Patient–Prosthesis Mismatch in Contemporary Small-Size Mechanical Prostheses Does Not Impact Survival at 10 Years
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Patient Population
2.3. Definitions
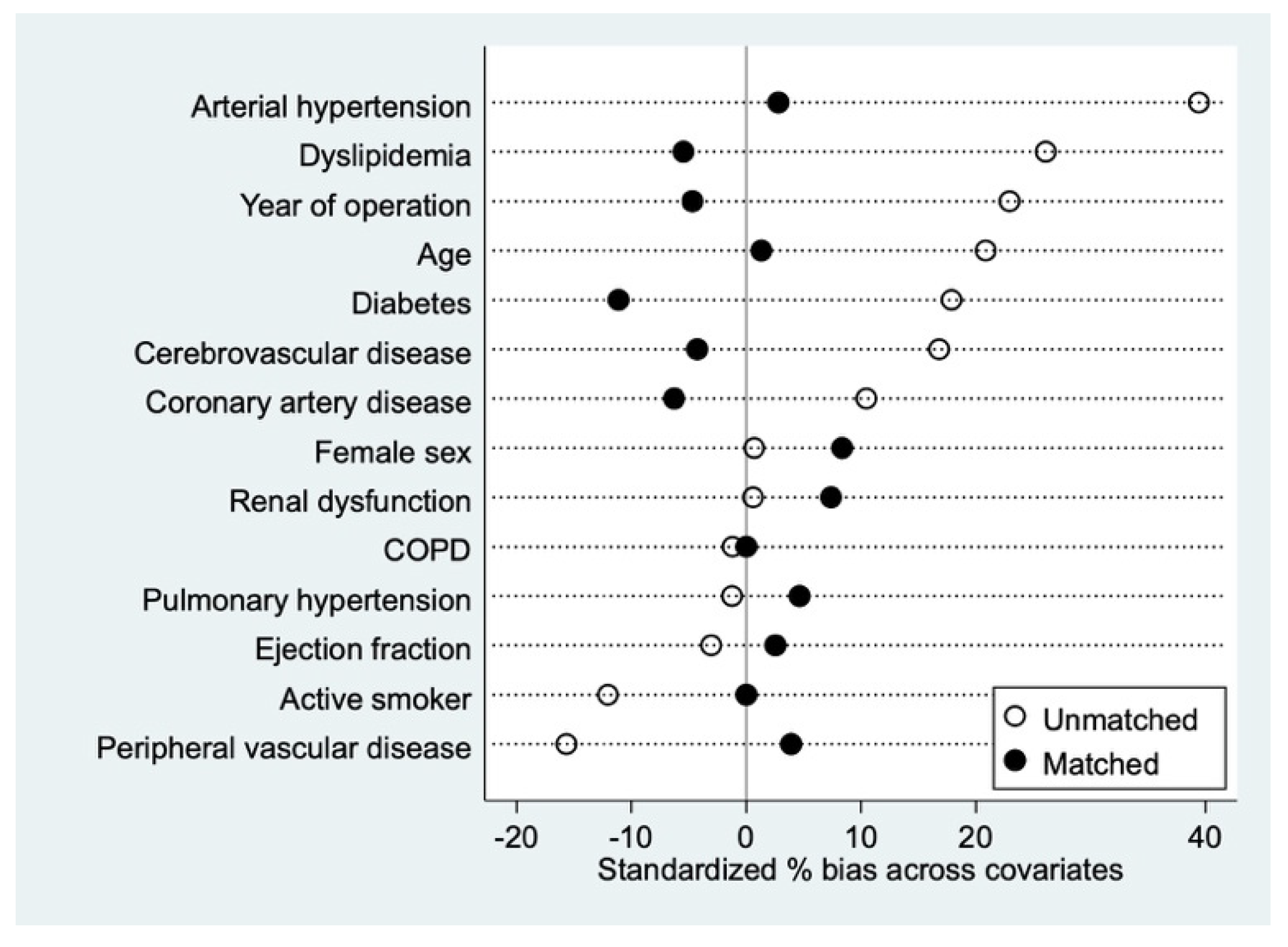
2.4. Propensity Matching
2.5. Objectives
2.6. Statistical Analysis
3. Results
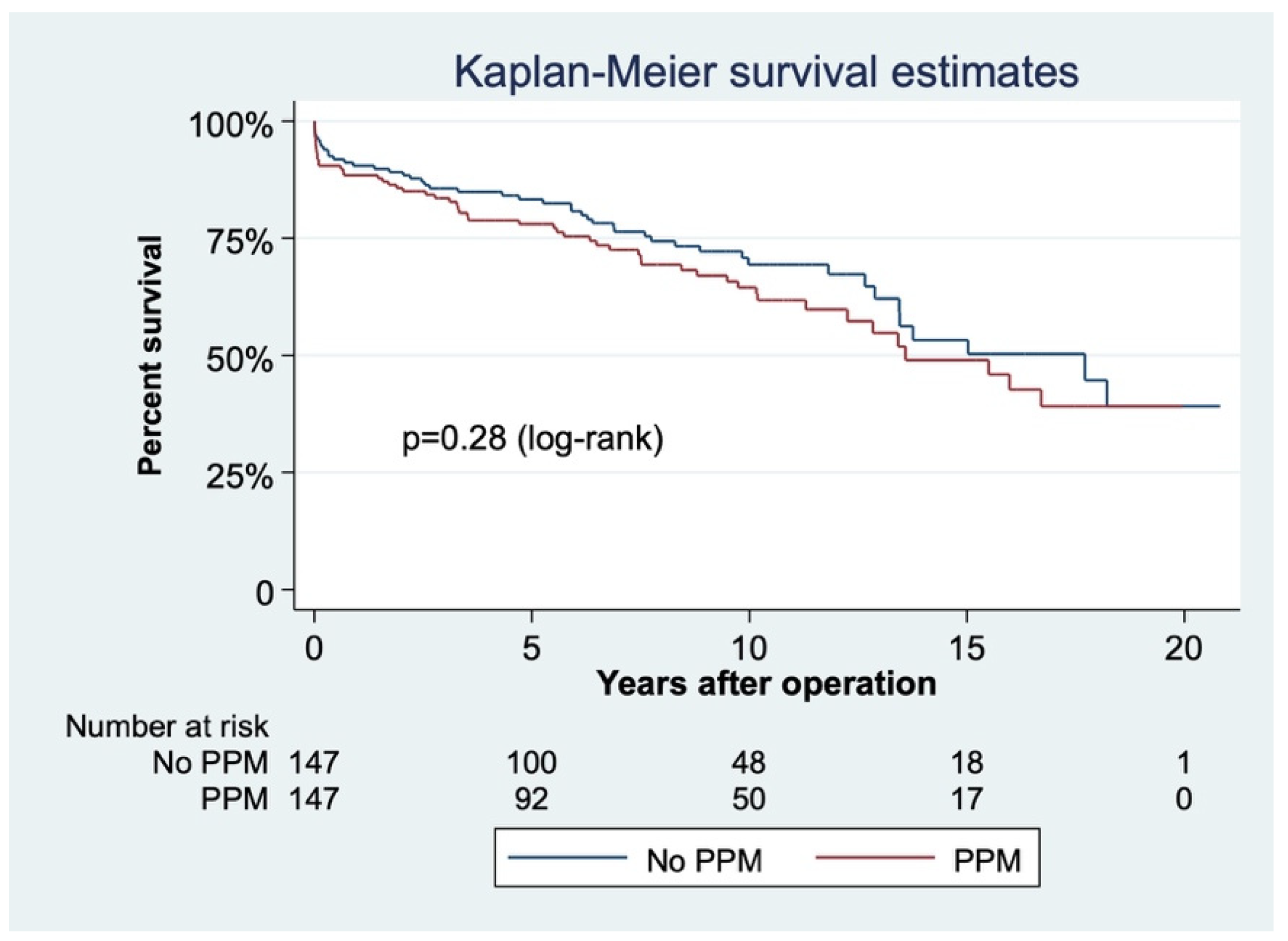
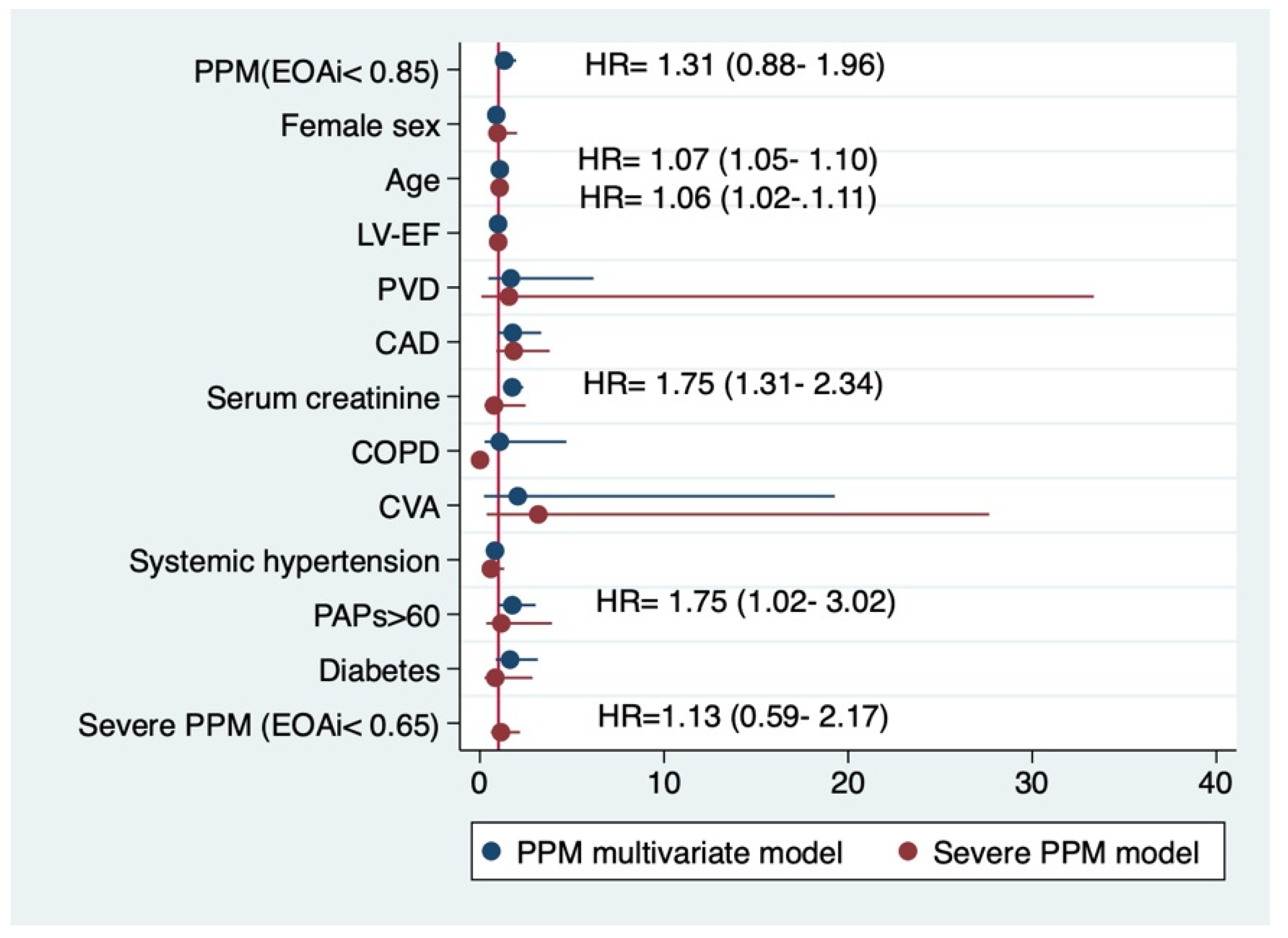
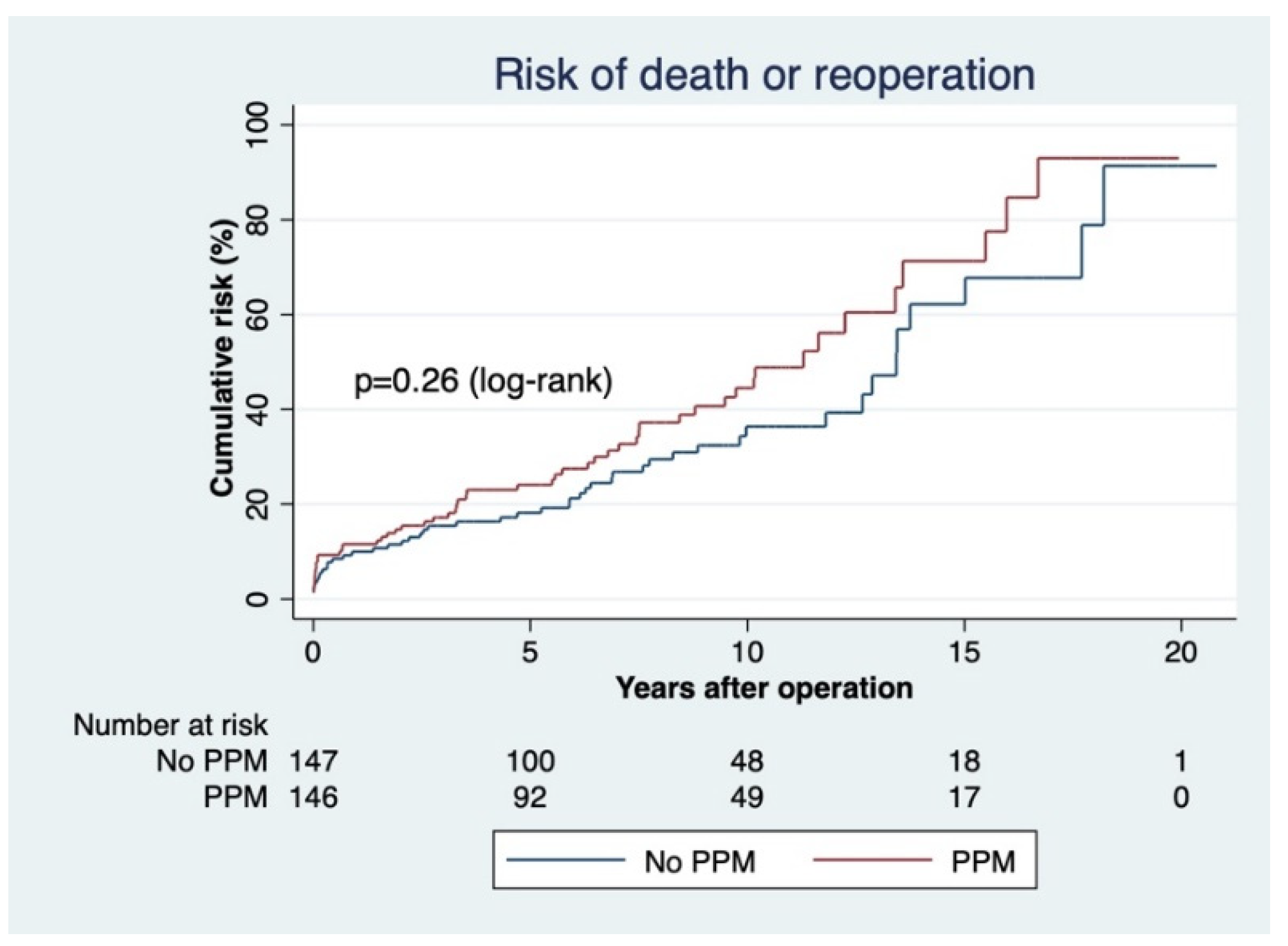
3.1. PPM
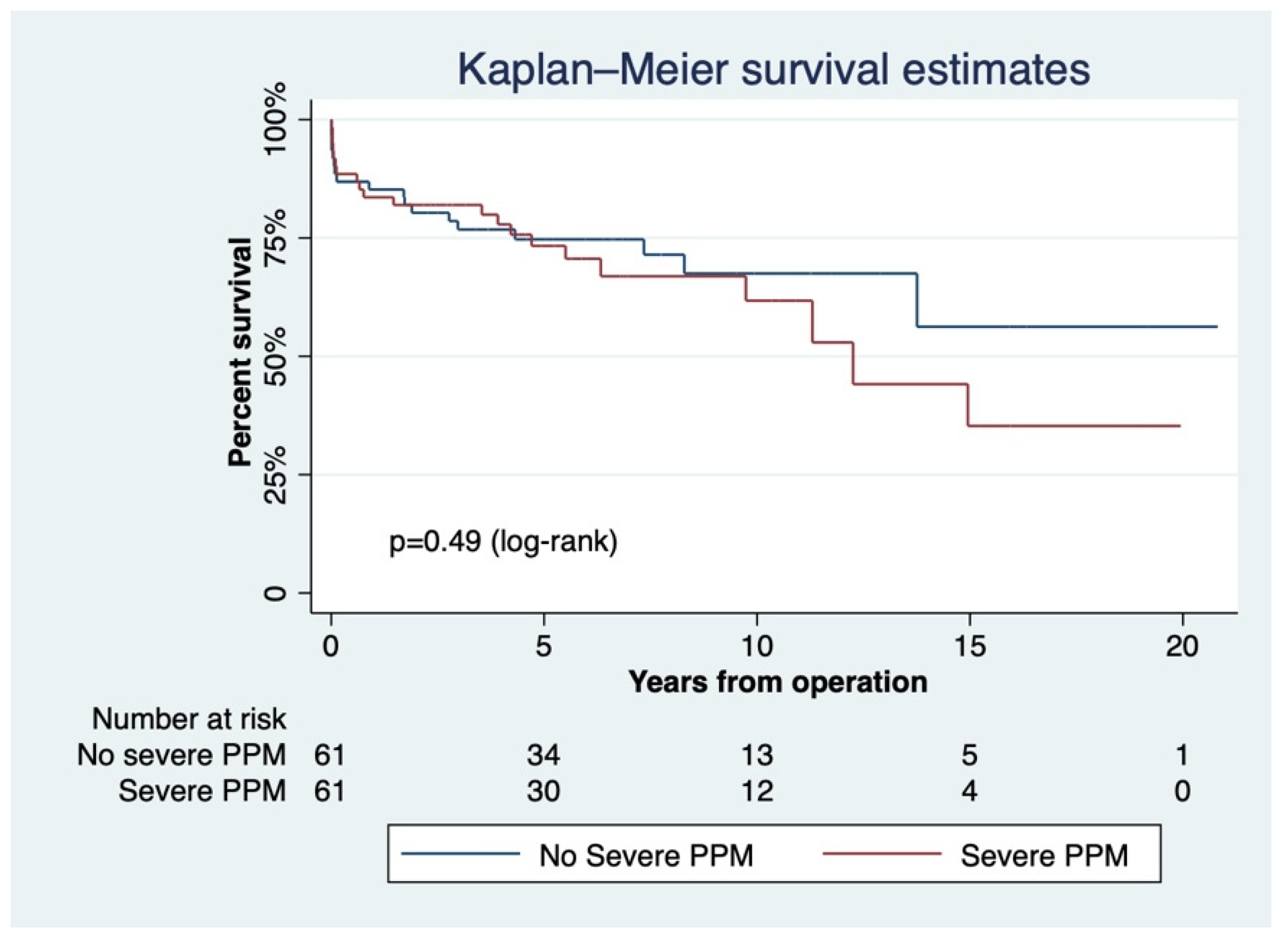
3.2. Severe PPM
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Eveborn, G.W.; Schirmer, H.; Heggelund, G.; Lunde, P.; Rasmussen, K. The evolving epidemiology of valvular aortic stenosis. The Tromsø study. Heart 2013, 99, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, ehab395. [Google Scholar] [CrossRef] [PubMed]
- Writing Committee Members; Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2021, 77, e25–e197. [Google Scholar] [CrossRef] [PubMed]
- Rahimtoola, S.H. The problem of valve prosthesis-patient mismatch. Circulation 1978, 58, 20–24. [Google Scholar] [CrossRef]
- Rao, V.; Jamieson, W.R.; Ivanov, J.; Armstrong, S.; David, T.E. Prosthesis-patient mismatch affects survival after aortic valve replacement. Circulation 2000, 102, III5–III9. [Google Scholar] [CrossRef]
- Walther, T.; Rastan, A.; Falk, V.; Lehmann, S.; Garbade, J.; Funkat, A.K.; Mohr, F.W.; Gummert, J.F. Patient prosthesis mismatch affects short- and long-term outcomes after aortic valve replacement. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2006, 30, 15–19. [Google Scholar] [CrossRef]
- Izzat, M.B.; Kadir, I.; Reeves, B.; Wilde, P.; Bryan, A.J.; Angelini, G.D. Patient-prosthesis mismatch is negligible with modern small-size aortic valve prostheses. Ann. Thorac. Surg. 1999, 68, 1657–1660. [Google Scholar] [CrossRef]
