Argyrophilic Nucleolar Organizer Regions as New Biomarkers in ST-Elevation Myocardial Infarction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Electrocardiography and Echocardiography
2.3. Coronary Angiography
2.4. AgNOR Staining
2.5. Image Analysis of Mean AgNOR Number and Total AgNOR Area/Total Nuclear Area (TAA/TNA) Ratio
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Damar, İ.H.; Eroz, R. The Association of Hereditary Prothrombotic Risk Factors with ST-Elevation Myocardial Infarction. Medeni. Med. J. 2020, 35, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, Z.; Deng, L.; Yuan, X.; Ma, Y.; Zhang, G.; Gantier, M.P.; Liu, J.P.; Shen, L.; Xu, D. Osteopontin promotes in flammation in patients with acute coronary syndrome through its activity on IL-17 producing cells. Eur. J. Immunol. 2012, 42, 2803–2814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frangogiannis, N.G.; Mendoza, L.H.; Lindsey, M.L.; Ballantyne, C.M.; Michael, L.H.; Smith, C.W.; Entman, M.L. IL-10 is induced in the reperfused myocardium and may modulate the reaction to injury. J. Immunol. 2000, 165, 2798–2808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spagnoli, L.G.; Bonanno, E.; Mauriello, A.; Palmieri, G.; Partenzi, A.; Sangiorgi, G.; Crea, F. Multicentric inflammation in epicardial coronary arteries of patients dying of acute myocardial infarction. J. Am. Coll. Cardiol. 2002, 40, 1579–1588. [Google Scholar] [CrossRef]
- Moreira, D.M.; da Silva, R.L.; Vieira, J.L.; Fattah, T.; Lueneberg, M.E.; Gottschall, C.A. Role of vascular inflammation in coronary artery disease: Potential of anti-inflammatory drugs in the prevention of atherothrombosis. Inflammation and anti-inflammatory drugs in coronary artery disease. Am. J. Cardiovasc. Drugs 2015, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Eroz, R.; Yilmaz, S.; Cucer, N. Argyrophilic nucleolar organizing region associated protein synthesis in hair root cells of humans at different developmental stages and sex. Biotech. Histochem. 2013, 88, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Selvi, B.; Demirtas, H.; Eroz, R.; Imamoglu, N. Reduction of the argyrophilic nucleolar organizing region associated protein synthesis with age in buccal epithelial cells of healthy individuals. Aging Clin. Exp. Res. 2015, 27, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Çolakoğlu, S.; Saritas, A.; Eroz, R.; Oktay, M.; Yaykasli, K.O.; Akoz, A.; Kaya, E.; Kandis, H. Is one-time carbon monoxide intoxication harmless? Evaluation by argyrophilic nucleolar-organizing regions staining method. Hum. Exp. Toxicol. 2015, 34, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Saritas, A.; Gunes, H.; Colakoglu, S.; Eroz, R.; Akoz, A.; Oktay, M.; Buyukkaya, A.; Kandis, H.; Ozkan, A. Are there any effects of chronic carbon monoxide exposure on argyrophilic nucleolar-organizing region-associated protein synthesis in rat myocardium? Hum. Exp. Toxicol. 2016, 35, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Kandiş, H.; Afacan, M.A.; Eröz, R.; Colakoglu, S.; Bayramoglu, A.; Oktay, M.; Saritas, A.; Colaki, S.; Kaya, M.; Kara, İ.H. Can argyrophilic nucleolar organizing region-associated protein amount be used for the detection of cardiac damage? Hum. Exp. Toxicol. 2016, 35, 323–331. [Google Scholar] [CrossRef]
- Gunes, H.; Saritas, A.; Eroz, R.; Colakoglu, S. Use of argyrophilic nucleolar–organizer region-associated protein synthesis in skeletal muscle cells for prediction of chronic carbon monoxide exposure. Toxin Rev. 2020, 39, 349–354. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; The Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction. J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benn, P.A.; Perle, M. Chromosome staining and banding techniques. In Human Cytogenetics, Constitutional Analysis, Practical Approach; Rooney, D.E., Czepulkowski, B.H., Eds.; Oxford University Press: Oxford, UK, 1986; Volume 1, pp. 91–118. [Google Scholar]
- Lindner, L.E. Improvements in the silver-staining technique for nucleolar organizer regions (AgNOR). J. Histochem. Cytochem. 1993, 41, 439–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasband, W.S. ImageJ; U.S. National Institutes of Health: Bethesda, MD, USA, 1997–2016. Available online: https://imagej.nih.gov/ij/ (accessed on 27 October 2021).
