Thoracoscopic Implantation of Epicardial Left Ventricular Lead for Cardiac Resynchronization Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population
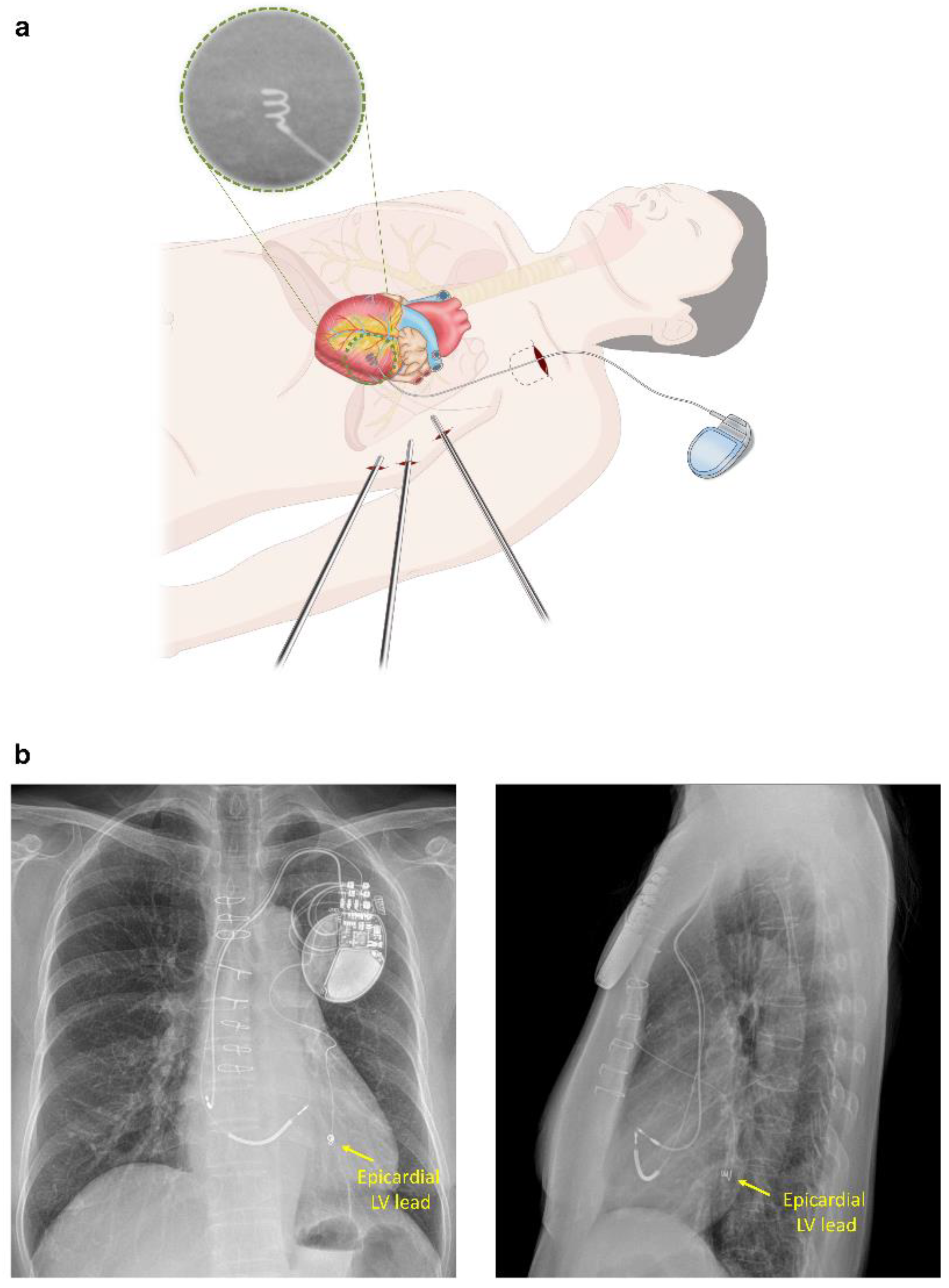
2.2. Epicardial LV Lead Implantation for CRT
2.3. Acute and Post-Discharge Outcomes
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Acute Outcomes after Epicardial LV Lead Implantation for CRT
3.3. Post-Discharge Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bradley, D.J.; Bradley, E.A.; Baughman, K.L.; Berger, R.D.; Calkins, H.; Goodman, S.N.; Kass, D.A.; Powe, N.R. Cardiac resynchronization and death from progressive heart failure: A meta-analysis of randomized controlled trials. JAMA 2003, 289, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Bristow, M.R.; Saxon, L.A.; Boehmer, J.; Krueger, S.; Kass, D.A.; De Marco, T.; Carson, P.; DiCarlo, L.; DeMets, D.; White, B.G.; et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N. Engl. J. Med. 2004, 350, 2140–2150. [Google Scholar] [CrossRef] [PubMed]
- Cleland, J.G.; Daubert, J.C.; Erdmann, E.; Freemantle, N.; Gras, D.; Kappenberger, L.; Tavazzi, L. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N. Engl. J. Med. 2005, 352, 1539–1549. [Google Scholar] [CrossRef] [Green Version]
- Curtis, A.B.; Worley, S.J.; Adamson, P.B.; Chung, E.S.; Niazi, I.; Sherfesee, L.; Shinn, T.; Sutton, M.S. Biventricular pacing for atrioventricular block and systolic dysfunction. N. Engl. J. Med. 2013, 368, 1585–1593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, A.S.; Wells, G.A.; Talajic, M.; Arnold, M.O.; Sheldon, R.; Connolly, S.; Hohnloser, S.H.; Nichol, G.; Birnie, D.H.; Sapp, J.L.; et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N. Engl. J. Med. 2010, 363, 2385–2395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAlister, F.A.; Ezekowitz, J.; Hooton, N.; Vandermeer, B.; Spooner, C.; Dryden, D.M.; Page, R.L.; Hlatky, M.A.; Rowe, B.H. Cardiac resynchronization therapy for patients with left ventricular systolic dysfunction: A systematic review. JAMA 2007, 297, 2502–2514. [Google Scholar] [CrossRef] [Green Version]
- Ahsan, S.Y.; Saberwal, B.; Lambiase, P.D.; Chaubey, S.; Segal, O.R.; Gopalamurugan, A.B.; McCready, J.; Rogers, D.P.; Lowe, M.D.; Chow, A.W. An 8-year single-centre experience of cardiac resynchronisation therapy: Procedural success, early and late complications, and left ventricular lead performance. Europace 2013, 15, 711–717. [Google Scholar] [CrossRef]
- Jeong, D.S.; Park, P.W.; Lee, Y.T.; Park, S.J.; Kim, J.S.; On, Y.K. Thoracoscopic left ventricular lead implantation in cardiac resynchronization therapy. J. Korean Med. Sci. 2012, 27, 1595–1597. [Google Scholar] [CrossRef]
