Abstract
Left ventricular noncompaction (LVNC) is a type of cardiomyopathy characterized anatomically by prominent ventricular trabeculation and deep intertrabecular recesses. The mortality associated with LVNC ranges from 5% to 47%. The etiology of LVNC is yet to be fully understood, although decades have passed since its recognition as a clinical entity globally. Furthermore, critical questions, i.e., whether LVNC represents an acquired pathology or has a congenital origin and whether the reduced contractile function in LVNC patients is a cause or consequence of noncompaction, remain to be addressed. In this study, to answer some of these questions, we analyzed the clinical features of LVNC patients. Out of 9582 subjects screened for abnormal cardiac functions, 45 exhibit the characteristics of LVNC, and 1 presents right ventricular noncompaction (RVNC). We found that 40 patients show valvular regurgitation, 39 manifest reduced systolic contractions, and 46 out of the 46 present different forms of arrhythmias that are not restricted to be caused by the noncompact myocardium. This retrospective examination of LVNC patients reveals some novel findings: LVNC is associated with regurgitation in most patients and arrhythmias in all patients. The thickness ratio of the trabecular layer to compact layer negatively correlates with fractional shortening, and reduced contractility might result from LVNC. This study adds evidence to support a congenital origin of LVNC that might benefit the diagnosis and subsequent characterization of LVNC patients.
1. Introduction
Left ventricular noncompaction (LVNC: OMIM No. 604169) is a type of cardiomyopathy anatomically characterized by prominent ventricular trabeculation and deep intertrabecular recesses [1,2,3]. It was reported for the first time in 1969, being addressed as a spongy myocardial condition back then [4]. In the following years, LVNC has gained tremendous attention due to the improvements in cardiac imaging techniques, primarily echocardiography and magnetic resonance imaging, that have enabled more detailed visualization and increased clinical awareness of this syndrome. Subsequently, many LVNC cardiomyopathy cases were reported, and AHA enlisted LVNC as a type of cardiomyopathy in 2006 [3]. LVNC shows highly variable clinical manifestations ranging from asymptomatic to symptomatic, and the major clinical features of LVNC are heart failure, arrhythmias, thromboembolic events, and sudden death [5]. Its symptoms are progressive, considered the 3rd most common cardiomyopathy in the pediatric population, and the mortality of patients with LVNC ranges from 5% to 47% [6,7,8]. Despite its clinical significance, the mechanism of trabecular compaction and the etiology of LVNC are unknown. Moreover, whether LVNC is acquired or congenital cardiomyopathy has been an unraveled controversy [9]. The focus of most clinical investigations on isolated adult LVNC patients failed to trace the anomalies in embryonic developmental stages, and the knowledge gap regarding the molecular level regulation of trabeculation could be the possible reason [10,11].
As abnormalities in trabecular and ventricular morphogenesis lead to LVNC, the revelation of the biological and physiological development of trabecular formation and ventricular compaction will help elucidate the etiology of LVNC [12,13]. Trabeculae are sheet-like structures extending from the myocardium to the heart lumen and function to increase surface area to support nutrition and oxygen supply when the coronary system is not yet established [14]. Many studies show that a lack of trabeculation causes embryonic demise, and excess trabeculation causes LVNC [2,6,11,13,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35]. The cellular and molecular mechanisms of trabecular formation have been revealed over the years. Recent studies demonstrated that the polarity-dependent oriented cell division (OCD) and directional migration of cardiomyocytes in the single-cell-thick myocardium contribute to trabecular initiation, resulting in myocardium with multiple-layer cells in mice [13,36,37,38,39]. However, in zebrafish, trabeculae are formed by polarity-dependent directional migration only [33,34,40]. Further study found that the cardiomyocyte in the outer and inner layers of the compact zone display different orientations during trabecular initiation, and disruption of their cellular orientation will result in trabecular initiation defects and LVNC [13,41]. Moreover, perpendicular OCD has contributed to trabecular specification and is responsible for trabecular cardiomyocytes being distinct from the cardiomyocytes in the compact zone [36]. After the trabecular initiation, the myocardium contains multiple layers of cardiomyocytes, and endocardial cells can burrow into the loose cardiomyocytes and separate them to form trabeculae [42,43].