- Eroz, R.; Saritas, A.; Colakoglu, S.; Oktay, M.; Kandiş, H. Evaluation of argyrophilic nucleolar organizing region–associated protein synthesis in femoral muscle cells of rats exposed 3000 ppm carbon monoxide gas. Konuralp Med. J. 2016, 8, 9–13. [Google Scholar]
- Caimi, G.; Lo Presti, R.; Canino, B.; Ferrera, E.; Hopps, E. Behaviour of the neutrophil to lymphocyte ratio in young subjects with acute myocardial infarction. Clin. Hemorheol. Microcirc. 2016, 62, 239–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.S.; Seo, K.W.; Choi, B.J.; Choi, S.Y.; Yoon, M.H.; Hwang, G.S.; Tahk, S.J.; Shin, J.H. Importance of prognostic value of neutrophil to lymphocyte ratio in patients with ST-elevation myocardial infarction. Medicine 2018, 97, e13471. [Google Scholar] [CrossRef] [PubMed]
- Meeuwsen, J.A.L.; Wesseling, M.; Hoefer, I.E.; de Jager, S.C.A. Prognostic Value of Circulating Inflammatory Cells in Patients with Stable and Acute Coronary Artery Disease. Front. Cardiovasc. Med. 2017, 14, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Ren, L.; Liu, N.; Lei, L.; Ye, H.; Peng, J. Association of monocyte count on admission with angiographic no-reflow after primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction. Kardiol. Pol. 2016, 74, 1160–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
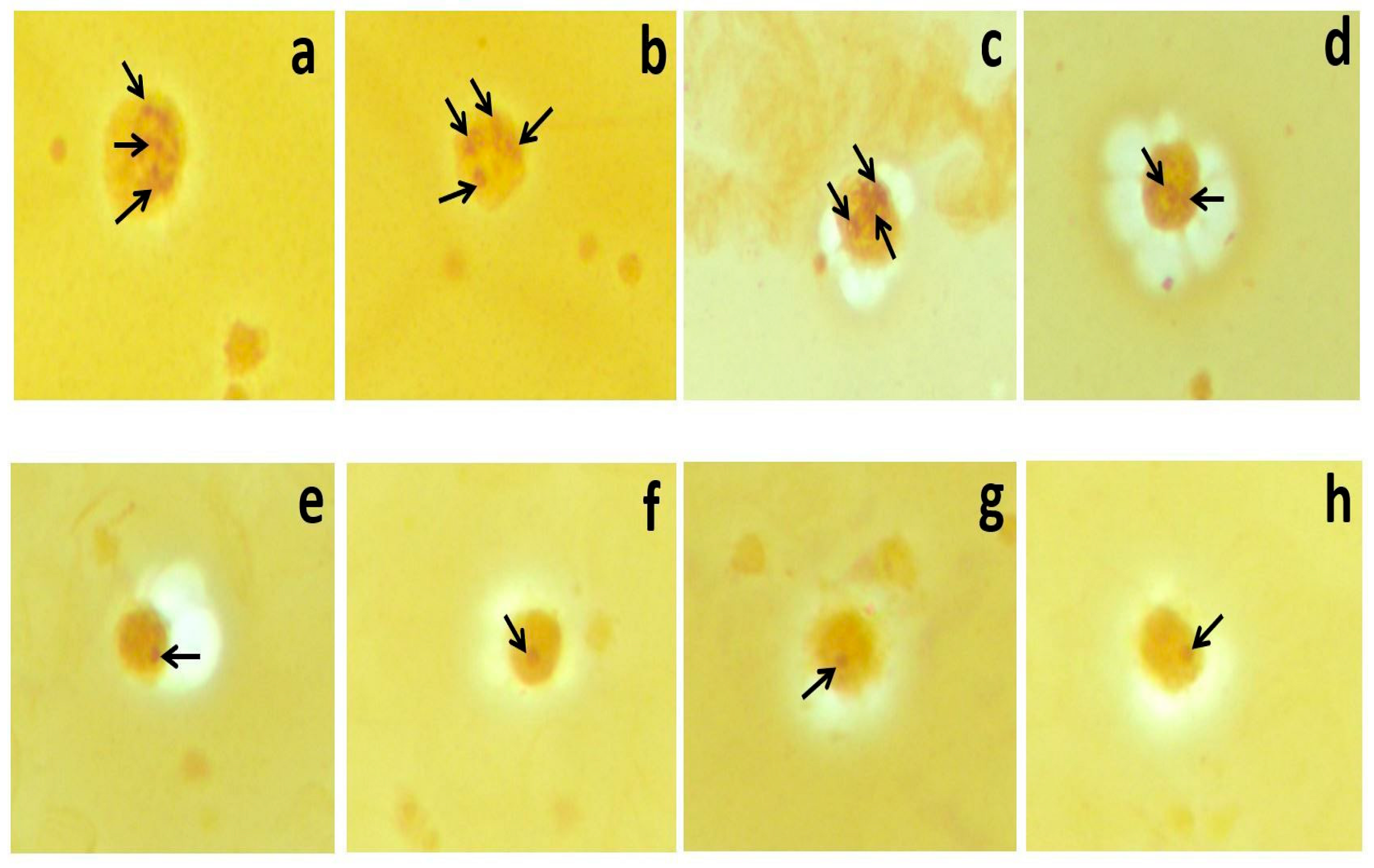
| STEMI (n = 75) Mean ± SD (Min–Max) (Median) | Control (n = 65) Mean ± SD (Min–Max) (Median) | χ2 | p | |
|---|---|---|---|---|
| Sex (M/F) (%) | 60 (80%)/15 (20%) | 52 (80%)/13 (20%) | 0.000 | 1.000 |
| Diabetes mellitus (Yes/No) (%) | 36 (48%)/39 (52%) | 21 (32.3%)/44 (67.7%) | 3.552 | 0.059 |
