Soluble (Pro)renin Receptor Is Adversely Associated with Indices of Left Ventricular Structure and Function: The African-PREDICT Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Demographic, Anthropometric and Physical Activity Measurements
2.3. Cardiovascular Measurements
2.4. Biological Sampling and Biochemical Analyses
2.5. Statistical Analyses
3. Results
3.1. Descriptive and Linear Regression Analyses
3.1.1. Characteristics of the Study Sample
3.1.2. Linear Regression Analyses
4. Discussion
Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skeggs, L.T.; Dorer, F.E.; Kahn, J.R.; Lentz, K.E.; Levine, M. The biochemistry of the renin-angiotensin system and its role in hypertension. Am. J. Med. 1976, 60, 737–748. [Google Scholar] [CrossRef]
- Volpe, M.; Savoia, C.; De Paolis, P.; Ostrowska, B.; Tarasi, D.; Rubattu, S. The renin-angiotensin system as a risk factor and therapeutic target for cardiovascular and renal disease. J. Am. Soc. Nephrol. 2002, 13, S173–S178. [Google Scholar] [CrossRef]
- Ma, T.K.; Kam, K.K.; Yan, B.P.; Lam, Y.Y. Renin–angiotensin–aldosterone system blockade for cardiovascular diseases: Current status. Br. J. Pharmacol. 2010, 160, 1273–1292. [Google Scholar] [CrossRef] [PubMed]
- Carey, R.M. Newly discovered components and actions of the renin–angiotensin system. Hypertension 2013, 62, 818–822. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fyhrquist, F.; Saijonmaa, O. Renin-angiotensin system revisited. J. Intern. Med. 2008, 264, 224–236. [Google Scholar] [CrossRef]
- Schmieder, R.E.; Langenfeld, M.R.; Friedrich, A.; Schobel, H.P.; Gatzka, C.D.; Weihprecht, H. Angiotensin II related to sodium excretion modulates left ventricular structure in human essential hypertension. Circulation 1996, 94, 1304–1309. [Google Scholar] [CrossRef] [PubMed]
- Klingbeil, A.U.; Schobel, H.; Langenfeld, M.R.; Hilgers, K.; Schäufele, T.; Schmieder, R.E. Hyper-responsiveness to angiotensin II is related to cardiac structural adaptation in hypertensive subjects. J. Hypertens. 1999, 17, 825–833. [Google Scholar] [CrossRef]
- Schlaich, M.P.; Schobel, H.P.; Langenfeld, M.R.; Hilgers, K.; Schmieder, R.E. Inadequate suppression of angiotensin II modulates left ventricular structure in humans. Clin. Nephrol. 1998, 49, 153–159. [Google Scholar]
- Varagic, J.; Ahmad, S.; Nagata, S.; Ferrario, C.M. ACE2: Angiotensin II/Angiotensin-(1–7) Balance in Cardiac and Renal Injury. Curr. Hypertens. Rep. 2014, 16, 420. [Google Scholar] [CrossRef]
- Brunner, H.R.; Gavras, H. Angiotensin blockade for hypertension: A promise fulfilled. Lancet 2002, 359, 990–992. [Google Scholar] [CrossRef]