Compared to the processes governing trabecular formation, the mechanism of ventricular compaction is mainly unknown. Recent studies report that endothelial initiated angiogenesis and Semaphorin 3E/PlexinD1 signaling might be required for ventricular compaction [44,45]. Another study along these lines shows that the trabecular cells coalesce with the compact zone to thicken and strengthen the compact zone [46]. However, the genetic networks and a clear description of the compaction process are not established yet. Furthermore, significant questions, i.e., whether the cause of LVNC is over-trabeculation or compaction arrest [12], whether LVNC represents an acquired pathology or has a congenital origin, whether LVNC patients associate with other clinical features, and whether the reduced contractile function is a cause or consequence of LVNC, are unknown. A thorough analysis of the clinical features of the LVNC will help answer these questions and identify the etiology of LVNC.
To answer some of these questions, we carefully examined the clinical features of LVNC patients. In this study, we explored a database of 9582 subjects screened for abnormal cardiac functions and found that 46 patients displayed the features of noncompaction. The hearts’ deformations were evaluated from the images harvested by echocardiography (ECHO) and/or cardiovascular magnetic resonance imaging (MRI). While echocardiography can be used as the first tracing tool in LVNC diagnosis, MRI-mediated evaluation can determine subclinical alteration in myocardial function and is more responsive to detect subtle functional changes and heart structures than ECHO [47].
The data show that 42 of the 46 patients display different extents of valvular or atrioventricular regurgitation. A total of 39 of the 46 patients present reduced systolic contraction, and 46 out of the 46 patients manifest different formats of arrhythmias that are not restricted to the noncompact myocardium. Interestingly, 1 out of the 46 patients manifests a right ventricular noncompaction (RVNC) but not a contractile defect, and 1 displays both LVNC and RVNC. This close retrospective examination of LVNC patients reveals some novel findings, including that LVNC is associated with regurgitation and arrhythmias in most of the patients and that the reduced contractility in LVNC patients correlates with the age of the patients and might be a consequence of LVNC. These findings suggest that regurgitation and arrhythmia might be clinical features for LVNC and add evidence to favor the notion that LVNC has a congenital origin.
2. Materials and Methods
2.1. Study Population and Clinical Data
The echocardiogram database, which includes 9582 unrelated patients screened for heart-related diseases at Second Affiliated Hospital of Nanchang University between November 2014 and November 2021, was searched for patients with the diagnosis of noncompaction. A total of 46 patients who display noncompaction were included in the analysis. Diagnosis of noncompaction was based on a consensus of re-evaluated echocardiography and MRI, according to the Jenni and Chin criteria and a dedicated participating cardiologist [15,48]. Clinical data were retrieved retrospectively from the medical records, including age, sex, cardiac diagnosis, electrocardiography, echocardiography, and cardiac MRI when available. The clinical symptoms, primary diagnosis, New York Heart Association classification (NYHA), associated dysmorphic features, valvular regurgitation, congenital heart disease, and presence of arrhythmia were documented. Echocardiograms were analyzed for ejection fraction, fractional shortening, and ventricular dimensions. Myocardial thickness was also determined at the site of the most prominent trabecular meshwork. The study design was approved by the Ethics Committee of the Nanchang University, Nanchang, China. All data used for this study were handled anonymously.
2.2. Diagnosis of LVNC and RVNC
Diagnosis of LVNC or RVNC is made using transthoracic echocardiography as a main diagnostic tool. Ventricular noncompaction is described by the compacted thin epicardial layer and a thicker noncompacted endocardial layer with a ratio between noncompacted to compacted myocardium >2 in end-systole of a short-axis slice [15,48]. Clinical data of cardiovascular MRI is frequently used to confirm or rule out the diagnosis.
2.3. Ventricular Systolic Funciton
Left ventricular (LV) systolic dysfunction was defined as LV ejection fraction of <50% or fractional shortening of <25% in both men and women on echocardiography.