| Hypertension (Yes/No) (%) | 40 (53.3%)/35 (46.7%) | 37 (56.9%)/28 (43.1%) | 0.81 | 0.670 |
| Hyperlipidemia (Yes/No) (%) | 38 (50.7%)/37 (49.3%) | 33 (50.8%)/32 (49.2%) | 0.000 | 0.990 |
| FHCD (Yes/No) (%) | 38 (50.7%)/37 (49.3%) | 27 (41.5%)/38 (58.5%) | 1.168 | 0.280 |
| Smoking (Yes/No) (%) | 37 (49.3%)/38 (50.7%) | 31 (47.7%)/34 (52.3%) | 0.038 | 0.846 |
| Z | p | |||
| Age (years) | 58.96 ± 10.37 (34–75) (59) | 56.29 ± 10.14 (36–75) (57) | −1.519 | 0.129 |
| BMI at diagnosis (kg/m2) | 27.86 ± 1.74 (21.78–32.08) (27.78) | 27.62 ± 3.16 (20.96–34.72) (27.31) | −1.216 | 0.224 |
| Systolic blood pressure (mmHg) | 135.33 ± 9.74 (100–160) (140) | 134.46 ± 12.09 (110–160) (140) | −0.362 | 0.717 |
| Diastolic blood pressure (mmHg) | 86.47 ± 6.03 (60–100) (90) | 85.62 ± 7.04 (70–100) (85) | −1.065 | 0.224 |
| Fasting blood sugar (mg/dL) | 117.81 ± 30.49 (76–175) (104) | 106.58 ± 27.44 (70–170) (95) | −1.996 | 0.046 |
| Creatinine (mg/dL) | 0.94 ± 0.21 (0.48–1.40) (0.91) | 0.85 ± 0.15 (0.5–1.2) (0.86) | −2.272 | 0.023 |
| LDL (mg/dL) | 112.55 ± 38.48 (28–211) (109) | 118.32 ± 36.27 (49–212) (118) | −0.986 | 0.324 |
| HDL (mg/dL) | 38.77 ± 9.13 (21–59) (38) | 44.63 ± 10.25 (23–70) (45) | −3.474 | 0.001 |
| Triglyceride (mg/dL) | 186.81 ± 148.73 (48–900) (144) | 159.54 ± 94.58 (47–594) (135) | −0.345 | 0.730 |
| Total cholesterol (mg/dL) | 186.25 ± 50.14 (93–359) (181) | 196.55 ± 44.17 (120–320) (193) | −1.617 | 0.106 |
| Hemoglobin (g/dL) | 13.54 ± 1.54 (10–16.9) | 14.20 ± 1.53 (10–16.8) | −2.663 | 0.008 |
| WBC (µL/mL) | 11,410.67 ± 2942.68 (5700–19,200) (12,000) | 7013.85 ± 1652.16 (4100–10,900) (6800) | −8.103 | <0.001 |
