Woodbury-Fariña, M.A.; Eaton, M.J. The role of glia in stress: Polyamines and brain disorders. Psychiatr. Clin. N. Am. 2014, 37, 653–678. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.F.; Jash, C.; Payghan, P.V.; Ghoshal, N.; Kumar, G.S. Polyamines and its analogue modulates amyloid fibrillation in lysozyme: A comparative investigation. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129557. [Google Scholar] [CrossRef] [PubMed]
- Guerra, G.P.; Rubin, M.A.; Mello, C.F. Modulation of learning and memory by natural polyamines. Pharmacol. Res. 2016, 112, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Kossorotow, A.; Wolf, H.U.; Seiler, N. Regulatory effects of polyamines on membrane-bound acetylcholinesterase. Biochem. J. 1974, 144, 21–27. [Google Scholar] [CrossRef]
- Kibe, S.; Kurihara, S.; Sakai, Y.; Suzuki, H.; Ooga, T.; Sawaki, E.; Muramatsu, K.; Nakamura, A.; Yamashita, A.; Kitada, Y.; et al. Upregulation of colonic luminal polyamines produced by intestinal microbiota delays senescence in mice. Sci. Rep. 2014, 4, 4548. [Google Scholar] [CrossRef]
- Matsumoto, M.; Kurihara, S.; Kibe, R.; Ashida, H.; Benno, Y. Longevity in mice is promoted by probiotic-induced suppression of colonic senescence dependent on upregulation of gut bacterial polyamine production. PLoS ONE 2011, 6, e23652. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).