- Hanayama, N.; Christakis, G.T.; Mallidi, H.R.; Joyner, C.D.; Fremes, S.E.; Morgan, C.D.; Mitoff, P.R.R.; Goldman, B.S. Patient prosthesis mismatch is rare after aortic valve replacement: Valve size may be irrelevant. Ann. Thorac. Surg. 2002, 73, 1822–1829. [Google Scholar] [CrossRef]
- Swinkels, B.M.; de Mol, B.A.; Kelder, J.C.; Vermeulen, F.E.; ten Berg, J.M. Prosthesis-Patient Mismatch after Aortic Valve Replacement: Effect on Long-Term Survival. Ann. Thorac. Surg. 2016, 101, 1388–1394. [Google Scholar] [CrossRef]
- Wilbring, M.; Alexiou, K.; Schumann, E.; Matschke, K.; Tugtekin, S.M. Isolated aortic valve replacement in patients with small aortic annulus-a high-risk group on long-term follow-up. Thorac. Cardiovasc. Surg. 2013, 61, 379–385. [Google Scholar] [CrossRef]
- Freitas-Ferraz, A.B.; Tirado-Conte, G.; Dagenais, F.; Ruel, M.; Al-Atassi, T.; Dumont, E.; Mohammadi, S.; Bernier, M.; Pibarot, P.; Rodés-Cabau, J. Aortic Stenosis and Small Aortic Annulus. Circulation 2019, 139, 2685–2702. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.; Cross, J.; Deverall, P.; Sowton, E. Echocardiographic description of the CarboMedics bileaflet prosthetic heart valve. J. Am. Coll. Cardiol. 1993, 21, 398–405. [Google Scholar] [CrossRef]
- Pibarot, P.; Dumesnil, J.G. Prosthetic heart valves: Selection of the optimal prosthesis and long-term management. Circulation 2009, 119, 1034–1048. [Google Scholar] [CrossRef] [PubMed]
- Head, S.J.; Mokhles, M.M.; Osnabrugge, R.L.J.; Pibarot, P.; Mack, M.J.; Takkenberg, J.J.M.; Bogers, A.J.J.C.; Kappetein, A.P. The impact of prosthesis-patient mismatch on long-term survival after aortic valve replacement: A systematic review and meta-analysis of 34 observational studies comprising 27 186 patients with 133 141 patient-years. Eur. Heart J. 2012, 33, 1518–1529. [Google Scholar] [CrossRef]