| Platelet (×103) | 249.39 ± 57.65 (137–432) (255) | 261.78 ± 61.98 (142–467) (253) | −0.913 | 0.361 |
| Lymphocyte (×103) | 2.35 ± 1.48 (0.37–8) (2) | 2.18 ± 0.61 (0.79–4.15) (2.18) | −0.493 | 0.622 |
| Monocyte (×103) | 0.80 ± 0.44 (0.1–2.6) (0.75) | 0.53 ± 0.16 (0.24–1.02) (0.53) | −4.607 | <0.001 |
| Neutrophil (×103) | 8.19 ± 2.77 (3.41–15.50) (8.2) | 4.06 ± 1.17 (1.29–6.97) (4.1) | −8.586 | <0.001 |
| Neutrophil/lymphocyte | 5.33 ± 5.26 (0.84–31.57) (3.68) | 1.99 ± 0.88 (0.57–6.97) (1.89) | −6.211 | <0.001 |
| Mean AgNOR number | 2.56 ± 0.8 (1.23–5) (2.5) | 1.32 ± 0.49 (1–3) (1) | −8.716 | <0.001 |
| TAA/TNA | 0.11 ± 0.03 (0.04–0.18) (0.11) | 0.03 ± 0.01 (0.02–0.06) (0.03) | −10.104 | <0.001 |
| Echocardiographic Findings | ||||
| STEMI Mean ± SD (Min–Max) | Control Mean ± SD (Min–Max) | Z | p | |
| IVST (cm) | 1.16 ± 0.12 (0.9–1.5) (1.2) | 1.08 ± 0.13 (0.9–1.5) (1) | −4.012 | <0.001 |
| EF (n %) | 47.69 ± 7.89 (20–60) (50) | 63.62 ± 2.91 (50–65) (65) | −10.051 | <0.001 |
| STEMI n (%) | Control n (%) | χ;2 | p | |
| MR (Yes/No) (%) | 52 (69.3%)/23 (30.7%) | 21 (32.3%)/44 (67.7%) | 19.130 | <0.001 |
| AR (Yes/No) (%) | 12 (17.3%)/62 (82.7%) | 7 (10.58%)/58 (89.2%) | 1.225 | 0.268 |
| PR (Yes/No) (%) | 12 (16%)/63 (84%) | 5 (7.7%)/60 (92.3%) | 2.253 | 0.133 |
| TR (Yes/No) (%) | 45 (60%)/30 (40%) | 24 (36.9%)/41 (63.1%) | 7.419 | 0.006 |
| Model Summary | Parameter Estimates | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Equation | R2 | F | df1 | df2 | sig | Constant | b1 | b2 | b3 |
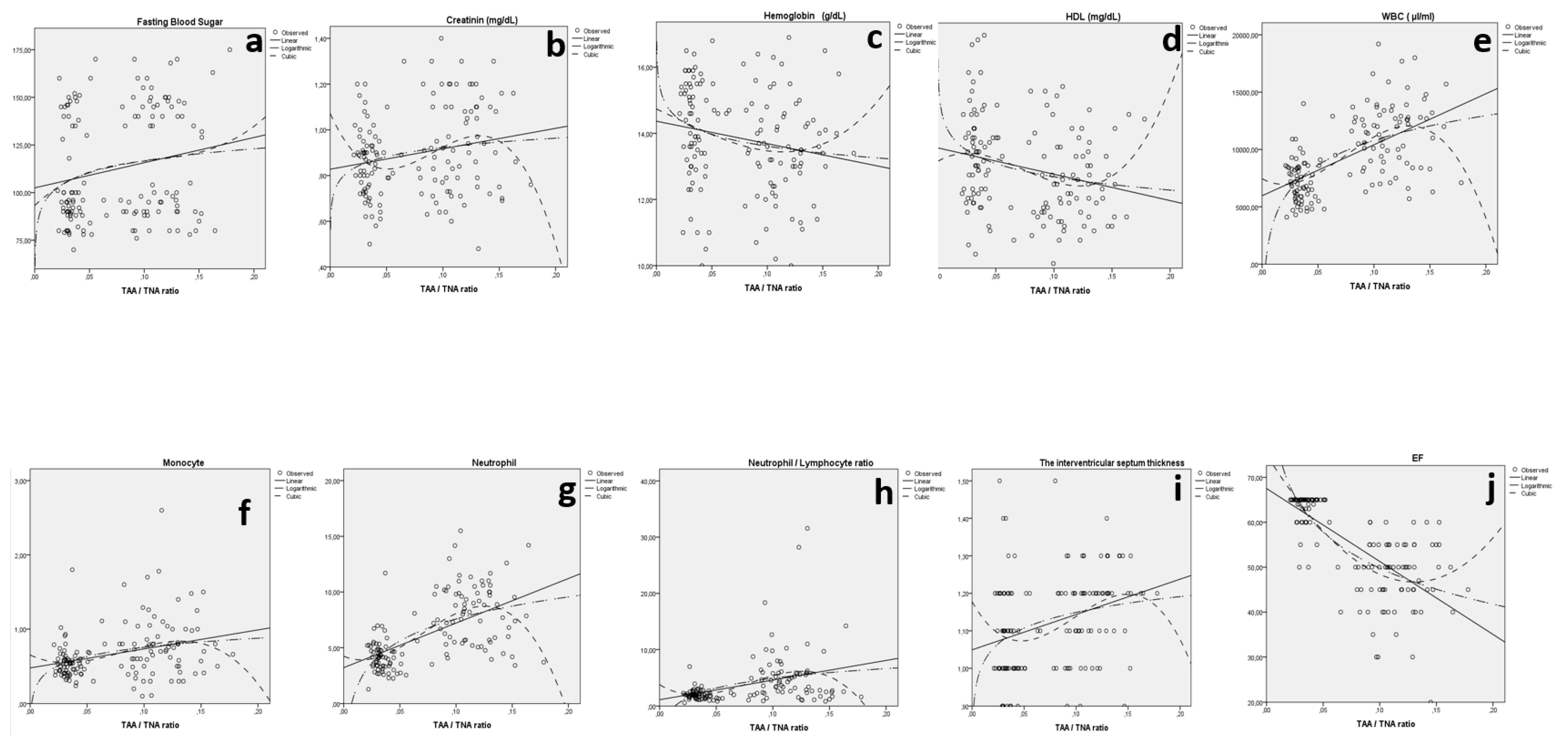
| IVST and TAA/TNA | Linear | 0.102 | 15.615 | 1 | 138 | <0.001 | 1.049 | 0.943 | ||
