Evaluation of the Association between Fetal Cardiac Disorders with Choroid Plexus Cyst in Fetuses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Protocol
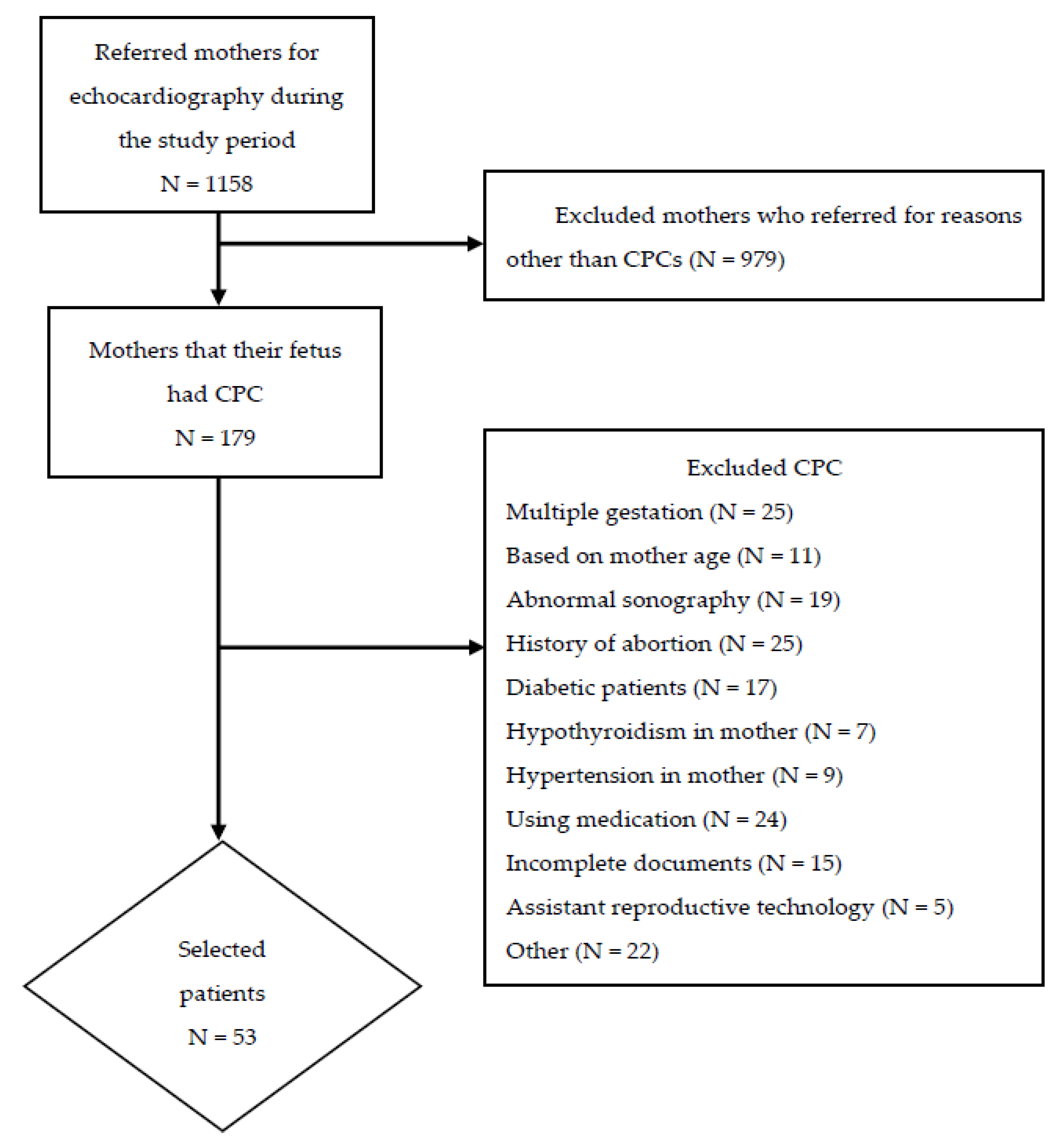
2.2. Study Participants
2.3. Echocardiography
2.4. Outcome
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walkinshaw, S.A. Fetal choroid plexus cysts: Are we there yet? Prenat. Diagn. 2000, 20, 657–662. [Google Scholar] [CrossRef]
- DiPietro, J.; Costigan, K.; Cristofalo, E.; Lu, Y.; Bird, C.; McShane, C.; Crino, J. Choroid plexus cysts do not affect fetal neurodevelopment. J. Perinatol. 2006, 26, 622–627. [Google Scholar] [CrossRef] [Green Version]
- Landy, H.J.; Bannon, P.; Zalud, I.; Collea, J.V.; Lewis, K.M.; Bartholomew, M.L.; Eglinton, G.S. Association of sex of the fetus in isolated fetal choroid plexus cysts. J. Ultrasound Med. 1999, 18, 769–771. [Google Scholar] [CrossRef] [Green Version]
- Demasio, K.; Canterino, J.; Ananth, C.; Fernandez, C.; Smulian, J.; Vintzileos, A. Isolated choroid plexus cyst in low-risk women less than 35 years old. Am. J. Obstet. Gynecol. 2002, 187, 1246–1249. [Google Scholar] [CrossRef]
- Filly, R.A.; Benacerraf, B.R.; Nyberg, D.A.; Hobbins, J.C. Choroid plexus cyst and echogenic intracardiac focus in women at low risk for chromosomal anomalies. J. Ultrasound Med. 2004, 23, 447–449. [Google Scholar] [CrossRef]
- DeVore, G.R. Second trimester ultrasonography may identify 77 to 97% of fetuses with trisomy 18. J. Ultrasound Med. 2000, 19, 565–576. [Google Scholar] [CrossRef] [Green Version]
- Ostlere, S.J.; Irving, H.; Lilford, R. Fetal choroid plexus cysts: A report of 100 cases. Radiology 1990, 175, 753–755. [Google Scholar] [CrossRef]
- Cristofalo, E.; Dipietro, J.; Costigan, K.; Nelson, P.; Crino, J. Women’s response to fetal choroid plexus cysts detected by prenatal ultrasound. J. Perinatol. 2006, 26, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Grijseels, E.; Cohen-Overbeek, T.; Adama van Scheltema, P.; Groenenberg, I.; Schoonderwaldt, E.; Steegers, E.; Wildschut, H. Sonomarkers: Subtle ultrasound findings in the 20-week ultrasound examination, which have a low association with some chromosomal and non-chromosomal abnormalities in the fetus. Ned. Tijdschr. Voor Geneeskd. 2008, 152, 2225–2231. [Google Scholar]
- Yang, Y.; Zhang, Y. Ultrasound soft markers and fetal cardiac structural assessment at 11-14 weeks. Zhonghua Fu Chan Ke Za Zhi 2014, 49, 188–192. [Google Scholar]
- Renna, M.D.; Pisani, P.; Conversano, F.; Perrone, E.; Casciaro, E.; Di Renzo, G.C.; Di Paola, M.; Perrone, A.; Casciaro, S. Sonographic markers for early diagnosis of fetal malformations. World J. Radiol. 2013, 5, 356. [Google Scholar] [CrossRef]