2.4. Vavular Regurditation
Valvular regurgitation severity is determined by the regurgitant fraction (RF) according to the published reference [49], and if the RF is less than 30%, it is defined as mild; if RF is between 30–39, it is defined as moderate; and if the RF is larger than 40, it is defined as severe.
2.5. Statistics
The differences in contractility among the groups of three different T/C ratios and between two age groups are compared via non-parametric tests for statistical comparison. A p-value of 0.05 or less was considered statistically significant.
2.6. Statement
All methods were carried out in accordance with relevant guidelines and regulations.
3. Results, Figures and Tables
3.1. Clinical Symptoms of LVNC Patients
The characterization of LVNC is evolving but remains incomplete, as many questions remain to be settled. One of them is whether LVNC patients associate with novel clinical features in addition to the reported symptoms. We searched the database for cardiac noncompaction from 9582 echocardiograms performed on subjects who were admitted to the hospital for further clinical examination and potential heart-related diseases. The abnormal compaction was identified based on Chin and Jenni criteria with a ratio of NC/C larger than two (Figure 1A,B, and Table 1) [15,48].
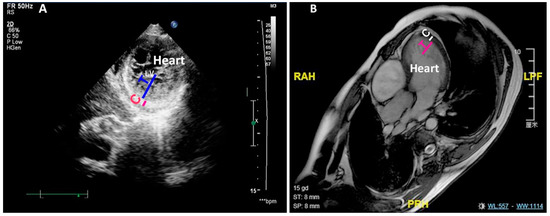
Figure 1.
LVNC is detected by ECHO and MRI. (A) Shows the noncompaction measured by ECHO of an LVNC patient. (B) Shows the noncompaction measured by MRI of an LVNC patient. C: compact zone; T: trabecular zone; LV: left ventricle; ECHO: echocardiography; MRI: magnetic resonance imaging.

Table 1.
Clinical features of the 45 LVNC and 1 RVNC patient.
Using these criteria, we identified 46 noncompaction cases. These patients account for 0.48% of all the cases searched in the database. Noncompaction occurs in different regions of the heart, primarily prominent in the apex of the left ventricle. Of the 46 patients, 44 display LVNC, 1 displays both LVNC and RVNC, and 1 displays RVNC. The age of the 46 patients ranges from 12 to 81 years old, and the average age is 52.2. Of the 46 patients, 32 were male, and 14 were female; 2 were younger than 15, and the other 44 patients were older than 20. The NYHA standard protocol was used to measure the severity of LVNC patients—24 patients showed grade I-II and 22 patients grade III-IV. Valvular regurgitation (40/46), reduced contractility (39/46), and arrhythmias (46/46) are the most common manifestations encountered in these patients. In this study, mild tricuspid and pulmonary regurgitation are not pathological, and they were not considered valvular regurgitation. Valvular Ebstein’s anomaly and atrial septal defects were also observed in some of the patients (Table 1). Unlike the previous report, about 80% of LVNC patients display neuromuscular disease [50]; only 1 out of 46 patients is associated with this defect. Cardiac functions, ventricular remodeling, and arrhythmias were examined and compared among patients (Table 1). We further characterize the features of the atrioventricular regurgitation, arrhythmias, reduced contractility, and right ventricular noncompaction of the 46 patients in the following sessions.
3.2. LVNC Patients Associate with Valvular Regurgitation
The valvular regurgitation in LVNC patients did not draw much attention until recently [6,51,52,53]. We examined the mitral valvular regurgitation (MVR), tricuspid valvular regurgitation (TVR), aortic regurgitation (AR), and pulmonary arterial regurgitation (PR) in all the patients based on the images/videos that were harvested via ECHO and/or MRI. Surprisingly, we found that 36 out of the 46 patients display MVR (Figure 2A and Table 1 and Table 2). A total of 36 out of 46 present TVR. Since mild TVR is not pathogenic, only the eight moderate or severe TVR are included to study the association of LVNC and valvular regurgitation (Figure 2A and Table 1 and Table 2). A total of 19 out of 46 have a clinical manifestation of AR (Table 1 and Table 2). A total of 6 out of the 46 display mild or moderate PR. Excluding the mild TVR and VR, we found that 40 patients showed clinical valvular regurgitation.
