Exercise Training and Interventions for Coronary Artery Disease
Abstract
1. Introduction
2. Bibliographic Search
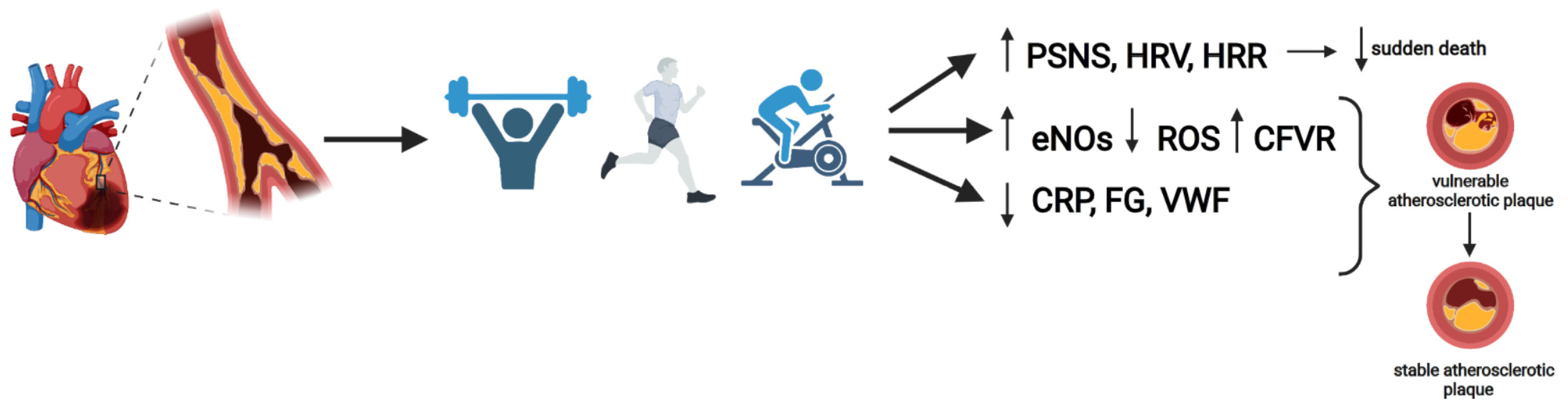
3. Effects of Exercise in CAD Patients
4. Aerobic Training (AT)
5. Resistance Training
6. Inspiratory Muscle Training
7. Exercise Prescription
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmadi, A.; Dabidi Roshan, V.; Jalali, A. Coronary vasomotion and exercise-induced adaptations in coronary artery disease patients: A systematic review and meta-analysis. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2020, 25, 76. [Google Scholar]
- Bruning, R.S.; Sturek, M. Benefits of exercise training on coronary blood flow in coronary artery disease patients. Prog. Cardiovasc. Dis. 2015, 57, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Racca, V.; Spezzaferri, R.; Modica, M.; Mazzini, P.; Jonsdottir, J.; De Maria, R.; Ferratini, M. Functioning and disability in ischaemic heart disease. Disabil. Rehabil. 2010, 32 (Suppl. 1), S42–S49. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Tsai, J.C.; Liou, Y.M.; Chan, P. Effectiveness of endurance exercise training in patients with coronary artery disease: A meta-analysis of randomised controlled trials. Eur. J. Cardiovasc. Nurs. 2017, 16, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Piepoli, M.F.; Corra, U.; Benzer, W.; Bjarnason-Wehrens, B.; Dendale, P.; Gaita, D.; McGee, H.; Mendes, M.; Niebauer, J.; Zwisler, A.-D.O.; et al. Secondary prevention through cardiac rehabilitation: From knowledge to implementation. A position paper from the Cardiac Rehabilitation Section of the European Association of Cardiovascular Prevention and Rehabilitation. Eur. J. Cardiovasc. Prev. Rehabil. 2010, 17, 1–17. [Google Scholar] [CrossRef]
- Anderson, L.; Oldridge, N.; Thompson, D.R.; Zwisler, A.D.; Rees, K.; Martin, N.; Taylor, R.S. Exercise-Based Cardiac Rehabilitation for Coronary Heart Disease: Cochrane Systematic Review and Meta-Analysis. J. Am. Coll. Cardiol. 2016, 67, 1–12. [Google Scholar] [CrossRef]
- Lawler, P.R.; Filion, K.B.; Eisenberg, M.J. Efficacy of exercise-based cardiac rehabilitation post-myocardial infarction: A systematic review and meta-analysis of randomized controlled trials. Am. Heart J. 2011, 162, 571–584.e2. [Google Scholar] [CrossRef]
- Linke, A.; Erbs, S.; Hambrecht, R. Effects of exercise training upon endothelial function in patients with cardiovascular disease. Front. Biosci. J. Virtual Libr. 2008, 13, 424–432. [Google Scholar] [CrossRef]
- Sattelmair, J.; Pertman, J.; Ding, E.L.; Kohl, H.W.; Haskell, W.; Lee, I.-M. Dose response between physical activity and risk of coronary heart disease. Circulation 2011, 124, 789–795. [Google Scholar] [CrossRef]
- Sturek, M. Ca2+ regulatory mechanisms of exercise protection against coronary artery disease in metabolic syndrome and diabetes. J. Appl. Physiol. 2011, 111, 573–586. [Google Scholar] [CrossRef]
- Libby, P.; Theroux, P. Pathophysiology of coronary artery disease. Circulation 2005, 111, 3481–3488. [Google Scholar] [CrossRef] [PubMed]