- Nguyen, G.; Delarue, F.; Burcklé, C.; Bouzhir, L.; Giller, T.; Sraer, J.D. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J. Clin. Investig. 2002, 109, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Verdecchia, P.; Angeli, F.; Mazzotta, G.; Gentile, G.; Reboldi, G. The renin angiotensin system in the development of cardiovascular disease: Role of aliskiren in risk reduction. Vasc. Health Risk Manag. 2008, 4, 971. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Wang, F.; Liu, X.; Hu, J.; Su, J.; Lu, X.; Lu, A.; Cho, J.M.; Symons, J.D.; Zou, C.J.; et al. Soluble (pro) renin receptor induces endothelial dysfunction and hypertension in mice with diet-induced obesity via activation of angiotensin II type 1 receptor. Clin. Sci. 2021, 135, 793–810. [Google Scholar] [CrossRef] [PubMed]
- Ichihara, A.; Kaneshiro, Y.; Takemitsu, T.; Sakoda, M.; Itoh, H. The (pro) renin receptor and the kidney. Semin. Nephrol. 2007, 27, 524–528. [Google Scholar] [CrossRef]
- Nguyen, G. The (pro) renin receptor: Pathophysiological roles in cardiovascular and renal pathology. Curr. Opin. Nephrol. Hypertens. 2007, 16, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Mohammadmoradi, S.; Thompson, J.; Su, W.; Gong, M.; Nguyen, G.; Yiannikouris, F. Adipocyte (pro) renin-receptor deficiency induces lipodystrophy, liver steatosis and increases blood pressure in male mice. Hypertension 2016, 68, 213–219. [Google Scholar] [CrossRef]
- Fukushima, A.; Kinugawa, S.; Matsushima, S.; Tsutsui, H.; Homma, T. Increased plasma soluble (pro) renin receptor levels are correlated with renal dysfunction in patients with heart failure. Int. J. Cardiol. 2013, 168, 4313–4314. [Google Scholar] [CrossRef]
- Nguyen, G.; Blanchard, A.; Curis, E.; Bergerot, D.; Chambon, Y.; Hirose, T.; Caumont-Prim, A.; Tabard, S.B.; Baron, S.; Frank, M.; et al. Plasma soluble (pro) renin receptor is independent of plasma renin, prorenin, and aldosterone concentrations but is affected by ethnicity. Hypertension 2014, 63, 297–302. [Google Scholar] [CrossRef]
- Gafane-Matemane, L.F.; Kruger, R.; Smith, W.; Mels, C.M.; Van Rooyen, J.M.; Mokwatsi, G.G.; Uys, A.S.; Brits, S.J.; Schutte, A.E. Characterization of the renin-angiotensin-aldosterone system in young healthy black adults: The african prospective study on the early detection and identification of hypertension and cardiovascular disease (African-PREDICT Study). Hypertension 2021, 78, 400–410. [Google Scholar] [CrossRef]
- Brewster, L.M.; Seedat, Y.K. Why do hypertensive patients of African ancestry respond better to calcium blockers and diuretics than to ACE inhibitors and β-adrenergic blockers? A systematic review. BMC Med. 2013, 11, 141. [Google Scholar] [CrossRef]
- Ojji, D.B.; Mayosi, B.; Francis, V.; Badri, M.; Cornelius, V.; Smythe, W.; Kramer, N.; Barasa, F.; Damasceno, A.; Dzudie, A.; et al. Comparison of dual therapies for lowering blood pressure in black Africans. N. Engl. J. Med. 2019, 380, 2429–2439. [Google Scholar] [CrossRef] [PubMed]
- Schutte, A.E.; Gona, P.N.; Delles, C.; Uys, A.S.; Burger, A.; Mels, C.M.; Kruger, R.; Smith, W.; Fourie, C.M.; Botha, S.; et al. The African Prospective study on the Early Detection and Identification of Cardiovascular Disease and Hypertension (African-PREDICT): Design, recruitment and initial examination. Eur. J. Prev. Cardiol. 2019, 26, 458–470. [Google Scholar] [CrossRef] [PubMed]