- Buiten, M.S.; van der Heijden, A.C.; Klautz, R.J.M.; Schalij, M.J.; van Erven, L. Epicardial leads in adult cardiac resynchronization therapy recipients: A study on lead performance, durability, and safety. Heart Rhythm 2015, 12, 533–539. [Google Scholar] [CrossRef]
- Jutley, R.S.; Waller, D.A.; Loke, I.; Skehan, D.; Ng, A.; Stafford, P.; Chin, D.; Spyt, T.J. Video-assisted thoracoscopic implantation of the left ventricular pacing lead for cardiac resynchronization therapy. Pacing Clin. Electrophysiol. 2008, 31, 812–818. [Google Scholar] [CrossRef]
- On, Y.K.; Park, K.M.; Jeong, D.S.; Park, P.W.; Lee, Y.T.; Park, S.J.; Kim, J.S. Electrophysiologic Results After Thoracoscopic Ablation for Chronic Atrial Fibrillation. Ann. Thorac. Surg. 2015, 100, 1595–1603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.K.; Kim, J.Y.; On, Y.K.; Jeong, D.S. Mid-Term Results of Totally Thoracoscopic Ablation in Patients with Recurrent Atrial Fibrillation after Catheter Ablation. Korean J. Thorac. Cardiovasc. Surg. 2020, 53, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Gamble, J.H.P.; Herring, N.; Ginks, M.; Rajappan, K.; Bashir, Y.; Betts, T.R. Procedural Success of Left Ventricular Lead Placement for Cardiac Resynchronization Therapy: A Meta-Analysis. JACC Clin. Electrophysiol. 2016, 2, 69–77. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, V.F.; Fanggiday, J.; Balt, J.C.; Wijffels, M.; Daeter, E.J.; Kelder, J.C.; Boersma, L.V.A. Effects of epicardial versus transvenous left ventricular lead placement on left ventricular function and cardiac perfusion in cardiac resynchronization therapy: A randomized clinical trial. J. Cardiovasc. Electrophysiol. 2017, 28, 917–923. [Google Scholar] [CrossRef]
- Giraldi, F.; Cattadori, G.; Roberto, M.; Carbucicchio, C.; Pepi, M.; Ballerini, G.; Alamanni, F.; Della Bella, P.; Pontone, G.; Andreini, D.; et al. Long-term effectiveness of cardiac resynchronization therapy in heart failure patients with unfavorable cardiac veins anatomy comparison of surgical versus hemodynamic procedure. J. Am. Coll. Cardiol. 2011, 58, 483–490. [Google Scholar] [CrossRef] [Green Version]
- Garikipati, N.V.; Mittal, S.; Chaudhry, F.; Musat, D.L.; Sichrovsky, T.; Preminger, M.; Arshad, A.; Steinberg, J.S. Comparison of endovascular versus epicardial lead placement for resynchronization therapy. Am. J. Cardiol. 2014, 113, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Rickard, J.; Johnston, D.R.; Price, J.; Tedford, R.; Baranowski, B.; Bassiouny, M.; Cantillon, D.; Grimm, R.A.; Tang, W.H.W.; Varma, N.; et al. Reverse ventricular remodeling and long-term survival in patients undergoing cardiac resynchronization with surgically versus percutaneously placed left ventricular pacing leads. Heart Rhythm 2015, 12, 517–523. [Google Scholar] [CrossRef]