- Cribier, A.; Eltchaninoff, H.; Bash, A.; Borenstein, N.; Tron, C.; Bauer, F.; Derumeaux, G.; Anselme, F.; Laborde, F.; Leon, M.B. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: First human case description. Circulation 2002, 106, 3006–3008. [Google Scholar] [CrossRef]
- Makkar, R.R.; Thourani, V.H.; Mack, M.J.; Kodali, S.K.; Kapadia, S.; Webb, J.G.; Yoon, S.-H.; Trento, A.; Svensson, L.G.; Herrmann, H.C.; et al. Five-Year Outcomes of Transcatheter or Surgical Aortic-Valve Replacement. N. Engl. J. Med. 2020, 382, 799–809. [Google Scholar] [CrossRef]
- Pibarot, P.; Salaun, E.; Dahou, A.; Avenatti, E.; Guzzetti, E.; Annabi, M.-S.; Toubal, O.; Bernier, M.; Beaudoin, J.; Ong, G.; et al. Echocardiographic Results of Transcatheter Versus Surgical Aortic Valve Replacement in Low-Risk Patients: The PARTNER 3 Trial. Circulation 2020, 141, 1527–1537. [Google Scholar] [CrossRef]
- Pibarot, P.; Dumesnil, J.G. Hemodynamic and clinical impact of prosthesis-patient mismatch in the aortic valve position and its prevention. J. Am. Coll. Cardiol. 2000, 36, 1131–1141. [Google Scholar] [CrossRef]
- Blais, C.; Dumesnil, J.G.; Baillot, R.; Simard, S.; Doyle, D.; Pibarot, P. Impact of valve prosthesis-patient mismatch on short-term mortality after aortic valve replacement. Circulation 2003, 108, 983–988. [Google Scholar] [CrossRef]
- Pibarot, P.; Dumesnil, J.G. Valve prosthesis-patient mismatch, 1978 to 2011: From original concept to compelling evidence. J. Am. Coll. Cardiol. 2012, 60, 1136–1139. [Google Scholar] [CrossRef]
- Dumesnil, J.G.; Pibarot, P. The problem of severe valve prosthesis-patient mismatch in aortic bioprostheses: Near extinction? J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2014, 27, 598–600. [Google Scholar] [CrossRef] [PubMed]
- Mohty, D.; Dumesnil, J.G.; Echahidi, N.; Mathieu, P.; Dagenais, F.; Voisine, P.; Pibarot, P. Impact of prosthesis-patient mismatch on long-term survival after aortic valve replacement: Influence of age, obesity, and left ventricular dysfunction. J. Am. Coll. Cardiol. 2009, 53, 39–47. [Google Scholar] [CrossRef]