| Log | 0.090 | 13.574 | 1 | 138 | <0001 | 1.289 | 0.061 | |||
| Cubic | 0.113 | 5.759 | 3 | 136 | 0.001 | 1.178 | −4.961 | 6.959 | −233.473 | |
| Fasting blood sugar and TAA/TNA | Linear | 0.039 | 5.564 | 1 | 138 | 0.020 | 102.487 | 132.121 | ||
| Log | 0.038 | 5.511 | 1 | 138 | 0.020 | 137.498 | 9.020 | |||
| Cubic | 0.040 | 1.892 | 3 | 136 | 0.134 | 93.230 | 536.768 | −4423.553 | 13,886.456 | |
| Creatinine and TAA/TNA | Linear | 0.043 | 6.237 | 1 | 138 | 0.014 | 0.828 | 0.894 | ||
| Log | 0.039 | 5.627 | 1 | 138 | 0.019 | 1.057 | 0.058 | |||
| Cubic | 0.070 | 3.387 | 3 | 136 | 0.020 | 1.071 | −10.944 | 148.978 | −542.843 | |
| HDL (mg/dL) and TAA/TNA | Linear | 0.061 | 9.020 | 1 | 138 | 0.003 | 45.829 | −56.646 | ||
| Log | 0.069 | 10.169 | 1 | 138 | 0.002 | 30.151 | −4.109 | |||
| Cubic | 0.088 | 4.379 | 3 | 136 | 0.006 | 42.925 | 145.671 | −3452.466 | 15,606.463 | |
| Hemoglobin (g/dL) and TAA/TNA | Linear | 0.036 | 5.204 | 1 | 138 | 0.024 | 14.368 | −6.789 | ||
| Log | 0.045 | 6.427 | 1 | 138 | 0.012 | 12.426 | −0.515 | |||
| Cubic | 0.052 | 2.462 | 3 | 136 | 0.065 | 14.738 | −15.514 | −26.853 | 554.055 | |
| WBC (µL/mL) and TAA/TNA | Linear | 0.358 | 76.924 | 1 | 138 | <0.001 | 5965.689 | 44,466.344 | ||
| Log | 0.373 | 81.993 | 1 | 138 | <0.001 | 17,959.856 | 3112.044 | |||
| Cubic | 0.406 | 31.038 | 3 | 136 | <0.001 | 7449.484 | −53,605.833 | 1,618,705.263 | −7,182,084.69 | |
| Monocyte (×103) and TAA/TNA | Linear | 0.096 | 14.727 | 1 | 138 | <0.001 | 0.478 | 2.560 | ||
| Log | 0.099 | 15.123 | 1 | 138 | <0.001 | 1.164 | 0.178 | |||