- Shah, R.V.; Lewis, E.F.; Givertz, M.M. Epicardial left ventricular lead placement for cardiac resynchronization therapy following failed coronary sinus approach. Congest. Heart Fail. 2006, 12, 312–316. [Google Scholar] [CrossRef] [Green Version]
- Marini, M.; Branzoli, S.; Moggio, P.; Martin, M.; Belotti, G.; Molon, G.; Guarracini, F.; Coser, A.; Quintarelli, S.; Pederzolli, C.; et al. Epicardial left ventricular lead implantation in cardiac resynchronization therapy patients via a video-assisted thoracoscopic technique: Long-term outcome. Clin. Cardiol. 2020, 43, 284–290. [Google Scholar] [CrossRef] [Green Version]
- Dekker, A.L.; Phelps, B.; Dijkman, B.; van der Nagel, T.; van der Veen, F.H.; Geskes, G.G.; Maessen, J.G. Epicardial left ventricular lead placement for cardiac resynchronization therapy: Optimal pace site selection with pressure-volume loops. J. Thorac. Cardiovasc. Surg. 2004, 127, 1641–1647. [Google Scholar] [CrossRef]
- Singh, J.P.; Klein, H.U.; Huang, D.T.; Reek, S.; Kuniss, M.; Quesada, A.; Barsheshet, A.; Cannom, D.; Goldenberg, I.; McNitt, S.; et al. Left ventricular lead position and clinical outcome in the multicenter automatic defibrillator implantation trial-cardiac resynchronization therapy (MADIT-CRT) trial. Circulation 2011, 123, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Butter, C.; Auricchio, A.; Stellbrink, C.; Fleck, E.; Ding, J.; Yu, Y.; Huvelle, E.; Spinelli, J. Effect of resynchronization therapy stimulation site on the systolic function of heart failure patients. Circulation 2001, 104, 3026–3029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceresa, F.; Sansone, F.; Calvagna, G.; Maiorana, M.P.; Busa, G.; Patane, S.; Evola, R.; Patane, F. Minimally invasive implantation of the myopore sutureless myocardial pacing lead. Innovations (Phila) 2012, 7, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.E.; Bates, M.G.; Turley, A.J.; Linker, N.J.; Owens, W.A. Video-assisted thoracoscopic left ventricular pacing in patients with and without previous sternotomy. Ann. Thorac. Surg. 2013, 95, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Navia, J.L.; Atik, F.A.; Grimm, R.A.; Garcia, M.; Vega, P.R.; Myhre, U.; Starling, R.C.; Wilkoff, B.L.; Martin, D.; Houghtaling, P.L.; et al. Minimally invasive left ventricular epicardial lead placement: Surgical techniques for heart failure resynchronization therapy. Ann. Thorac. Surg. 2005, 79, 1536–1544, discussion 1536–1544. [Google Scholar] [CrossRef] [PubMed]



| No. | Age (years) | Sex | Etiology of Heart Failure | NYHA | QRSd (ms) | QRS Morphology | LVEF (%) | Previous Surgery | History of Cardiac Surgery | Surgical Technique |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 49 | F | Non-ICMP | III | 224 | LBBB | 17.7 | Absence of suitable lateral cardiac vein branches | Thoracoscopy | |