- Boden, W.E.; Franklin, B.; Berra, K.; Haskell, W.L.; Calfas, K.J.; Zimmerman, F.H.; Wenger, N.K. Exercise as a therapeutic intervention in patients with stable ischemic heart disease: An underfilled prescription. Am. J. Med. 2014, 127, 905–911. [Google Scholar] [CrossRef]
- Thompson, G.; Davison, G.W.; Crawford, J.; Hughes, C.M. Exercise and inflammation in coronary artery disease: A systematic review and meta-analysis of randomised trials. J. Sports Sci. 2020, 38, 814–826. [Google Scholar] [CrossRef] [PubMed]
- Winzer, E.B.; Woitek, F.; Linke, A. Physical Activity in the Prevention and Treatment of Coronary Artery Disease. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2018, 7, e007725. [Google Scholar] [CrossRef] [PubMed]
- Gielen, S.; Schuler, G.; Hambrecht, R. Exercise training in coronary artery disease and coronary vasomotion. Circulation 2001, 103, E1–E6. [Google Scholar] [CrossRef] [PubMed]
- Hambrecht, R.; Wolf, A.; Gielen, S.; Linke, A.; Hofer, J.; Erbs, S.; Schoene, N.; Schuler, G. Effect of Exercise on Coronary Endothelial Function in Patients with Coronary Artery Disease. N. Engl. J. Med. 2000, 342, 454–460. [Google Scholar] [CrossRef]
- Vanhoutte, P.M.; Shimokawa, H.; Feletou, M.; Tang, E.H.C. Endothelial dysfunction and vascular disease—A 30th anniversary update. Acta Physiol. 2017, 219, 22–96. [Google Scholar] [CrossRef]
- Wienbergen, H.; Hambrecht, R. Physical exercise and its effects on coronary artery disease. Curr. Opin. Pharmacol. 2013, 13, 218–225. [Google Scholar] [CrossRef]
- Laughlin, M.H.; Newcomer, S.C.; Bender, S.B. Importance of hemodynamic forces as signals for exercise-induced changes in endothelial cell phenotype. J. Appl. Physiol. 2008, 104, 588–600. [Google Scholar] [CrossRef]
- Vita, J.A.; Keaney, J.F. Endothelial function: A barometer for cardiovascular risk? Circulation 2002, 106, 640–642. [Google Scholar] [CrossRef]
- Gielen, S.; Schuler, G.; Adams, V. Cardiovascular effects of exercise training: Molecular mechanisms. Circulation 2010, 122, 1221–1238. [Google Scholar] [CrossRef] [PubMed]
- Duncker, D.J.; Bache, R.J. Regulation of coronary blood flow during exercise. Physiol. Rev. 2008, 88, 1009–1086. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.S.; Chen, L.S.; Cao, J.M.; Sharifi, B.; Karagueuzian, H.S.; Fishbein, M.C. Sympathetic nerve sprouting, electrical remodeling and the mechanisms of sudden cardiac death. Cardiovasc. Res. 2001, 50, 409–416. [Google Scholar] [CrossRef]
- Figueiredo, T.D.G.; Souza, H.C.M.D.; Neves, V.R.; do Rêgo Barros, A.E.V.; de Andrade, A.D.F.D.; Brandão, D.C. Effects of physical exercise on the autonomic nervous system in patients with coronary artery disease: A systematic review. Expert Rev. Cardiovasc. Ther. 2020, 18, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Manresa-Rocamora, A.; Ribeiro, F.; Sarabia, J.M.; Íbias, J.; Oliveira, N.L.; Vera-García, F.J.; Moya-Ramón, M. Exercise-based cardiac rehabilitation and parasympathetic function in patients with coronary artery disease: A systematic review and meta-analysis. Clin. Auton. Res. 2021, 31, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Peçanha, T.; Silva-Júnior, N.D.; de Moraes Forjaz, C.L. Heart rate recovery: Autonomic determinants, methods of assessment and association with mortality and cardiovascular diseases. Clin. Physiol. Funct. Imaging 2014, 34, 327–339. [Google Scholar] [CrossRef]