- du Toit, W.L.; Schutte, A.E.; Gafane-Matemane, L.F.; Kruger, R.; Mels, C.M. The renin-angiotensin-system and left ventricular mass in young adults: The African-PREDICT study. Blood Press. 2021, 30, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Gafane-Matemane, L.F.; Mokae, N.L.; Breet, Y.; Poglitsch, M.; Schutte, A.E. Associations of central and peripheral blood pressure with the renin-angiotensin-aldosterone system in healthy young adults: The African-PREDICT study. Hypertens. Res. 2021, 44, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Van der Westhuizen, B.; Schutte, A.E.; Gafane-Matemane, L.F.; Kruger, R. Left ventricular mass independently associates with 24-hour sodium excretion in young masked hypertensive adults: The African-PREDICT study. Int. J. Cardiol. 2019, 276, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Strauss-Kruger, M.; Kruger, R.; Smith, W.; Gafane-Matemane, L.F.; Mokwatsi, G.; Wei, W.; Fedorova, O.V.; Schutte, A.E. The cardiotonic steroid marinobufagenin is a predictor of increased left ventricular mass in obesity: The African-PREDICT study. Nutrients 2020, 12, 3185. [Google Scholar] [CrossRef]
- Patro, B.K.; Jeyashree, K.; Gupta, P.K. Kuppuswamy’s socioeconomic status scale 2010—The need for periodic revision. Indian. J. Pediatr. 2012, 79, 395–396. [Google Scholar] [CrossRef]
- Marfell-Jones, T.; Stewart, A.; Olds, T. Kinanthropometry IX: Proceedings of the 9th International Conference of the International Society for the Advancement of Kinanthropometry; Routledge-Taylor & Francis: London, UK; New York, NY, USA, 2006. [Google Scholar]
- Mosteller, R. Simplified calculation of body-surface area. N. Engl. J. Med. 1987, 317, 1098. [Google Scholar] [CrossRef]
- Takken, T.; Stephens, S.; Balemans, A.; Tremblay, M.S.; Esliger, D.W.; Schneiderman, J.; Biggar, D.; Longmuir, P.; Wright, V.; McCrindle, B. Validation of the Actiheart activity monitor for measurement of activity energy expenditure in children and adolescents with chronic disease. Eur. J. Clin. Nutr. 2010, 64, 1494–1500. [Google Scholar] [CrossRef][Green Version]
- de Simone, G.; Devereux, R.B.; Daniels, S.R.; Mureddu, G.; Roman, M.J.; Kimball, T.R.; Greco, R.; Witt, S.; Contaldo, F. Stroke volume and cardiac output in normotensive children and adults: Assessment of relations with body size and impact of overweight. Circulation 1997, 95, 1837–1843. [Google Scholar] [CrossRef]
- de Simone, G.; Devereux, R.B.; Ganau, A.; Hahn, R.T.; Saba, P.S.; Mureddu, G.F.; Roman, M.J.; Howard, B.V. Estimation of left ventricular chamber and stroke volume by limited M-mode echocardiography and validation by two-dimensional and Doppler echocardiography. Am. J. Cardiol. 1996, 78, 801–807. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J.-Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef] [PubMed]