- Fallon, J.M.; DeSimone, J.P.; Brennan, J.M.; O’Brien, S.; Thibault, D.P.; DiScipio, A.W.; Pibarot, P.; Jacobs, J.P.; Malenka, D.J. The Incidence and Consequence of Prosthesis-Patient Mismatch after Surgical Aortic Valve Replacement. Ann. Thorac. Surg. 2018, 106, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Ruel, M.; Al-Faleh, H.; Kulik, A.; Chan, K.L.; Mesana, T.G.; Burwash, I.G. Prosthesis-patient mismatch after aortic valve replacement predominantly affects patients with preexisting left ventricular dysfunction: Effect on survival, freedom from heart failure, and left ventricular mass regression. J. Thorac. Cardiovasc. Surg. 2006, 131, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.R.; Quiroga, J.S.; Fernandez, M.V.; Nazar, B.A.; Sampedro, F.G.; Martinez Comendador, J.M.; Martinez Cereijo, J.M.; Alves Perez, M.T. Up to twenty-five-year survival after aortic valve replacement with size 19 mm valves. Interact. Cardiovasc. Thorac. Surg. 2010, 10, 32–35. [Google Scholar] [CrossRef][Green Version]
- Koene, B.M.; Soliman Hamad, M.A.; Bouma, W.; Mariani, M.A.; Peels, K.C.; van Dantzig, J.-M.; van Straten, A.H. Impact of prosthesis-patient mismatch on early and late mortality after aortic valve replacement. J. Cardiothorac. Surg. 2013, 8, 96. [Google Scholar] [CrossRef]
- Koch, C.G.; Khandwala, F.; Estafanous, F.G.; Loop, F.D.; Blackstone, E.H. Impact of prosthesis-patient size on functional recovery after aortic valve replacement. Circulation 2005, 111, 3221–3229. [Google Scholar] [CrossRef]
- Van Slooten, Y.J.; van Melle, J.P.; Freling, H.G.; Bouma, B.J.; van Dijk, A.P.; Jongbloed, M.R.; Post, M.C.; Sieswerda, G.T.; Huis In’t Veld, A.; Ebels, T.; et al. Aortic valve prosthesis-patient mismatch and exercise capacity in adult patients with congenital heart disease. Heart Br. Card. Soc. 2016, 102, 107–113. [Google Scholar] [CrossRef]