| Cubic | 0.111 | 5.670 | 3 | 136 | 0.001 | 0.652 | −7.112 | 139.691 | −569.051 | |
| Neutrophil (×103) and TAA/TNA | Linear | 0.344 | 72.240 | 1 | 138 | <0.001 | 3.210 | 40.017 | ||
| Log | 0.372 | 81.584 | 1 | 138 | <0.001 | 14.151 | 2.854 | |||
| Cubic | 0.425 | 33.512 | 3 | 136 | <0.001 | 4.238 | −43.604 | 1550.651 | −7314.028 | |
| Neutrophil/ lymphocyte and TAA/TNA | Linear | 0.130 | 20.698 | 1 | 138 | <0.001 | 1.128 | 34.698 | ||
| Log | 0.139 | 22.363 | 1 | 138 | <0.001 | 10.577 | 2.461 | |||
| Cubic | 0.172 | 9.410 | 3 | 136 | <0.001 | 3.799 | −122.341 | 2379.721 | −10,018.401 | |
| EF and TAA/TNA | Linear | 0.509 | 142.807 | 1 | 138 | 0.000 | 67.518 | −162.426 | ||
| Log | 0.549 | 167.926 | 1 | 138 | 0.000 | 23.139 | −11.573 | |||
| Cubic | 0.572 | 60.493 | 3 | 136 | 0.000 | 75.085 | −405.136 | 1204.423 | 1787.206 | |
| Model Summary | Parameter Estimates | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Equation | R2 | F | df1 | df2 | sig | Constant | b1 | b2 | b3 |
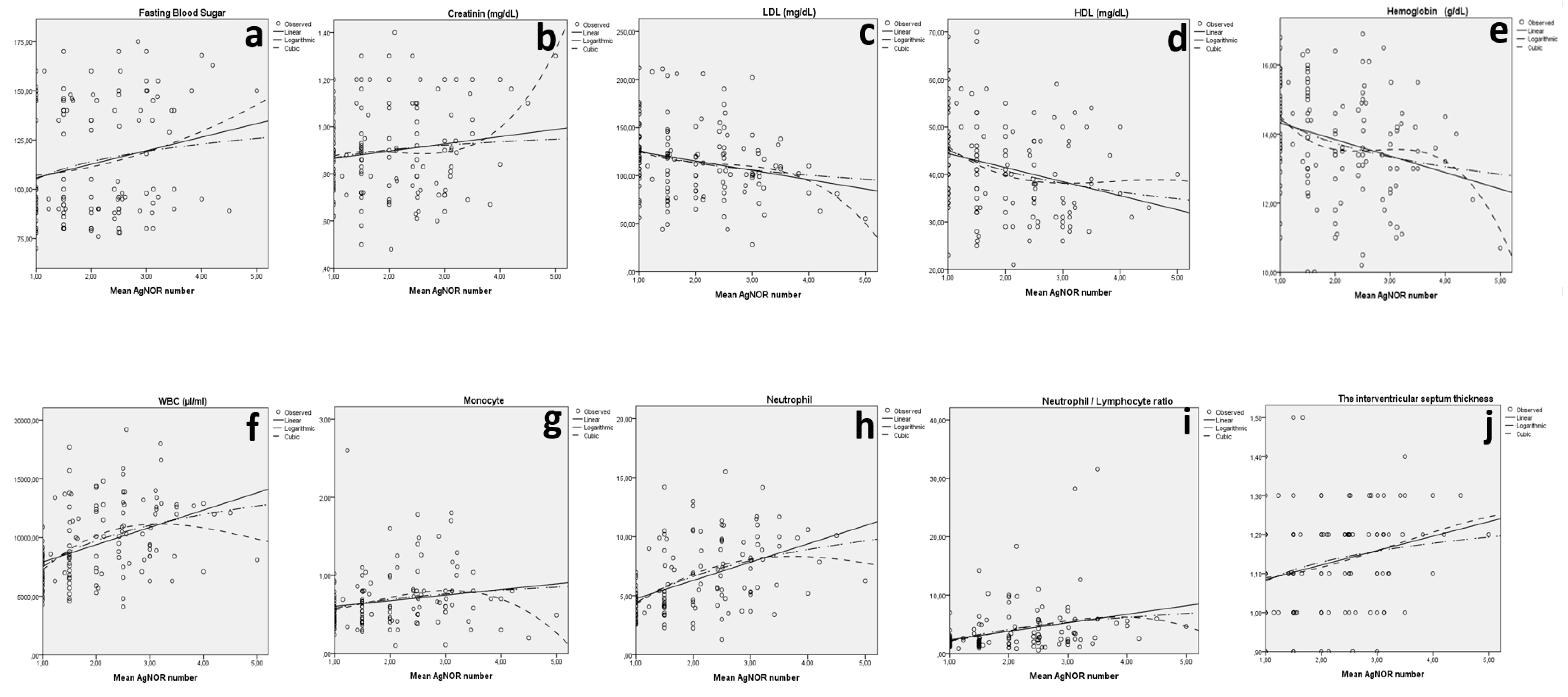
| IVST and mean AgNOR | Linear | 0.068 | 10.014 | 1 | 138 | 0.002 | 1.048 | 0.037 | ||
| Log | 0.061 | 8.983 | 1 | 138 | 0.003 | 1.081 | 0.070 | |||
| Cubic | 0.069 | 3.360 | 3 | 136 | 0.021 | 1.093 | −0.021 | 0.021 | −0.002 | |
| Fasting blood sugar and mean AgNOR | Linear | 0.045 | 6.565 | 1 | 138 | 0.011 | 98.958 | 6.875 | ||
| Log | 0.040 | 5.719 | 1 | 138 | 0.018 | 105.125 | 12.857 | |||
| Cubic | 0.048 | 2.290 | 3 | 136 | 0.081 | 106.272 | −0.889 | 1.763 | −0.024 | |
| Creatinine and mean AgNOR | Linear | 0.022 | 3.113 | 1 | 138 | 0.080 | 0.835 | 0.031 | ||
| Log | 0.014 | 2.022 | 1 | 138 | 0.157 | 0.867 | 0.050 | |||