- Schulz, A.; Jankowski, J.; Zidek, W.; Jankowski, V. Absolute quantification of endogenous angiotensin II levels in human plasma using ESI-LC-MS/MS. Clin. Proteom. 2014, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Olkowicz, M.; Radulska, A.; Suraj, J.; Kij, A.; Walczak, M.; Chlopicki, S.; Smolenski, R.T. Development of a sensitive, accurate and robust liquid chromatography/mass spectrometric method for profiling of angiotensin peptides in plasma and its application for atherosclerotic mice. J. Chromatogr. A 2015, 1393, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.; Castro, A.F., III; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Stevens, L.A.; Claybon, M.A.; Schmid, C.H.; Chen, J.; Horio, M.; Imai, E.; Nelson, R.G.; Van Deventer, M.; Wang, H.-Y.; Zuo, L.; et al. Evaluation of the chronic kidney disease epidemiology collaboration equation for estimating the glomerular filtration rate in multiple ethnicities. Kidney Int. 2011, 79, 555–562. [Google Scholar] [CrossRef]
- World Health Organization and the Pan American Health Organization Group for Cardiovascular Disease Prevention through Population-Wide Dietary Salt Reduction. Protocol for Population Level Sodium Determination in 24-h Urine Samples. 2010. Available online: https://www.paho.org/hq/dmdocuments/2013/24h-urine-Protocol-eng.pdf (accessed on 18 April 2022).
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic; World Health Organization: Geneva, Switzerland, 2000; p. 9. ISBN 9241208945. [Google Scholar]
- Ekoru, K.; Murphy, G.; Young, E.; Delisle, H.; Jerome, C.; Assah, F.; Longo-Mbenza, B.; Nzambi, J.; On’Kin, J.; Buntix, F.; et al. Deriving an optimal threshold of waist circumference for detecting cardiometabolic risk in Sub-Saharan Africa. Int. J. Obes. 2018, 42, 487–494. [Google Scholar] [CrossRef]
- Tu, W.; Eckert, G.J.; Pratt, J.H.; Jan Danser, A.H. Plasma levels of prorenin and renin in blacks and whites: Their relative abundance and associations with plasma aldosterone concentration. Am. J. Hypertens. 2012, 25, 1030–1034. [Google Scholar] [CrossRef]
- Danser, A.J.; Deinum, J. Renin, prorenin and the putative (pro) renin receptor. Hypertension 2005, 46, 1069–1076. [Google Scholar] [CrossRef]
- Hirose, T.; Hashimoto, M.; Totsune, K.; Metoki, H.; Asayama, K.; Kikuya, M.; Sugimoto, K.; Katsuya, T.; Ohkubo, T.; Hashimoto, J. Association of (pro) renin receptor gene polymorphism with blood pressure in Japanese men: The Ohasama study. Am. J. Hypertens. 2009, 22, 294–299. [Google Scholar] [CrossRef]
- Ott, C.; Schneider, M.P.; Delles, C.; Schlaich, M.P.; Hilgers, K.F.; Schmieder, R.E. Association of (pro) renin receptor gene polymorphism with blood pressure in Caucasian men. Pharmacogenet. Genom. 2011, 21, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Amari, Y.; Morimoto, S.; Suda, C.; Iida, T.; Okuda, H.; Yurugi, T.; Oyama, Y.; Aoyama, N.; Nakajima, F. Serum soluble (pro) renin receptor level as a prognostic factor in patients undergoing maintenance hemodialysis. Sci. Rep. 2021, 11, 17402. [Google Scholar] [CrossRef] [PubMed]