- Petit-Eisenmann, H.; Epailly, E.; Velten, M.; Radojevic, J.; Eisenmann, B.; Kremer, H.; Kindo, M. Impact of Prosthesis-Patient Mismatch on Long-term Functional Capacity after Mechanical Aortic Valve Replacement. Can. J. Cardiol. 2016, 32, 1493–1499. [Google Scholar] [CrossRef]
- Tasca, G.; Mhagna, Z.; Perotti, S.; Centurini, P.B.; Sabatini, T.; Amaducci, A.; Brunelli, F.; Cirillo, M.; Dalla Tomba, M.; Quaini, E.; et al. Impact of prosthesis-patient mismatch on cardiac events and midterm mortality after aortic valve replacement in patients with pure aortic stenosis. Circulation 2006, 113, 570–576. [Google Scholar] [CrossRef]
- Mohty-Echahidi, D.; Malouf, J.F.; Girard, S.E.; Schaff, H.V.; Grill, D.E.; Enriquez-Sarano, M.E.; Miller, F.A., Jr. Impact of Prosthesis-Patient Mismatch on Long-Term Survival in Patients with Small St Jude Medical Mechanical Prostheses in the Aortic Position. Circulation 2006, 113, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Kohsaka, S.; Mohan, S.; Virani, S.; Lee, V.-V.; Contreras, A.; Reul, G.J.; Coulter, S.A. Prosthesis-patient mismatch affects long-term survival after mechanical valve replacement. J. Thorac. Cardiovasc. Surg. 2008, 135, 1076–1080. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kappetein, A.P.; Head, S.J.; Généreux, P.; Piazza, N.; van Mieghem, N.M.; Blackstone, E.H.; Brott, T.G.; Cohen, D.J.; Cutlip, D.E.; van Es, G.-A.; et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: The Valve Academic Research Consortium-2 consensus document (VARC-2). Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2012, 42, S45–S60. [Google Scholar] [CrossRef] [PubMed]