- Kannankeril, P.J.; Le, F.K.; Kadish, A.H.; Goldberger, J.J. Parasympathetic effects on heart rate recovery after exercise. J. Investig. Med. Off. Publ. Am. Fed. Clin. Res. 2004, 52, 394–401. [Google Scholar]
- Coote, J.H. Recovery of heart rate following intense dynamic exercise. Exp. Physiol. 2010, 95, 431–440. [Google Scholar] [CrossRef]
- Goldberger, J.J.; Johnson, N.P.; Subacius, H.; Ng, J.; Greenland, P. Comparison of the physiologic and prognostic implications of the heart rate versus the RR interval. Heart Rhythm. 2014, 11, 1925–1933. [Google Scholar] [CrossRef]
- Peçanha, T.; Bartels, R.; Brito, L.; Paula-Ribeiro, M.; Oliveira, R.S.; Goldberger, J.J. Methods of assessment of the post-exercise cardiac autonomic recovery: A methodological review. Int. J. Cardiol. 2017, 227, 795–802. [Google Scholar] [CrossRef]
- von Haehling, S.; Steinbeck, L.; Doehner, W.; Springer, J.; Anker, S.D. Muscle wasting in heart failure: An overview. Int. J. Biochem. Cell Biol. 2013, 45, 2257–2265. [Google Scholar] [CrossRef] [PubMed]
- Heineke, J.; Auger-Messier, M.; Xu, J.; Sargent, M.; York, A.; Welle, S.; Molkentin, J.D. Genetic deletion of myostatin from the heart prevents skeletal muscle atrophy in heart failure. Circulation 2010, 121, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Hotta, K.; Ota, E.; Mori, R.; Matsunaga, A. Effects of resistance training on muscle strength, exercise capacity, and mobility in middle-aged and elderly patients with coronary artery disease: A meta-analysis. J. Cardiol. 2016, 68, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, G.F.; Ades, P.A.; Kligfield, P.; Arena, R.; Balady, G.J.; Bittner, V.; Coke, L.A.; Fleg, J.L.; Forman, D.E.; Gerber, T.C.; et al. Exercise standards for testing and training: A scientific statement from the American Heart Association. Circulation 2013, 128, 873–934. [Google Scholar] [CrossRef]
- Gomes-Neto, M.; Durães, A.R.; dos Reis, H.F.C.; Neves, V.R.; Martinez, B.P.; Carvalho, V.O. High-intensity interval training versus moderate-intensity continuous training on exercise capacity and quality of life in patients with coronary artery disease: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2017, 24, 1696–1707. [Google Scholar] [CrossRef]
- Mann, T.N.; Webster, C.; Lamberts, R.P.; Lambert, M.I. Effect of exercise intensity on post-exercise oxygen consumption and heart rate recovery. Eur. J. Appl. Physiol. 2014, 114, 1809–1820. [Google Scholar] [CrossRef]
- Claes, J.; Buys, R.; Budts, W.; Smart, N.; Cornelissen, V.A. Longer-term effects of home-based exercise interventions on exercise capacity and physical activity in coronary artery disease patients: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2017, 24, 244–256. [Google Scholar] [CrossRef]