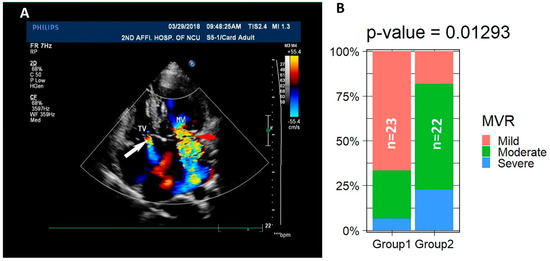
Figure 2.
A total of 40 of the 45 LVNC patients are associated with valvular regurgitation. (A) ECHO images show the TVR indicated by the white arrow and MVR indicated by the red arrow. (B) The percentages of mild, moderate, and severe regurgitation in the two age groups are significantly different.

Table 2.
A total of 40 of the 45 LVNC patients display valvular regurgitation.
One of the major causes of regurgitation is the abnormal structure of the valves. We found that only #14 displays Ebstein’s anomaly with anterior longer and bigger leaflet, septal leaflet prolapse, and posterior leaflet deformity. The structure and components of the tricuspid and mitral valves of the other patients were not visibly abnormal. To determine if the symptoms are age-dependent, patients were divided into two groups: 23 patients in group 1 with age younger than the median age at 59 and 22 patients in group 2 with age equal to or older than 59. We analyzed the severity of MVR between the two age groups. It was well accepted that the prevalence of valvular heart diseases increases by age [54], and the severity of regurgitation between the two groups is significantly different based on Fisher’s Exact Test of the percentages of mild, moderate, and severe patients in the two groups (Table 2) (Figure 2B), suggesting that regurgitation is an age-related remodeling of the valves in the LVNC patients. These data imply that valvular regurgitation is associated with the onset of LVNC and might be a clinical feature for LVNC during the diagnosis.
3.3. The LVNC Patients Display Reduced Contractility and Thickness Ratio of Trabecular Layer to Compact Layer Negatively Correlates with Cardiac Contractility
Accumulating pieces of evidence indicate a crucial role for cardiac contraction and the resulting hemodynamic force on ventricular wall compaction [52,55]. Impaired contractile function and sluggish blood flow likely cause thrombotic formation in deep intertrabecular recesses of the left ventricle, which might underlie the frequent thrombotic events in LVNC. A prior study found that about 82% of the genetic mutations that caused LVNC occurred in genes that encode sarcomere components. Among them, MYH7, MYBPC3, and TTN are the most frequently mutated genes, and their mutations account for 71% of the LVNC cases [52]. Therefore, it has been speculated that the contractile function is required for trabecular compaction [56]. We examined the clinical data to determine the association between contractile dysfunction and LVNC. A total of 39 out of the 45 LVNC patients manifest reduced contractility as indicated by the reduced F.S. and E.F. (Table 1), suggesting that contractile dysfunction might be a feature of LVNC but is not necessarily the onset of LVNC. We compared the contractile functions between the two age groups to determine if reduced contractility correlates with age in LVNC patients. Compared to the normal range, 17 out of 23 patients in the young group show a contractile defect, indicated by reduced E.F. and F.S. (Figure 3A,B and Table 1), while 22 out of 22 patients in the old-age group display reduced contractility (Figure 3A,B and Table 1). Patients in the old-age group exhibited weaker contractility with a reduced ejection fraction (EF) and fractional shortening (FS), while the patients younger than 59 have a higher contractile function, but the difference is not significant based on the Mann–Whitney non-parametric test (Figure 3A,B). We further examined if the thickness ratio of trabecular layer to compact layer is correlated with the reduced systolic function. The 45 patients were divided into three groups: T/C < 3/1, T/C = 3/1, and T/C > 3/1. We compared the contractility of the three groups via the Kruskal–Wallis rank sum test and pairwise comparisons using Wilcoxon rank sum test. It was found that FS is significantly different, suggesting that a larger thickness ratio negatively correlates with the left ventricular systolic function (Figure 3C).