| 2 | 45 | M | Non-ICMP | III | 184 | LBBB | 24.7 | Absence of suitable lateral cardiac vein branches | Thoracoscopy | |
| 3 | 62 | M | Non-ICMP | III | 214 | LBBB | 23.0 | AVR | Tortuous target branches | Thoracoscopy |
| 4 | 62 | M | ICMP | II | 168 | LBBB | 26.3 | CABG, MVR | Absence of suitable lateral cardiac vein branches | Thoracoscopy |
| 5 | 70 | F | Non-ICMP | II | 208 | LBBB | 23.3 | Recurrent LV lead dislodgement & PNS | Thoracoscopy | |
| 6 | 73 | M | ICMP | III | 182 | LBBB | 24.3 | CABG | Recurrent LV lead dislodgement & PNS | Thoracoscopy with mini-thoracotomy |
| 7 | 64 | M | Non-ICMP | IV | 194 | LBBB | 23.4 | MVR | Anomalous cardiac veins | Thoracoscopy |
| 8 | 53 | M | Non-ICMP | III | 214 | RBBB | 25.0 | Tortuous coronary sinus and target branches | Thoracoscopy with mini-thoracotomy | |
| 9 | 70 | F | Non-ICMP | III | 250 | LBBB | 30.0 | Absence of suitable lateral cardiac vein branches | Thoracoscopy with mini-thoracotomy | |
| 10 | 61 | F | Non-ICMP | III | 166 | LBBB | 32.0 | Absence of suitable lateral cardiac vein branches | Thoracoscopy | |
| 11 | 62 | M | ICMP | II | 145 | LBBB | 30.1 | CABG | Coronary sinus atresia with unroofed coronary sinus | Thoracoscopy with mini-thoracotomy |
| 12 | 74 | F | Non-ICMP | II | 168 | LBBB | 28.4 | Tortuous coronary sinus and target branches | Thoracoscopy | |
| 13 | 59 | F | Non-ICMP | II | 151 | LBBB | 35.0 | VSD closure | Anomalous cardiac veins | Thoracoscopy with mini-thoracotomy |
| Epicardial LV Lead (n = 13) | Endocardial LV Lead (n = 243) | p-Value | |
|---|---|---|---|
| Age, years | 61.9 ± 8.9 | 66.2 ± 12.7 | 0.225 |
| Male gender | 6 (46.2%) | 152 (62.6%) | 0.236 |
| BMI (kg/m2) | 24.2 ± 2.9 | 23.3 ± 3.7 | 0.318 |
| Hypertension | 6 (46.2%) | 134 (55.1%) | 0.526 |
| Diabetes | 4 (30.8%) | 89 (36.6%) | 0.669 |
| Chronic kidney disease | 1 (7.7%) | 33 (13.6%) | 0.542 |
| Stroke | 3 (23.1%) | 23 (9.5%) | 0.113 |
| Previous PCI | 1 (7.7%) | 47 (19.4%) | 0.294 |
| Previous cardiac surgery | 5 (38.5%) | 42 (17.3%) | 0.055 |
| Ischemic cardiomyopathy | 4 (30.8%) | 65 (26.7%) | 0.750 |
| LBBB | 12 (92.3%) | 210 (86.4%) | 0.542 |
| QRS duration (msec) | 190 ± 31 | 170 ± 36 | 0.019 |
| LVEF (%) | 26.4 ± 4.6 | 25.6 ± 6.2 | 0.701 |
| LVEDV (ml) | 230 ± 46 | 233 ± 77 | 0.848 |
| LVESV (ml) | 181 ± 55 | 175 ± 66 | 0.615 |
| CRT-defibrillator | 12 (92.3%) | 233 (95.9%) | 0.538 |
| Lateral LV pacing site | 13 (100%) | 227 (93.4%) | 0.339 |
| Non-apical LV pacing sites | 13 (100%) | 242 (99.6%) | 0.817 |
| LV pacing threshold (V) | 1.5 ± 1.0 | 1.3 ± 0.8 | 0.651 |
| LV pacing pulse width (ms) | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.538 |