- Ichihara, A.; Suzuki, F.; Nakagawa, T.; Kaneshiro, Y.; Takemitsu, T.; Sakoda, M.; Nabi, A.N.; Nishiyama, A.; Sugaya, T.; Hayashi, M. Prorenin receptor blockade inhibits development of glomerulosclerosis in diabetic angiotensin II type 1a receptor–deficient mice. J. Am. Soc. Nephrol. 2006, 17, 1950–1961. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, N.; Isobe, S.; Ishigaki, S.; Suzuki, T.; Iwakura, T.; Ono, M.; Fujikura, T.; Tsuji, T.; Otsuka, A.; Ishii, Y. Plasma soluble (pro) renin receptor reflects renal damage. PLoS ONE 2016, 11, e0156165. [Google Scholar] [CrossRef] [PubMed]
- Hamada, K.; Taniguchi, Y.; Shimamura, Y.; Inoue, K.; Ogata, K.; Ishihara, M.; Horino, T.; Fujimoto, S.; Ohguro, T.; Yoshimoto, Y. Serum level of soluble (pro) renin receptor is modulated in chronic kidney disease. Clin. Exp. Nephrol. 2013, 17, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Hirose, T.; Hashimoto, M.; Totsune, K.; Metoki, H.; Hara, A.; Satoh, M.; Kikuya, M.; Ohkubo, T.; Asayama, K.; Kondo, T.; et al. Association of (pro) renin receptor gene polymorphisms with lacunar infarction and left ventricular hypertrophy in Japanese women: The Ohasama study. Hypertens. Res. 2011, 34, 530–535. [Google Scholar] [CrossRef]
- Yoshikawa, A.; Aizaki, Y.; Kusano, K.I.; Kishi, F.; Susumu, T.; Iida, S.; Ishiura, S.; Nishimura, S.; Shichiri, M.; Senbonmatsu, T. The (pro) renin receptor is cleaved by ADAM19 in the Golgi leading to its secretion into extracellular space. Hypertens. Res. 2011, 34, 599–605. [Google Scholar] [CrossRef]

| s(P)RR Q1 (n = 296) <20.40 ng/mL | s(P)RR Q2 (n = 290) 20.4–22.69 ng/mL | s(P)RR Q3 (n = 294) 22.70–25.31 ng/mL | s(P)RR Q4 (n = 292) ≥25.32 ng/mL | p Trend | |
|---|---|---|---|---|---|
| Sociodemographics | |||||
| Age, years | 24.7± 3.07 | 24.3 ± 3.08 | 24.3 ± 3.15 | 24.9 ± 3.13 | 0.032 * |
| Ethnicity/Black n (%) | 200 (67.6) | 156 (53.8) | 131 (44.6) | 100 (34.3) | <0.001 |
| Sex/Female n (%) | 172 (58.1) | 151 (52.1) | 158 (53.4) | 129 (44.2) | 0.008 |
| Socioeconomic status | 0.060 | ||||
| Low, n (%) | 121 (40.9) | 119 (41) | 121 (41.2) | 102 (34.9) | |
| Middle, n (%) | 89 (30.1) | 86 (29.7) | 90 (30.6) | 74 (25.3) | |
| High, n (%) | 86 (29.1) | 85 (29.3) | 83 (28.2) | 116 (39.7) | |
| Anthropometric measurements | |||||
| Waist circumference, cm | 76.5 ± 9.70 | 78.0 ± 10.6 | 80.9 ± 12.6 | 84.9 ± 13.5 | <0.001 ‡ |
| Body weight, kg | 66.1 ± 13.4 | 68.9 ± 15.4 | 72.0 ± 18.1 | 77.6 ± 18.4 | <0.001 ‡ |
| Body height, cm | 167 ± 8.61 | 169 ± 9.45 | 168 ± 9.67 | 171 ± 9.89 | <0.001 ‡ |
| Body mass index, kg/m2 | 23.8 ± 4.56 | 24.2 ± 5.05 | 25.4 ± 5.42 | 26.8 ± 6.29 | <0.001 ‡ |
| Blood Pressure and Echocardiographic variables | |||||
| 24 h systolic BP, mmHg | 114 ± 9.50 | 116 ± 8.85 | 117 ± 9.30 | 120 ± 8.88 | <0.001 ‡ |
| 24 h diastolic BP, mmHg | 67.9 ± 5.97 | 68.0 ± 5.63 | 68.7 ±5.83 | 70.2 ± 5.89 | <0.001 ‡ |
| 24 h heart rate, bpm | 74.8 ± 10.3 | 73.4 ± 10.9 | 74.7 ± 9.85 | 74.7 ± 11.0 | 0.33 |
| Relative wall thickness, cm | 0.37 ± 0.07 | 0.37 ± 0.07 | 0.37 ± 0.07 | 0.38 ± 0.07 | 0.28 |
| Interventricular septal thickness at diastole, cm | 0.82 ± 0.17 | 0.82 ± 0.16 | 0.83 ± 0.17 | 0.84 ± 0.17 | 0.52 |