- Mahjoub, H.; Mathieu, P.; Larose, E.; Dahou, A.; Sénéchal, M.; Dumesnil, J.-G.; Després, J.-P.; Pibarot, P. Determinants of aortic bioprosthetic valve calcification assessed by multidetector CT. Heart Br. Card. Soc. 2015, 101, 472–477. [Google Scholar] [CrossRef]
- Tasca, G.; Brunelli, F.; Cirillo, M.; DallaTomba, M.; Mhagna, Z.; Troise, G.; Quaini, E. Impact of valve prosthesis-patient mismatch on left ventricular mass regression following aortic valve replacement. Ann. Thorac. Surg. 2005, 79, 505–510. [Google Scholar] [CrossRef]
- Pibarot, P.; Magne, J.; Leipsic, J.; Côté, N.; Blanke, P.; Thourani, V.H.; Hahn, R. Imaging for Predicting and Assessing Prosthesis-Patient Mismatch after Aortic Valve Replacement. JACC Cardiovasc. Imaging 2019, 12, 149–162. [Google Scholar] [CrossRef]
- Mooney, J.; Sellers, S.L.; Blanke, P.; Pibarot, P.; Hahn, R.T.; Dvir, D.; Douglas, P.S.; Weissman, N.J.; Kodali, S.K.; Thourani, V.H.; et al. CT-Defined Prosthesis-Patient Mismatch Downgrades Frequency and Severity, and Demonstrates No Association with Adverse Outcomes after Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2017, 10, 1578–1587. [Google Scholar] [CrossRef]
| Variable | All Patients | Matched Cohort | ||||
|---|---|---|---|---|---|---|
| PPM (n = 242) | No PPM (n = 174) | p Value | PPM (n = 147) | No PPM (n = 147) | p Value | |
| n (%) | n (%) | n (%) | n (%) | |||
| Age | 60.24 ± 11.04 | 57.81 ± 13.15 | 0.04 | 58.82 ± 12.19 | 58.66 ± 12.12 | 0.90 |
| Female sex | 147 (60.74) | 107 (61.49) | 0.87 | 94 (63.95) | 88 (59.86) | 0.47 |
| Aortic stenosis | 222 (91.74) | 163 (93.68) | 0.45 | 135 (91.84) | 137 (93.20) | 0.65 |
| Systemic hypertension | 162 (66.94) | 81 (46.55) | <0.01 | 76 (51.70) | 74 (50.34) | 0.81 |
| Severe pulmonary hypertension | 21 (8.68) | 16 (9.20) | 0.85 | 17 (11.56) | 15 (10.20) | 0.70 |
| Diabetes | 47 (19.42) | 23 (13.22) | 0.09 | 12 (8.16) | 18 (12.24) | 0.24 |
| CAD 1 | 33 (13.64) | 18 (10.34) | 0.31 | 12 (8.16) | 15 (10.20) | 0.54 |