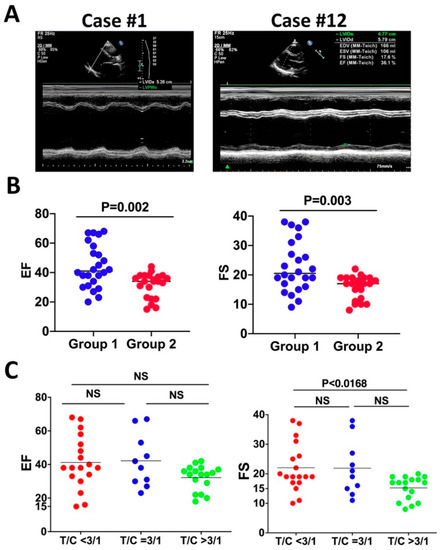
Figure 3.
The LVNC patients display reduced contractility and thickness ratio of trabecular layer to compact layer negatively correlates with cardiac contractility. (A) Representative M-mode of left ventricular long-axis views of echocardiography. Case #1 displays reduced contractility, and Case #12 manifests normal contractility. (B) The ejection fraction (EF) and fractional shortening (FS) of group #1 (age younger than 59) are greater than group #2 (age older than 59), and the difference is not significant. (C) FS of patients with a T/C ratio higher than 3/1 is significantly less than patients with a T/C ratio smaller than 3/1.
3.4. All LVNC Patients Are Associated with Arrhythmias
A key factor responsible for sudden death in LVNC patients is arrhythmia, a significant feature of LVNC. Ventricular arrhythmias are the standard prominent clinical components of LVNC [57]. It was thought that the deep intramyocardial invagination, which carries the Purkinje system deeper into the myocardium, might result in delayed depolarization and inhomogeneous repolarization and subsequently cause arrhythmias in LVNC [58].
However, arrhythmias in the 46 patients are not restricted to the noncompacted myocardium, as atrial fibrillation (AF) is observed in LVNC patients [58]. LVNC patients bear mutations in RYR2 [59] and KCNH2/KCNQ1 [60] associated with cardiac arrhythmia syndromes. The range of percentages of arrhythmias in LVNC patients varies from 26 to 94 in different studies [48,52,61,62,63,64,65,66,67]. The major types of arrhythmias include ventricular tachycardia (VT) and AF. In this study, all the patients, including LVNC and RVNC patients, display arrhythmias and, in addition to VT and AF, supraventricular tachycardia (SVT), left bundle branch block (LBBB), atrial tachycardia (AT), atrial premature contraction (APC), sick sinus syndrome (SSS), right bundle branch block (RBBB), ventricular premature contraction (VPC), atrial ventricular block (AVB), and malignant arrhythmia (MA) were observed (Table 1 and Figure 4). Therefore, our data suggest that arrhythmias in LVNC patients are not restricted to the noncompacted myocardium.
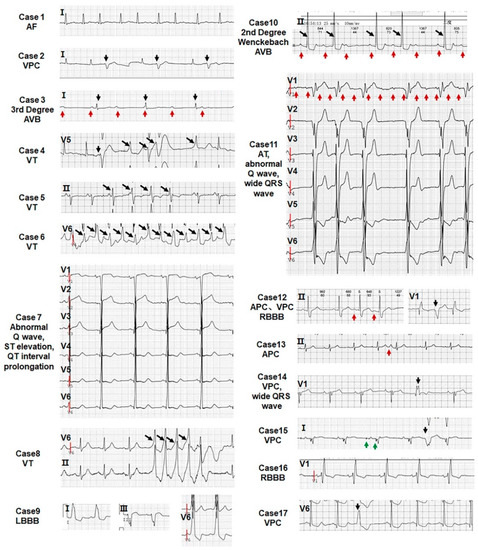
Figure 4.