| Interventricular septal thickness at systole, cm | 1.07 ± 0.19 | 1.06 ± 0.18 | 1.08 ± 0.18 | 1.10 ± 0.18 | 0.10 |
| LV internal diameter diastole, cm/m | 2.74 ± 0.23 | 2.77 ± 0.23 | 2.81 ± 0.23 | 2.80 ± 0.25 | 0.002 * |
| LV posterior wall thickness diastole, cm/m | 0.51 ± 0.08 | 0.51 ± 0.09 | 0.52 ± 0.08 | 0.53 ± 0.08 | 0.005 * |
| End systolic volume index, mL/m | 19.5 ± 5.53 | 20.5 ± 5.59 | 22.1 ± 12.8 | 21.8 ± 7.17 | <0.001 * |
| End diastolic volume index, mL/m | 58.1 ± 12.1 | 60.1 ± 12.4 | 62.2 ± 13.2 | 62.9 ± 13.6 | <0.001 |
| Left ventricular mass index, g/m2 | 71.5 ± 18.0 | 73.2 ± 17.1 | 74.4 ± 16.7 | 74.6 ± 17.4 | 0.12 |
| LV ejection fraction, % | 66.3 ± 5.95 | 66.2 ± 5.81 | 65.6 ± 6.27 | 65.7 ± 6.26 | 0.36 |
| Stroke volume index, mL/m2.04 | 23.2 ± 5.16 | 23.8 ± 5.25 | 24.2 ± 5.19 | 24.2 ± 5.45 | 0.055 |
| E/A ratio | 2.14 ± 0.55 | 2.22 ± 0.60 | 2.14 ± 0.57 | 2.08 ± 0.52 | 0.029 |
| E/e’ ratio | 6.35 ± 1.17 | 6.28 ± 1.14 | 6.39 ± 1.17 | 6.28 ± 1.13 | 0.59 |
| LA/Ao ratio | 1.06 ± 0.15 | 1.04 ± 0.15 | 1.07 ± 0.15 | 1.08 ± 0.14 | 0.022 |
| Kidney variables | |||||
| Soluble (pro)renin receptor, ng/mL | 18.4 (15.9–20.3) | 21.6 (20.5–22.6) | 23.9 (22.8–25.2) | 28.4 (25.5–35.0) | <0.001 ‡ |
| a Prorenin, ng/mL | 0.71 (0.09–2.66) | 0.92 (0.29–3.07) | 0.84 (0.12–3.89) | 0.90 (0.14–4.84) | 0.013 * |
| Plasma renin activity-S, pmol/L | 72.1 (13.1–269) | 84.7 (13.9–272) | 92.0 (15.1–303) | 114 (23.1–373) | <0.001 ‡ |
| eq Angiotensin II, pmol/L | 54.7 (9.10–214) | 62.9 (10.1–202) | 68.9 (11.9–218) | 83.2 (18.5–280) | <0.001 ‡ |
| 24 h urinary Na/K ratio | 3.28 (1.24–7.62) | 3.32 (1.45–7.03) | 3.06 (1.21–6.69) | 2.96 (1.08–6.44) | 0.035 |
| eGFR, mL/min/1.73 m2 | 124 ± 17.1 | 121 ± 19.3 | 120 ± 17.7 | 113 ± 19.0 | <0.001 ‡ |
| Metabolic variables | |||||
| Glucose, mmol/L | 3.73 (2.37–5.39) | 3.81(2.52–5.32) | 3.89 (2.42–5.47) | 4.39 (2.77–5.76) | <0.001 ‡ |
| LDL-cholesterol, mmol/L | 2.05 (0.99–3.81) | 2.14 (0.98–3.74) | 2.28 (1.10–4.26) | 2.60 (1.36–4.53) | <0.001 ‡ |
| HDL-cholesterol, mmol/L | 1.08 (0.58–1.87) | 1.08 (0.55–1.84) | 1.08 (0.60–1.88) | 1.10 (0.56–2.03) | 0.92 |
| C-reactive protein, mg/L | 0.63 (0.05–5.85) | 0.71 (0.09–9.08) | 1.02 (0.12–8.58) | 1.34 (0.11–13.7) | <0.001 ‡ |
| Interleukin-6, mg/L | 0.95 (0.33–2.91) | 1.01 (0.38–3.98) | 1.09 (0.40–3.70) | 1.28 (0.47–4.38) | <0.001 ‡ |
| Tumor Necrosis Factor-α, mg/L | 0.91(0.34–2.41) | 0.98 (0.38–2.09) | 1.19 (0.62–2.84) | 1.22 (0.60–2.66) | <0.001 ‡ |
| Gamma-glutamyl transferase, U/L | 16.5 (5.40–54.8) | 16.7 (5.80–45.5) | 18.3 (6.20–58.5) | 21.9 (7.90–77.5) | <0.001 ‡ |
| Lifestyle factors | |||||
| Self-reported smoking, n (%) | 63 (21.5) | 56 (19.3) | 81 (27.6) | 76 (26) | 0.063 |
| Self-reported alcohol use, n (%) | 153 (52.4) | 153 (52.8) | 167 (57.2) | 176 (60.7) | 0.14 |
| AEE, kCal/kg/day | 6.31 ± 2.57 | 6.21 ± 3.16 | 6.0 ± 2.91 | 5.11 ± 2.79 | <0.001 ‡ |
| Black n = 587 | White n = 585 | p-Value | |
|---|---|---|---|
| Sociodemographics | |||
| Age, years | 24.5 ± 3.17 | 24.6 ± 3.07 | 0.59 |