- Arbit, B.; Azarbal, B.; Hayes, S.W.; Gransar, H.; Germano, G.; Friedman, J.D.; Thomson, L.; Berman, D.S. Prognostic Contribution of Exercise Capacity, Heart Rate Recovery, Chronotropic Incompetence, and Myocardial Perfusion Single-Photon Emission Computerized Tomography in the Prediction of Cardiac Death and All-Cause Mortality. Am. J. Cardiol. 2015, 116, 1678–1684. [Google Scholar] [CrossRef]
- Myers, J.; Prakash, M.; Froelicher, V.; Do, D.; Partington, S.; Atwood, J.E. Exercise Capacity and Mortality among Men Referred for Exercise Testing. N. Engl. J. Med. 2002, 346, 793–801. [Google Scholar] [CrossRef]
- Laukkanen, J.A.; Rauramaa, R.; Salonen, J.T.; Kurl, S. The predictive value of cardiorespiratory fitness combined with coronary risk evaluation and the risk of cardiovascular and all-cause death. J. Intern. Med. 2007, 262, 263–272. [Google Scholar] [CrossRef]
- Dibben, G.; Faulkner, J.; Oldridge, N.; Rees, K.; Thompson, D.R.; Zwisler, A.-D.; Taylor, R.S. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst. Rev. 2021, 11, CD001800. [Google Scholar] [PubMed]
- Sakellariou, X.M.; Papafaklis, M.I.; Domouzoglou, E.M.; Katsouras, C.S.; Michalis, L.K.; Naka, K.K. Exercise-mediated adaptations in vascular function and structure: Beneficial effects in coronary artery disease. World J. Cardiol. 2021, 13, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Hambrecht, R.; Adams, V.; Erbs, S.; Linke, A.; Kränkel, N.; Shu, Y.; Baither, Y.; Gielen, S.; Thiele, H.; Gummert, J.F.; et al. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation 2003, 107, 3152–3158. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, S.L.; Lopes, S.; Bohn, L.; Cavero-Redondo, I.; Álvarez-Bueno, C.; Viamonte, S.; Santos, M.; Oliveira, J.; Ribeiro, F. Effects of exercise on endothelial progenitor cells in patients with cardiovascular disease: A systematic review and meta-analysis of randomized controlled trials. Rev. Port. Cardiol. 2019, 38, 817–827. [Google Scholar] [CrossRef]
- Harold Laughlin, M.; Bowles, D.K.; Duncker, D.J. The coronary circulation in exercise training. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H10–H23. [Google Scholar] [CrossRef]
- Swardfager, W.; Herrmann, N.; Cornish, S.; Mazereeuw, G.; Marzolini, S.; Sham, L.; Lanctôt, K.L. Exercise intervention and inflammatory markers in coronary artery disease: A meta-analysis. Am. Heart J. 2012, 163, 666–676.e3. [Google Scholar] [CrossRef]
- Kraal, J.J.; Vromen, T.; Spee, R.; Kemps, H.M.C.; Peek, N. The influence of training characteristics on the effect of exercise training in patients with coronary artery disease: Systematic review and meta-regression analysis. Int. J. Cardiol. 2017, 245, 52–58. [Google Scholar] [CrossRef]
- Vromen, T.; Kraal, J.; Kuiper, J.; Spee, R.; Peek, N.; Kemps, H. The influence of training characteristics on the effect of aerobic exercise training in patients with chronic heart failure: A meta-regression analysis. Int. J. Cardiol. 2016, 208, 120–127. [Google Scholar] [CrossRef]
- Xanthos, P.D.; Gordon, B.A.; Kingsley, M.I.C. Implementing resistance training in the rehabilitation of coronary heart disease: A systematic review and meta-analysis. Int. J. Cardiol. 2017, 230, 493–508. [Google Scholar] [CrossRef]