| Cubic | 0.062 | 2.997 | 3 | 136 | 0.033 | 0.718 | 0.269 | −0.132 | 0.021 | |
| LDL (mg/dL) and mean AgNOR | Linear | 0.056 | 8.205 | 1 | 138 | 0.005 | 134.453 | −9.686 | ||
| Log | 0.051 | 7.408 | 1 | 138 | 0.007 | 125.951 | −18.438 | |||
| Cubic | 0.068 | 3.283 | 3 | 136 | 0.023 | 162.666 | −56.427 | 22.083 | −3.059 | |
| HDL (mg/dL) and mean AgNOR | Linear | 0.072 | 10.783 | 1 | 138 | 0.001 | 47.366 | −2.960 | ||
| Log | 0.086 | 12.950 | 1 | 138 | <0.001 | 45.233 | −6.433 | |||
| Cubic | 0.094 | 4.702 | 3 | 136 | 0.004 | 58.798 | −17.121 | 4.00 | −0.396 | |
| Hemoglobin (g/dL) and mean AgNOR | Linear | 0.078 | 11.638 | 1 | 138 | 0.001 | 14.796 | −0.477 | ||
| Log | 0.083 | 12.499 | 1 | 138 | 0.001 | 14.422 | −0.986 | |||
| Cubic | 0.103 | 5.201 | 3 | 136 | 0.002 | 17.819 | −4.878 | 1.14 | −0.221 | |
| WBC (µL/mL) and mean AgNOR | Linear | 0.170 | 28.298 | 1 | 138 | <0.001 | 6444.631 | 1473.871 | ||
| Log | 0.205 | 35.527 | 1 | 138 | <0.001 | 7491.555 | 3229.917 | |||
| Cubic | 0.222 | 12.970 | 3 | 136 | <0.001 | 1688.337 | 7049.536 | −1626.10 | 108.735 | |
| Monocyte (×103) and mean AgNOR | Linear | 0.033 | 4.706 | 1 | 138 | 0.032 | 0.531 | 0.072 | ||
| Log | 0.046 | 6.694 | 1 | 138 | 0.011 | 0.575 | 0.170 | |||
| Cubic | 0.079 | 3.915 | 3 | 136 | 0.010 | 0.452 | 0.035 | 0.091 | −0.021 | |
| Neutrophil (×103) and mean AgNOR | Linear | 0.223 | 39.523 | 1 | 138 | <0.001 | 3.201 | 1.548 | ||
| Log | 0.250 | 46.118 | 1 | 138 | <0.001 | 4.366 | 3.281 | |||
| Cubic | 0.256 | 15.635 | 3 | 136 | <0.001 | 0.078 | 5.073 | −0.944 | 0.047 | |
| Neutrophil/lymphocyte ratio and mean AgNOR | Linear | 0.099 | 15.237 | 1 | 138 | <0.001 | 0.894 | 1.456 | ||