| Sex/Female n (%) | 303 (51.6)) | 307 (52.5) | 0.77 |
| Socioeconomic status | <0.001 | ||
| Low, n (%) | 345 (58.8) | 118 (20.2) | |
| Middle, n (%) | 161 (27.4)) | 178 (30.4) | |
| High, n (%) | 81 (13.8) | 289 (49.4)) | |
| Anthropometric measurements | |||
| Waist circumference, cm | 77.8 ± 10.8 | 82.3 ± 12.9 | <0.001 |
| * Men, n (%) | 77 (27.1) | 83 (30.3) | 0.41 |
| * Women, n (%) | 116 (38.3) | 75 (24.4) | <0.001 |
| Body weight, kg | 66.2 ± 14.5 | 76.2 ± 17.8 | <0.001 |
| Body height, cm | 164 ± 8.40 | 173 ± 8.79 | <0.001 |
| Body mass index, kg/m2 | 24.6 ± 5.70 | 25.5 ± 5.21 | 0.005 |
| Blood Pressure and Echocardiographic Variables | |||
| 24 h systolic BP, mmHg | 116 ± 8.99 | 118 ± 9.63 | 0.001 |
| 24 h diastolic BP, mmHg | 68.8 ± 5.94 | 68.6 ± 5.86 | 0.44 |
| 24 h heart rate, bpm | 75.3 ± 10.7 | 73.6 ± 10.3 | 0.010 |
| Relative wall thickness, cm | 0.38 ± 0.07 | 0.36 ± 0.07 | <0.001 |
| Interventricular septal thickness at diastole, cm | 0.84 ± 0.17 | 0.82 ± 0.16 | 0.16 |
| Interventricular septal thickness at systole, cm | 1.08 ± 0.18 | 1.08 ± 0.18 | 0.71 |
| LV internal diameter diastole/height, cm/m | 2.76 ± 0.24 | 2.80 ± 0.24 | 0.001 |
| LV posterior wall thickness diastole/height, cm/m | 0.53 ± 0.08 | 0.50 ± 0.08 | <0.001 |
| End systolic volume index, mL/m | 19.8 ± 9.72 | 22.1 ± 6.54 | <0.001 |
| Left ventricular mass index, g/m2 | 73.6 ± 18.4 | 73.2 ± 16.2 | 0.70 |
| LV ejection fraction, % | 66.4 ± 6.23 | 65.5 ± 5.89 | 0.014 |
| Stroke volume index, mL/m2.04 | 23.5 ± 5.26 | 24.3 ± 5.27 | 0.010 |
| End diastolic volume index, mL/m | 58.0 ±11.9 | 63.9 ± 13.3 | <0.001 |
| E/A ratio | 2.25 ± 0.61 | 2.04 ± 0.48 | <0.001 |
| E/e’ ratio | 6.55 ± 1.18 | 6.10 ± 1.07 | <0.001 |
| LA/Ao ratio | 1.06 ± 0.15 | 1.06 ± 0.15 | 0.45 |
| Kidney Variables | |||
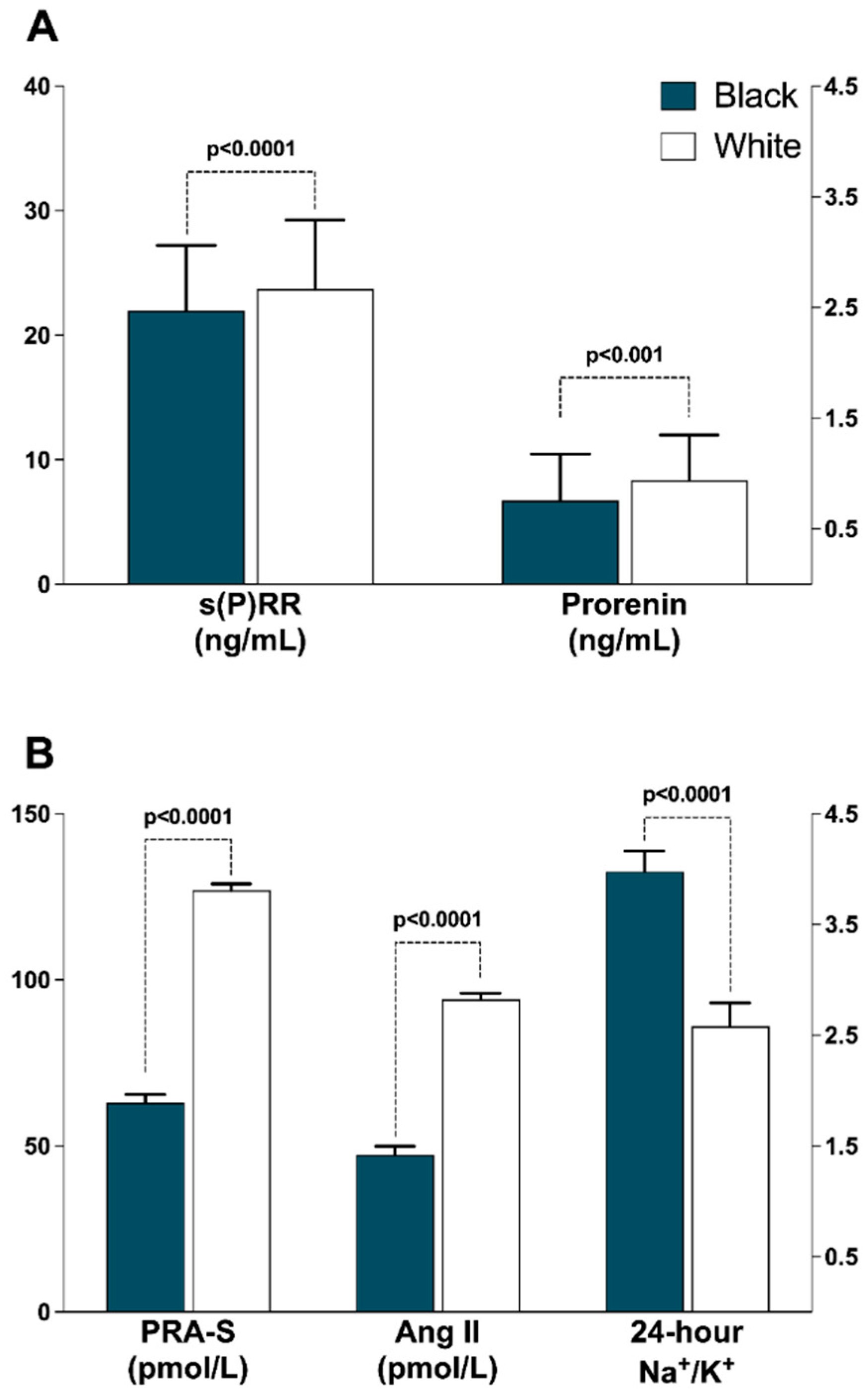
| Soluble (pro)renin receptor, ng/ml | 21.9 (19.9–29.2) | 23.7 (17.9–31.6) | <0.001 |
| Prorenin, ng/mL | 0.76 (0.12–2.78) | 0.94 (0.28–4.31) | 0.001 |
| Plasma renin activity-S, pmol/L | 63.0 (11.4–263) | 127 (39.0–337) | <0.001 |
| eq Angiotensin II, pmol/L | 47.3 (8.70–186) | 94.1 (29.3–253) | <0.001 |
| 24 h urinary Sodium, mmol/L | 114 (39.7–250) | 102 (35.8–207) | 0.001 |
| 24 h urinary Potassium, mmol/L | 34.8 (13.4–101) | 51.3 (22.8–112) | <0.001 |
| 24 h urinary Na/K ratio | 3.98 (1.94–7.96) | 2.58 (1.11–5.31) | <0.001 |
| 24 h urinary Creatinine, mmol/L | 8.83 (3.02–21.7) | 9.34 (4.03–20.9) | 0.11 |
| eGFR, mL/min/1.73 m2 | 123 ± 16.2 | 116 ± 20.4 | <0.001 |
| Metabolic Variables | |||
| Glucose, mmol/L | 3.79 (2.35–5.44) | 4.11 (2.61–5.58) | <0.001 |
| LDL-cholesterol, mmol/L | 2.07 (0.99–3.70) | 2.46 (1.23–4.42) | <0.001 |
| HDL-cholesterol, mmol/L | 1.08 (0.58–1.82) | 1.09 (0.57–2.01) | 0.78 |
| C-reactive protein, mg/L | 1.00 (0.10–10.1) | 0.78 (0.08–8.08) | 0.003 |
| Interleukin-6, mg/L | 1.24 (0.46–3.98) | 0.93 (0.32–3.04) | <0.001 |
| Tumor Necrosis Factor-α, mg/L | 0.96 (0.37–2.43) | 1.18 (0.59–2.51) | <0.001 |
| Gamma-glutamyl transferase, U/L | 22.3 (8.50–33.2) | 14. 9 (5.40–47.0) | <0.001 |