- Hollings, M.; Mavros, Y.; Freeston, J.; Fiatarone Singh, M. The effect of progressive resistance training on aerobic fitness and strength in adults with coronary heart disease: A systematic review and meta-analysis of randomised controlled trials. Eur. J. Prev. Cardiol. 2017, 24, 1242–1259. [Google Scholar] [CrossRef]
- Marzolini, S.; Oh, P.I.; Brooks, D. Effect of combined aerobic and resistance training versus aerobic training alone in individuals with coronary artery disease: A meta-analysis. Eur. J. Prev. Cardiol. 2012, 19, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Yu, M.; Li, J.; Zhang, H.; Liu, Q.; Zhao, L.; Wang, T.; Xu, H. Efficacy and Safety of Resistance Training for Coronary Heart Disease Rehabilitation: A Systematic Review of Randomized Controlled Trials. Front. Cardiovasc. Med. 2021, 8, 754794. [Google Scholar] [CrossRef] [PubMed]
- Vona, M.; Codeluppi, G.; Iannino, T.; Ferrari, E.; Bogousslavsky, J.; Von Segesser, L. Effects of Different Types of Exercise Training Followed by Detraining on Endothelium-Dependent Dilation in Patients with Recent Myocardial Infarction. Circulation 2009, 119, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.R.; Taylor, B.J. Inspiratory muscle weakness in cardiovascular diseases: Implications for cardiac rehabilitation. Prog. Cardiovasc. Dis. 2021, 70, 49–57. [Google Scholar] [CrossRef]
- Fernández-Rubio, H.; Becerro-de-Bengoa-Vallejo, R.; Rodríguez-Sanz, D.; Calvo-Lobo, C.; Vicente-Campos, D.; Chicharro, J.L. Unraveling the Role of Respiratory Muscle Metaboloreceptors under Inspiratory Training in Patients with Heart Failure. Int. J. Environ. Res. Public Health 2021, 18, 1697. [Google Scholar] [CrossRef]
- Fernandez-Rubio, H.; Becerro-De-Bengoa-Vallejo, R.; Rodríguez-Sanz, D.; Calvo-Lobo, C.; Vicente-Campos, D.; Chicharro, J.L. Inspiratory Muscle Training in Patients with Heart Failure. J. Clin. Med. 2020, 9, 1710. [Google Scholar] [CrossRef]
- Cahalin, L.P.; Arena, R.; Guazzi, M.; Myers, J.; Cipriano, G.; Chiappa, G.; Lavie, C.J.; Forman, D.E. Inspiratory muscle training in heart disease and heart failure: A review of the literature with a focus on method of training and outcomes. Expert Rev. Cardiovasc. Ther. 2013, 11, 161–177. [Google Scholar] [CrossRef]
- Wu, J.; Kuang, L.; Fu, L. Effects of inspiratory muscle training in chronic heart failure patients: A systematic review and meta-analysis. Congenit. Heart Dis. 2018, 13, 194–202. [Google Scholar] [CrossRef]
- Cahalin, L.P.; Arena, R.A. Breathing exercises and inspiratory muscle training in heart failure. Heart Fail. Clin. 2015, 11, 149–172. [Google Scholar] [CrossRef]
- Dos Santos, T.D.; Pereira, S.N.; Portela, L.O.C.; Cardoso, D.M.; Lago, P.D.; Dos Santos Guarda, N.; Moresco, R.N.; Pereira, M.B.; Albuquerque, I.M. Moderate-to-high intensity inspiratory muscle training improves the effects of combined training on exercise capacity in patients after coronary artery bypass graft surgery: A randomized clinical trial. Int. J. Cardiol. 2019, 279, 40–46. [Google Scholar] [CrossRef]