All patients show abnormal ECG. The type of arrhythmia of 17 LVNC patients is indicated in each echocardiogram. The red arrow points to the atrial wave, the black arrow points to the ventricular wave, and the green arrow directs to the pacemaker signal.
3.5. RVNC Does Not Associate with Reduced Contractility
While the most common site of noncompaction is the left ventricle, the noncompaction in the right ventricle was rarely reported. It is likely that noncompaction in the left ventricle has more prominent clinical symptoms than noncompaction in the right ventricle which rendered noncompaction in the right ventricle to receive less attention being explored. Another possibility is that the rate of noncompaction in the right ventricle is low and rarely detected. In this study, of the 46 patients who display noncompaction, a 55-year-old male displays RVNC but not LVNC (Figure 5), and a 49-year-old male displays both RVNC and LVNC, which is considered as LVNC for the analysis.
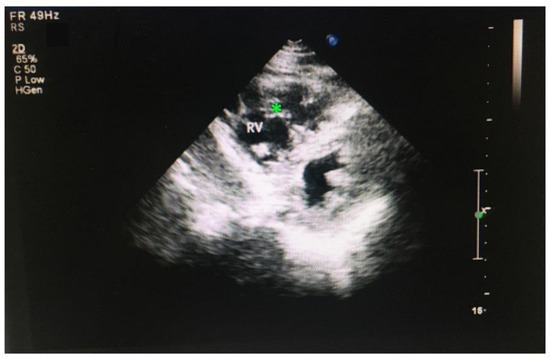
Figure 5.
ECHO image of an RVNC. * indicates the trabecula in RV.
Further examination revealed that RVNC is associated with dilated RV (>35 mm, normal range <25 mm), enlarged RA (>43, normal range 30–40 mm), and increased pulmonary artery pressure. The interventricular septum displays a normal thickness. The right ventricular wall is thinner. Trabeculae are prominent in the right ventricle and the apex of the right ventricle displays a beehive structure due to the abundance of trabeculae (Figure 5). The patient also displays atrial premature contraction (APC) (Figure 4) and regurgitation in the tricuspid valve (Table 1 and data not shown). However, left ventricle remodeling and cardiac functions are not affected (Table 1). The systolic and diastolic functions of the left ventricle are in the normal ranges (Table 1 and data not shown), suggesting that this patient does not display a reduced contractile defect in the left ventricle, which happens to be consistent with a previous study reporting that a RVNC patient displays normal contractile function in the RV and LV [68]. The patient has been smoking and drinking for more than 30 years. The patient denied further MRI examination.
4. Discussion
With the data reported in this study and data published by other groups, we would like to discuss further whether LVNC has congenital or acquired originations and whether the reduced contractility is a cause or a consequence of LVNC.
4.1. Congenital LVNC or Acquired LVNC
LVNC is characterized by hypertrabeculation and noncompaction in myocardial anatomy associated with deep intertrabecular recesses [1,2,3,69]. Clinical presentation of LVNC is progressive and heterogeneous, and some symptoms are not recognized and accepted, which prevents setting a universal standard to characterize LVNC and to determine the etiology of LVNC. A significant block for elucidating the etiology of LVNC is whether it represents an acquired pathology or has a congenital origin. Understanding the trabecular formation and subsequent compaction will help identify the origins and etiologies of LVNC. There are two major steps in trabecular formation. The first step is the polarity-dependent OCD, cell orientation, and directional migration of cardiomyocytes in the early myocardium, which contribute to trabecular initiation, resulting in myocardium with multiple-layer cells in mice [13,36,37,38,39,41]. Subsequently, the endocardial cells burrow into the multiple-layer myocardium and separate cardiomyocytes to form trabeculae [42,43]. After trabecular formation, the myocardium will undergo compaction through gradually compacting inwards from the base to the apex [61,70], and the trabeculae will coalesce with the compact zone to form the thickened compact zone [46]. Therefore, the etiology of LVNC can be based either on over-trabeculation or compaction arrestment. Both over-trabeculation and compaction arrest occur during the cardiac developmental stage and are thereby considered to bear a congenital origin [12]. Several pediatric studies indisputably demonstrated that LVNC possesses a congenital origin in children [1,20]. However, it was also found that LVNC can be acquired as many LVNC patients did not display noncompaction defects until a later stage, suggesting an acquired origin of LVNC. Therefore, it is likely that there are two types of LVNC: congenital LVNC and acquired LVNC.