- Barboza, J.M.; Dajani, N.K.; Glenn, L.G.; Angtuaco, T.L. Prenatal diagnosis of congenital cardiac anomalies: A practical approach using two basic views. Radiographics 2002, 22, 1125–1138. [Google Scholar] [CrossRef]
- Allan, L. Antenatal diagnosis of heart disease. Heart 2000, 83, 367. [Google Scholar] [CrossRef] [Green Version]
- Sharland, G. Fetal cardiac screening and variation in prenatal detection rates of congenital heart disease: Why bother with screening at all? Future Cardiol. 2012, 8, 189–202. [Google Scholar] [CrossRef]
- Norton, K.I.; Rai, B.; Desai, H.; Brown, D.; Cohen, M. Prevalence of choroid plexus cysts in term and near-term infants with congenital heart disease. Am. J. Roentgenol. 2011, 196, W326–W329. [Google Scholar] [CrossRef]
- Eronen, M. Outcome of fetuses with heart disease diagnosed in utero. Arch. Dis. Child. -Fetal Neonatal Ed. 1997, 77, F41–F46. [Google Scholar] [CrossRef] [Green Version]
- Bonnet, D.; Coltri, A.; Butera, G.; Fermont, L.; Le Bidois, J.; Kachaner, J.; Sidi, D. Detection of transposition of the great arteries in fetuses reduces neonatal morbidity and mortality. Circulation 1999, 99, 916–918. [Google Scholar] [CrossRef] [Green Version]
- Tworetzky, W.; McElhinney, D.B.; Reddy, V.M.; Brook, M.M.; Hanley, F.L.; Silverman, N.H. Improved surgical outcome after fetal diagnosis of hypoplastic left heart syndrome. Circulation 2001, 103, 1269–1273. [Google Scholar] [CrossRef] [Green Version]
- Wen, S.W.; Miao, Q.; Taljaard, M.; Lougheed, J.; Gaudet, L.; Davies, M.; Lanes, A.; Leader, A.; Corsi, D.J.; Sprague, A.E.; et al. Associations of Assisted Reproductive Technology and Twin Pregnancy With Risk of Congenital Heart Defects. JAMA Pediatr 2020, 174, 446–454. [Google Scholar] [CrossRef]
- Messing, B.; Porat, S.; Imbar, T.; Valsky, D.; Anteby, E.; Yagel, S. Mild tricuspid regurgitation: A benign fetal finding at various stages of pregnancy. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2005, 26, 606–610. [Google Scholar] [CrossRef]
- Respondek, M.L.; Kammermeier, M.; Ludomirsky, A.; Weil, S.R.; Huhta, J.C. The prevalence and clinical significance of fetal tricuspid valve regurgitation with normal heart anatomy. Am. J. Obstet. Gynecol. 1994, 171, 1265–1270. [Google Scholar] [CrossRef]
- Gembruch, U.; Smrcek, J. The prevalence and clinical significance of tricuspid valve regurgitation in normally grown fetuses and those with intrauterine growth retardation. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 1997, 9, 374–382. [Google Scholar] [CrossRef]
| Case | Control | p-value | |