- Hulzebos, E.H.J.; Van Meeteren, N.L.U.; Van Den Buijs, B.J.W.M.; De Bie, R.A.; Rivière, A.B.D.; Helders, P.J.M. Feasibility of preoperative inspiratory muscle training in patients undergoing coronary artery bypass surgery with a high risk of postoperative pulmonary complications: A randomized controlled pilot study. Clin. Rehabil. 2006, 20, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Hulzebos, E.H.; Helders, P.J.; Favié, N.J.; De Bie, R.A.; de la Riviere, A.B.; Van Meeteren, N.L. Preoperative intensive inspiratory muscle training to prevent postoperative pulmonary complications in high-risk patients undergoing CABG surgery: A randomized clinical trial. J. Am. Med. Assoc. 2006, 296, 1851–1857. [Google Scholar] [CrossRef] [PubMed]
- Stein, R.; Maia, C.P.; Silveira, A.D.; Chiappa, G.R.; Myers, J.; Ribeiro, J.P. Inspiratory Muscle Strength as a Determinant of Functional Capacity Early After Coronary Artery Bypass Graft Surgery. Arch. Phys. Med. Rehab. 2009, 90, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
- Turky, K.; Afify, A.M.A. Effect of Preoperative Inspiratory Muscle Training on Alveolar-Arterial Oxygen Gradients After Coronary Artery Bypass Surgery. J. Cardiopulm. Rehabil. Prev. 2017, 37, 290–294. [Google Scholar] [CrossRef]
- Valkenet, K.; Trappenburg, J.C.A.; Hulzebos, E.H.; van Meeteren, N.L.U.; Backx, F.J.G. Effects of a pre-operative home-based inspiratory muscle training programme on perceived health-related quality of life in patients undergoing coronary artery bypass graft surgery. Physiotherapy 2017, 103, 276–282. [Google Scholar] [CrossRef]
- Savci, S.; Degirmenci, B.; Saglam, M.; Arikan, H.; Inal-Ince, D.; Turan, H.N.; Demircin, M. Short-term effects of inspiratory muscle training in coronary artery bypass graft surgery: A randomized controlled trial. Scand. Cardiovasc. J. 2011, 45, 286–293. [Google Scholar] [CrossRef]
- Weiner, P.; Zeidan, F.; Zamir, D.; Pelled, B.; Waizman, J.; Beckerman, M.; Weiner, M. Prophylactic inspiratory muscle training in patients undergoing coronary artery bypass graft. World J. Surg. 1998, 22, 427–431. [Google Scholar] [CrossRef]
- Miozzo, A.P.; Stein, C.; Marcolino, M.Z.; Sisto, I.R.; Hauck, M.; Coronel, C.C.; Plentz, R.D.M. Effects of High-Intensity Inspiratory Muscle Training Associated with Aerobic Exercise in Patients Undergoing CABG: Randomized Clinical Trial. Braz. J. Cardiovasc. Surg. 2018, 33, 376–383. [Google Scholar] [CrossRef]
- Matheus, G.B.; Dragosavac, D.; Trevisan, P.; Costa, C.E.; Lopes, M.M.; Ribeiro, G.C. Inspiratory muscle training improves tidal volume and vital capacity after CABG surgery. Rev. Bras. Cir. Cardiovasc. 2012, 27, 362–369. [Google Scholar] [CrossRef][Green Version]
- Hermes, B.M.; Cardoso, D.M.; Gomes, T.J.; Santos, T.D.; Vicente, M.S.; Pereira, S.N.; Barbosa, V.A.; Albuquerque, I.M. Short-term inspiratory muscle training potentiates the benefits of aerobic and resistance training in patients undergoing CABG in phase II cardiac rehabilitation program. Rev. Bras. Cir. Cardiovasc. 2015, 30, 474–481. [Google Scholar]