| COPD 2 | 6 (2.48) | 3 (1.72) | 0.60 | 3 (2.04) | 3 (2.04) | 1.00 |
| Dyslipidemia | 131 (54.13) | 70 (40.23) | <0.01 | 58 (39.46) | 62 (42.18) | 0.63 |
| PVD 3 | 6 (2.48) | 7 (4.02) | 0.37 | 4 (2.72) | 3 (2.04) | 0.70 |
| Cerebrovascular disease | 9 (3.72) | 3 (1.72) | 0.23 | 1 (0.68) | 2 (1.36) | 0.56 |
| LVEF 4 | 51.82 ± 9.28 | 52.14 ± 11.37 | 0.75 | 52.21 ± 10.19 | 51.94 ± 11.34 | 0.83 |
| Moderate mitral regurgitation | 52 (21.49) | 30 (17.24) | 0.28 | 27 (18.37) | 29 (19.73) | 0.76 |
| BSA 5 | 1.84 ± 0.19 | 1.72 ± 0.20 | <0.01 | 1.82 ± 0.20 | 1.73 ± 0.20 | <0.01 |
| Mean aortic gradient | 52.51 ± 21.02 | 55.29 ± 20.71 | 0.19 | 53 ± 21.90 | 55.49 ± 20.60 | 0.32 |
| BMI 6 | 27.92 ± 4.83 | 25.36 ± 4.62 | <0.01 | 27.76 ± 4.76 | 25.58 ± 4.77 | <0.01 |
| Producer | Valve Type | Unmatched Cohort | Total | |||
|---|---|---|---|---|---|---|
| <19 mm (n) | EOA (cm2) | 21 mm (n) | EOA (cm2) | |||
| St. Jude | Masters | 26 | 1 ± 0.2 | 87 | 1.4 ± 0.2 | 113 |
| Regent | 5 | 1.6 ± 0.4 | 25 | 2 ± 0.7 | 30 | |
| HP | 1 | 1.3 ± 0.2 | 0 | 1 | ||
| Carbomedics | TopHat | 25 | 1 | 96 | 1.4 | 121 |
| Orbis | 14 | 1 | 29 | 1.5 | 43 | |
| Standard | 9 | 1 ± 0.4 | 6 | 1.5 ± 0.3 | 15 | |
| Sorin | Bicarbon | 11 | 1.5 | 23 | 1.9 | 34 |
| Allcarbon | 3 | 1 | 2 | 1.2 | 5 | |
| Medtronic | OpenPivot | 6 | 1.55 | 31 | 2.02 | 37 |
| Medtronic-Hall | 0 | 11 | 1.3 ± 0.2 | 11 | ||
| Advantage | 0 | 4 | 1.7 ± 0.2 | 4 | ||
| Cryolife | On-X | 0 | 2 | 1.7 ± 0.4 | 2 | |
| Total | 100 | 316 | 416 | |||
| Variable | PPM (n = 147) | No PPM (n = 147) | p |
|---|---|---|---|
| Operative Data | |||
| Bypass time (mean ± SD) | 108.05 ± 61.01 | 100.33 ± 38.06 | 0.19 |
| Cross-clamp time (mean ± SD) | 70.76 ± 25.52 | 67.37 ± 24.33 | 0.24 |
| Postoperative length of stay (mean ± SD) | 9.02 ± 4.54 | 9 ± 5.00 | 0.97 |
| Early mortality (<30 days) | 12 (8.16) | 5 (3.40) | 0.08 |