| Lifestyle factors | |||
| Self-reported smoking, n (%) | 147 (25.1) | 129 (22.1) | 0.22 |
| Self-reported alcohol use, n (%) | 325 (56.0)) | 324 (55.5) | 0.85 |
| ExpenditureAEE, kCal/kg/day | 6.54 ± 2.91 | 5.38 ± 2.64 | <0.001 |
| Soluble (Pro)renin Receptor (ng/mL) | |||
|---|---|---|---|
| Total (n = 1172) | Black (n = 587) | White (n = 585) | |
| Relative wall thickness, cm | r = 0.062; p = 0.036 | r = −0.025; p = 0.552 | r = 0.154; p < 0.001 |
| Left ventricular mass index, g/m2 | r = 0.018; p = 0.540 | r = 0.078; p = 0.063 | r = 0.042; p = 0.317 |
| Left ventricular ejection fraction, % | r = −0.009; p = 0.765 | r = 0.071; p = 0.085 | r = −0.094; p = 0.024 |
| Stroke volume index, ml/m2.04 | r = −0.01; p = 0.659 | r = 0.11; p = 0.008 | r = −0.142; p = 0.001 |
| Dependent Variables | Soluble (Pro)renin Receptor (ng/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total (n = 1172) | Black (n = 587) | White (n = 585) | |||||||
| a R2 | β (95% C.I.) | p | a R2 | β (95% C.I.) | p | R2 | β (95% C.I.) | p | |
| Relative wall thickness, cm | 0.075 | 0.073 (−0.002; 0.138) | 0.056 | 0.012 | −0.001 (−0.12; 0.10) | 0.86 | 0.101 | 0.141 (0.039; 0.218) | 0.005 |
| Left ventricular mass index, g/m2 | 0.244 | 0.037 (−7.13; 24.0) | 0.29 | 0.272 | 0.081 (−3.91; 45.5) | 0.102 | 0.213 | −0.012 (−22.6; 17.33) | 0.79 |
| Left ventricular ejection fraction, % | 0.06 | −0.031 (−8.62; 3.55) | 0.42 | 0.049 | 0.059 (−4.49; 14.6) | 0.30 | 0.072 | −0.123 (−0.22; −0.023) | 0.016 |
| Stroke volume index, ml/m2.04 | 0.071 | −0.029 (−28.2; 1.91) | 0.449 | 0.104 | 0.104 (−6.36; 37.6) | 0.067 | 0.247 | −0.144 (−0.24; −0.045) | 0.004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gafane-Matemane, L.F.; Kruger, R.; Van Rooyen, J.M.; Gona, P.N.; Schutte, A.E. Soluble (Pro)renin Receptor Is Adversely Associated with Indices of Left Ventricular Structure and Function: The African-PREDICT Study. J. Cardiovasc. Dev. Dis. 2022, 9, 130. https://doi.org/10.3390/jcdd9050130
Gafane-Matemane LF, Kruger R, Van Rooyen JM, Gona PN, Schutte AE. Soluble (Pro)renin Receptor Is Adversely Associated with Indices of Left Ventricular Structure and Function: The African-PREDICT Study. Journal of Cardiovascular Development and Disease. 2022; 9(5):130. https://doi.org/10.3390/jcdd9050130
Chicago/Turabian StyleGafane-Matemane, Lebo F., Ruan Kruger, Johannes M. Van Rooyen, Philimon N. Gona, and Aletta E. Schutte. 2022. "Soluble (Pro)renin Receptor Is Adversely Associated with Indices of Left Ventricular Structure and Function: The African-PREDICT Study" Journal of Cardiovascular Development and Disease 9, no. 5: 130. https://doi.org/10.3390/jcdd9050130
APA StyleGafane-Matemane, L. F., Kruger, R., Van Rooyen, J. M., Gona, P. N., & Schutte, A. E. (2022). Soluble (Pro)renin Receptor Is Adversely Associated with Indices of Left Ventricular Structure and Function: The African-PREDICT Study. Journal of Cardiovascular Development and Disease, 9(5), 130. https://doi.org/10.3390/jcdd9050130