- Darnley, G.M.; Gray, A.C.; McClure, S.J.; Neary, P.; Petrie, M.; McMurray, J.J.V.; MacFarlane, N.G. Effects of resistive breathing on exercise capacity and diaphragm function in patients with ischaemic heart disease. Eur. J. Heart Fail. 1999, 1, 297–300. [Google Scholar] [CrossRef]
- Muammer, K.; Mutluay, F.; Demir, R.; Özkan, A.A. Effects of peripheral and different inspiratory muscle training methods in coronary artery disease patients with metabolic syndrome: A randomized-controlled trial. Respir. Med. 2020, 172, 106119. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, P.H.; Ross, J.H.; Joo, K.C. Contemporary Approaches to Prescribing Exercise in Coronary Artery Disease Patients. Am. J. Lifestyle Med. 2016, 12, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Mytinger, M.; Nelson, R.K.; Zuhl, M. Exercise Prescription Guidelines for Cardiovascular Disease Patients in the Absence of a Baseline Stress Test. J. Cardiovasc. Dev. Dis. 2020, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.-T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts): Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. J. Prev. Cardiol. 2016, 23, NP1–NP96. [Google Scholar] [PubMed]
- Price, K.J.; Gordon, B.A.; Bird, S.R.; Benson, A.C. A review of guidelines for cardiac rehabilitation exercise programmes: Is there an international consensus? Eur. J. Prev. Cardiol. 2016, 23, 1715–1733. [Google Scholar] [CrossRef] [PubMed]
| Studies | Groups | Parameters Improving Significantly (p < 0.05) with Aerobic Training | Parameters Not Improving Significantly (p < 0.05) with Aerobic Training |
|---|---|---|---|
| Chen, 2017 | AT vs. CG | Peak VO2, LDL-C, HDL-C, LVEF and SBP | DBP, triglycerides and total cholesterol levels |
| Kraal, 2017 | AT vs. CG | Peak VO2 | - |
| Studies | Groups | Parameters Improving Significantly (p < 0.05) with Resistance Training | Parameters Not Improving Significantly (p < 0.05) with Resistance Training |
|---|---|---|---|
| Yamamoto, 2016 | (Older) RCT vs. AT | Peak VO2, lower extremity strength, time of exercise and mobility | - |
| (Middle-aged) RCT vs. AT | Peak VO2, lower extremity strength and time of exercise | Mobility | |
| Fan, 2021 | RT vs. CG | Peak VO2, quality of life, LVEF and LVEDD | - |
| RT vs. AT | Anaerobic threshold and LVEF | Peak VO2 and quality of life | |
| CT vs. AT | Peak VO2, quality of life in the physical and global component, upper and lower body muscle strength, anaerobic threshold and LVEF | Maximal VO2, the emotional component of quality of life, and LVEDD | |
| Hollings, 2017 | RT vs. CG | Upper and lower body muscle strength | - |
| RT vs. AT | - | Peak VO2 and work capacity | |
| CT vs. AT | Maximal work capacity and upper and lower body muscle strength | Peak VO2 | |
| Xanthos, 2017 | RT vs. AT | - | Peak VO2 and muscle strength |
| CT vs. AT | Peak VO2, maximal work capacity and muscle strength | - | |
| Marzolini, 2012 | CT vs. AT | Maximal exercise capacity, ventilatory threshold, fat-free mass and upper and lower body muscle strength | Peak VO2 |