| EOAi (cm2/m2) | 0.71 ± 0.09 | 1.03 ± 0.17 | <0.01 |
| Euroscore 2 risk score | 1.85 ± 1.97 | 1.74 ± 1.45 | 0.59 |
| Follow-up (years) (mean ± SD) | 6.54 ± 4.60 | 5.98 ± 4.47 | 0.32 |
| _t | Coefficient | Robust SE | z | p | 95% CI | |
|---|---|---|---|---|---|---|
| ATE (PPM vs. No PPM) | 0.49 | 1.64 | 0.30 | 0.76 | 2.73 | 3.71 |
| POmean (PPM vs. No PPM) | 8.59 | 1.06 | 8.09 | 0.00 | 6.51 | 10.68 |
| _t | Coefficient | Robust SE | z | p | 95% CI | |
|---|---|---|---|---|---|---|
| ATE (Severe PPM vs. No severe PPM) | 4.03 | 2.53 | 1.59 | 0.11 | 0.93 | 9.01 |
| POmean (PPM vs. No PPM) | 4.14 | 1.17 | 3.52 | 0.00 | 1.83 | 6.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feier, H.; Mocan, M.; Grigorescu, A.; Falnita, L.; Gaspar, M.; Luca, C.-T. Patient–Prosthesis Mismatch in Contemporary Small-Size Mechanical Prostheses Does Not Impact Survival at 10 Years. J. Cardiovasc. Dev. Dis. 2022, 9, 48. https://doi.org/10.3390/jcdd9020048
Feier H, Mocan M, Grigorescu A, Falnita L, Gaspar M, Luca C-T. Patient–Prosthesis Mismatch in Contemporary Small-Size Mechanical Prostheses Does Not Impact Survival at 10 Years. Journal of Cardiovascular Development and Disease. 2022; 9(2):48. https://doi.org/10.3390/jcdd9020048
Chicago/Turabian StyleFeier, Horea, Mihaela Mocan, Andrei Grigorescu, Lucian Falnita, Marian Gaspar, and Constantin-Tudor Luca. 2022. "Patient–Prosthesis Mismatch in Contemporary Small-Size Mechanical Prostheses Does Not Impact Survival at 10 Years" Journal of Cardiovascular Development and Disease 9, no. 2: 48. https://doi.org/10.3390/jcdd9020048
APA StyleFeier, H., Mocan, M., Grigorescu, A., Falnita, L., Gaspar, M., & Luca, C.-T. (2022). Patient–Prosthesis Mismatch in Contemporary Small-Size Mechanical Prostheses Does Not Impact Survival at 10 Years. Journal of Cardiovascular Development and Disease, 9(2), 48. https://doi.org/10.3390/jcdd9020048