| Log | 0.104 | 16.073 | 1 | 138 | <0.001 | 2.051 | 2.980 | |||
| Cubic | 0.108 | 5.515 | 3 | 136 | 0.001 | 1.545 | −0.244 | 1.076 | −0.181 | |
| EF and mean AgNOR | Linear | 0.260 | 48.550 | 1 | 138 | <0.001 | 66.169 | −5.585 | ||
| Log | 0.315 | 63.584 | 1 | 138 | <0.001 | 62.228 | −1.285 | |||
| Cubic | 0.351 | 24.483 | 3 | 136 | <0.001 | 92.315 | −38.908 | 11.372 | −1.070 | |
| Groups | AUC (95%) | Cut-Off | p | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|---|---|
| Mean AgNOR number | STEMI: 75 and Control: 65 | 0.923 (0.879–0.967) | 1.523 | 0.000 | 86.7 | 86.2 |
| TAA/TNA | 0.996 (0.988–1) | 0.054 | 0.000 | 98.7 | 98.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damar, İ.H.; Eroz, R. Argyrophilic Nucleolar Organizer Regions as New Biomarkers in ST-Elevation Myocardial Infarction. J. Cardiovasc. Dev. Dis. 2022, 9, 58. https://doi.org/10.3390/jcdd9020058
Damar İH, Eroz R. Argyrophilic Nucleolar Organizer Regions as New Biomarkers in ST-Elevation Myocardial Infarction. Journal of Cardiovascular Development and Disease. 2022; 9(2):58. https://doi.org/10.3390/jcdd9020058
Chicago/Turabian StyleDamar, İbrahim Halil, and Recep Eroz. 2022. "Argyrophilic Nucleolar Organizer Regions as New Biomarkers in ST-Elevation Myocardial Infarction" Journal of Cardiovascular Development and Disease 9, no. 2: 58. https://doi.org/10.3390/jcdd9020058
APA StyleDamar, İ. H., & Eroz, R. (2022). Argyrophilic Nucleolar Organizer Regions as New Biomarkers in ST-Elevation Myocardial Infarction. Journal of Cardiovascular Development and Disease, 9(2), 58. https://doi.org/10.3390/jcdd9020058