| Studies | Groups | Parameters Improving Significantly (p < 0.05) with Inspiratory Muscle Training | Parameters Not Improving Significantly (p < 0.05) with Inspiratory Training |
|---|---|---|---|
| Dos Santos, 2019 | IMT vs. CG | MIP, peak VO2, 6MWT, FRAP and MLHFQ | SMIP, CRP, NOx and AOPP |
| Hulzebos, 2006 [61] | IMT vs. CG | MIP and post-operative pulmonary complications | FVC, FEV1, VC and length of hospital stay |
| Hulzebos, 2006 [62] | IMT vs. CG | MIP, SMIP, post-operative pulmonary complications and length of hospital stay | - |
| Stein, 2009 | IMT vs. CG | MIP, MEP, peak VO2, 6MWT, FVC and FEV1 | - |
| Turky, 2017 | IMT vs. CG | MIP, alveolar-arterial oxygen gradient and oxygen saturation | - |
| Valkenet, 2017 | IMT vs. CG | MIP, post-operative pulmonary complications and length of hospital stay | SF-36 questionnaire and EQ-5D-3L |
| Savci, 2011 | IMT vs. CG | MIP, 6MWT, length of hospital stay, duration in intensive care, NHP (sleep) and HADS (anxiety) | FVC, FEV1, FEV1/FVC, NHP (emotional reactions, pain, energy, social isolation and physical mobility) and HADS (depression) |
| Weiner, 1998 | IMT vs. CG | MIP and SMIP | FVC and FEV1 |
| Miozzo, 2018 | AT + IMT vs. AT | MIP | MEP, peak VO2, 6MWT, muscle strength and SF-36 questionnaire |
| Matheus, 2012 | IMT vs. CG | VC and TV | MIP, MEP and MEF |
| Hermes, 2015 | IMT | MIP, MEP, peak VO2 and MLHFQ | - |
| Variable | Recommendation, CAD Patient | Recommendation, Healthy Subject |
|---|---|---|
| Mode | Continuous aerobic training | |
| Frequency | Three to five sessions per week | |
| Duration | 20–60 min per session | |
| Intensity |
|
|
| Variable | Recommendation, CAD Patient | Recommendation, Healthy Subject |
|---|---|---|
| Frequency | Two or three times per week | Two times per week |
| Sets/Repetitions | 1–2 sets/12–15 repetitions | 2–3 sets/8–12 repetitions |
| Loads | 40–50% of the 1 RM | 60–80% of the 1 RM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Rubio, H.; Becerro-de-Bengoa-Vallejo, R.; Rodríguez-Sanz, D.; Calvo-Lobo, C.; Vicente-Campos, D.; Chicharro, J.L. Exercise Training and Interventions for Coronary Artery Disease. J. Cardiovasc. Dev. Dis. 2022, 9, 131. https://doi.org/10.3390/jcdd9050131
Fernández-Rubio H, Becerro-de-Bengoa-Vallejo R, Rodríguez-Sanz D, Calvo-Lobo C, Vicente-Campos D, Chicharro JL. Exercise Training and Interventions for Coronary Artery Disease. Journal of Cardiovascular Development and Disease. 2022; 9(5):131. https://doi.org/10.3390/jcdd9050131
Chicago/Turabian StyleFernández-Rubio, Hugo, Ricardo Becerro-de-Bengoa-Vallejo, David Rodríguez-Sanz, César Calvo-Lobo, Davinia Vicente-Campos, and José López Chicharro. 2022. "Exercise Training and Interventions for Coronary Artery Disease" Journal of Cardiovascular Development and Disease 9, no. 5: 131. https://doi.org/10.3390/jcdd9050131
APA StyleFernández-Rubio, H., Becerro-de-Bengoa-Vallejo, R., Rodríguez-Sanz, D., Calvo-Lobo, C., Vicente-Campos, D., & Chicharro, J. L. (2022). Exercise Training and Interventions for Coronary Artery Disease. Journal of Cardiovascular Development and Disease, 9(5), 131. https://doi.org/10.3390/jcdd9050131