| LV lead impedance (Ω) | 354 ± 51 | 673 ± 267 | <0.001 |
| Discharge Medications | |||
| Beta-blocker | 9 (69.2%) | 181 (74.5%) | 0.673 |
| ARB/ACEi | 11 (84.6%) | 20 (84.4%) | 0.980 |
| Spironolactone | 9 (69.2%) | 178 (73.3%) | 0.750 |
| Epicardial LV Lead (n = 13) | Endocardial LV Lead (n = 243) | p Value | |||||
|---|---|---|---|---|---|---|---|
| Baseline | Last Follow-Up | p Value * | Baseline | Last Follow-Up | p Value * | ||
| Follow-Up | |||||||
| Follow-up duration, month | 34.3 ± 28.6 | 37.1 ± 32.7 | 0.913 | ||||
| BiV pacing percentage (%) | 96 ± 7 | 94 ± 16 | 0.772 | ||||
| LV Lead Performance | |||||||
| Threshold (V) | 1.5 ± 1.0 | 1.7 ± 0.7 | 0.154 | 1.3 ± 0.8 | 1.5 ± 1.0 | <0.001 | 0.365 † |
| Pulse width (ms) | 0.5 ± 0.1 | 0.5 ± 0.2 | 0.168 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.433 | 0.500 † |
| Impedance (Ω) | 354 ± 51 | 349 ± 62 | 0.859 | 673 ± 267 | 694 ± 309 | 0.231 | 0.483 † |
| Electrocardiographic Outcomes | |||||||
| QRS duration (ms) | 190 ± 31 | 150 ± 31 | <0.001 | 170 ± 36 | 141 ± 22 | <0.001 | 0.602 † |
| Echocardiographic Outcomes | |||||||
| LVEF (%) | 25.7 ± 3.9 | 37.7 ± 15.1 | 0.023 | 25.6 ± 6.2 | 38.1 ± 14.5 | <0.001 | 0.866 † |
| LVEDV (ml) | 218 ± 44 | 192 ± 83 | 0.484 | 236 ± 76 | 192 ± 91 | <0.001 | 0.592 † |
| LVESV (ml) | 174 ± 60 | 118 ± 60 | 0.036 | 177 ± 64 | 132 ± 82 | <0.001 | 0.768 † |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.R.; Lim, K.; Park, S.-J.; Park, J.-S.; Kim, J.Y.; Chung, S.; Jung, D.-S.; Park, K.-M.; On, Y.K.; Kim, J.S. Thoracoscopic Implantation of Epicardial Left Ventricular Lead for Cardiac Resynchronization Therapy. J. Cardiovasc. Dev. Dis. 2022, 9, 160. https://doi.org/10.3390/jcdd9050160
Kim HR, Lim K, Park S-J, Park J-S, Kim JY, Chung S, Jung D-S, Park K-M, On YK, Kim JS. Thoracoscopic Implantation of Epicardial Left Ventricular Lead for Cardiac Resynchronization Therapy. Journal of Cardiovascular Development and Disease. 2022; 9(5):160. https://doi.org/10.3390/jcdd9050160
Chicago/Turabian StyleKim, Hye Ree, Kyunghee Lim, Seung-Jung Park, Jong-Sung Park, Ju Youn Kim, Suryeun Chung, Dong-Seop Jung, Kyoung-Min Park, Young Keun On, and June Soo Kim. 2022. "Thoracoscopic Implantation of Epicardial Left Ventricular Lead for Cardiac Resynchronization Therapy" Journal of Cardiovascular Development and Disease 9, no. 5: 160. https://doi.org/10.3390/jcdd9050160
APA StyleKim, H. R., Lim, K., Park, S.-J., Park, J.-S., Kim, J. Y., Chung, S., Jung, D.-S., Park, K.-M., On, Y. K., & Kim, J. S. (2022). Thoracoscopic Implantation of Epicardial Left Ventricular Lead for Cardiac Resynchronization Therapy. Journal of Cardiovascular Development and Disease, 9(5), 160. https://doi.org/10.3390/jcdd9050160