|---|---|---|---|
| Number | 53 | 47 | |
| Mothers’ Age (Mean ± SD) | 28.06 ± 3.69 | 27.02 ± 4.26 | 0.663 |
| Gestational Age (Mean week ± SD) | 25.53 ± 5.65 | 25.58 ± 5.65 | 0.94 |
| Number of Pregnancy (Mean ± SD) | 1.11 ± 0.32 | 1.07 ± 0.33 | 0.213 |
| Index (mean ± SD) | Case n = 53 | Control n = 47 | p-Value |
|---|---|---|---|
| CT ratio | 0.43 ± 0.01 | 0.43 ± 0.02 | 0.633 |
| LVPWd * | 2.0(1.40–3.90) | 2.0(1.59–3.40) | 0.903 |
| IVSd * | 2.1(1.50–4.10) | 2.10(1.67–3.70) | 0.621 |
| RV wall thickness * | 2.16(1.49–4.30) | 2.16(1.38–3.90) | 0.721 |
| LVEF | 64.73 ± 2.41 | 64.97 ± 1.87 | 0.582 |
| RVEF | 68.94 ± 2.54 | 68.47 ± 2.63 | 0.374 |
| LCOP | 580.17 ± 95.74 | 592.8 ± 99.29 | 0.521 |
| RCOP | 600.89 ± 98.09 | 610.09 ± 98.66 | 0.644 |
| LV-MPI | 0.43 ± 0.02 | 0.51 ± 0.52 | 0.273 |
| RV-MPI | 0.37 ± 0.02 | 0.38 ± 0.02 | 0.063 |
| TAPSE* | 0.41(0.38–0.53) | 0.41(0.37–0.45) | 0.036 |
| MAPSE | 0.46 ± 0.02 | 0.45 ± 0.02 | 0.50 |
| Mv E/A | 0.6 ± 0.01 | 0.61 ± 0.008 | 0.302 |
| TV E/A | 0.61 ± 0.01 | 0.61 ± 0.08 | 0.638 |
| LV-DFP | 40.2 ± 0.71 | 40.46 ± 0.83 | 0.097 |
| RV-DFP | 40.34 ± 0.84 | 40.42 ± 0.73 | 0.641 |
| TR velocity | 0.75 ± 0.25 | 0.76 ± 0.09 | 0.951 |
| Echocardiography | Case (n = 53) | Control (n = 47) | p-Value |
|---|---|---|---|
| Tricuspid Regurgitation | 0.43 | ||
| Trivial | 6 | 5 | |
| Mild | 2 | 0 | |
| Speed (m/s) (mean ± SD) | 0.75 ± 0.25 | 0.76 ± 0.09 | 0.951 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dakkali, M.S.; Karimi Behnagh, A.; Ghasemi Assl, S.; Kimiaeifar, A.; Radgoodarzi, M. Evaluation of the Association between Fetal Cardiac Disorders with Choroid Plexus Cyst in Fetuses. J. Cardiovasc. Dev. Dis. 2022, 9, 60. https://doi.org/10.3390/jcdd9020060
Dakkali MS, Karimi Behnagh A, Ghasemi Assl S, Kimiaeifar A, Radgoodarzi M. Evaluation of the Association between Fetal Cardiac Disorders with Choroid Plexus Cyst in Fetuses. Journal of Cardiovascular Development and Disease. 2022; 9(2):60. https://doi.org/10.3390/jcdd9020060
Chicago/Turabian StyleDakkali, Mohammad Sedigh, Arman Karimi Behnagh, Shakiba Ghasemi Assl, Atiyeh Kimiaeifar, and Mohammad Radgoodarzi. 2022. "Evaluation of the Association between Fetal Cardiac Disorders with Choroid Plexus Cyst in Fetuses" Journal of Cardiovascular Development and Disease 9, no. 2: 60. https://doi.org/10.3390/jcdd9020060
APA StyleDakkali, M. S., Karimi Behnagh, A., Ghasemi Assl, S., Kimiaeifar, A., & Radgoodarzi, M. (2022). Evaluation of the Association between Fetal Cardiac Disorders with Choroid Plexus Cyst in Fetuses. Journal of Cardiovascular Development and Disease, 9(2), 60. https://doi.org/10.3390/jcdd9020060