Nevertheless, we would provide a different opinion: LVNC is congenital, but the clinical symptoms can be manifested in embryos/childhood or adulthood depending on the underlying genetic mutations. Although it was shown that coronary angiogenesis [44], Semaphorin 3E/PlexinD1 [45], Notch1 signaling [71], and many genes, including Numb [13] and DAAM1 [72], are involved in ventricular compaction, the genetic networks that regulate trabecular compaction are still not established yet, and a unifying description of the compaction process is not available. However, it is agreed that the ventricular compaction is a complicated process and regulated by complex signaling networks, and different gene mutations present different phenotypes. Some genes are required for trabecular compaction and embryonic development, and the mutated animals bearing mutations of these genes will display LVNC at an early postnatal stage or even die during pregnancy. While some genes regulate the maintenance of compaction and cardiac homeostasis, their mutations will cause LVNC at a later stage. Therefore, we infer that LVNC is congenital, and the clinical symptoms we observed in human subjects are manifestations of abnormal/mutated gene function at different stages.
The two different manifestations of LVNC can be modeled in the mouse. LVNC, with the congenital onset, usually will cause embryonic lethality or early neonatal death in the mouse. The adult-onset of LVNC was not modeled in the mouse until a recent report that compound heterozygous mutant mice provide an adult-onset mouse model [73]. This study shows that the adult-onset LVNC mouse model can be established unless the gene-based dosage is not lethal so that the animals can survive to the adult stage and display LVNC. In summary, we propose that LVNC is a congenital defect, and the manifestation can be congenital and adult onsets depending on the functions of the disrupted genes.
In this study, our results support the notion that LVNC is congenital, and the clinical symptoms are manifested at congenital or adult stages. With a detailed clinical diagnosis of the 46 noncompaction patients, we found some novel features of LVNC; most of the noncompaction patients are associated with regurgitation, and all the patients are associated with arrhythmias. The LVNC was supposed to be associated with ventricular related arrhythmias such as VPC and VT but not atrial related arrhythmias such as AT, APC, SSS, AVB, and AF. Some of the arrhythmias might be acquired due to ventricular remodeling. However, this could only explain the acquisition of ventricular arrhythmias but not the etiology of atrial related arrhythmias. A possible explanation is that some of the arrhythmias result from cardiac remodeling, but even this could not explain the etiology of SSS, VPC, AVB, and other types of arrhythmias observed in this study. Instead, these data suggest a congenital formation of these arrhythmias that associate with LVNC. In summary, manifestations of different types of arrhythmias suggest that the arrhythmias, or at least some of the arrhythmias in LVNC patients, are congenital.
One of the major causes of regurgitation is the valvular structural defect. Of the 45 LVNC patients, only #14 displays Ebstein’s anomaly. The structure and components of the tricuspid and mitral valves of the other patients were not noticed to be abnormal. However, many components of the valve, including the annulus, leaflets, chords, papillary muscles, and ventricular function and geometry can contribute to regurgitation. A more detailed examination of the valves in the LVNC patients will be needed to determine if the regurgitation is caused by congenital structural defects or remodeled structural changes. An alternative way to determine if the regurgitation symptoms are acquired by remodeling or are congenital is to determine whether the regurgitation symptoms are being associated with age or not. We analyzed the severity of regurgitation between the two age groups. It was well accepted that the prevalence of valvular heart diseases increases by age [54], and the severity of regurgitation between the two groups is significantly different based on the percentage of mild, moderate, and severe patients in the two groups (Table 2), suggesting that regurgitation is an age-related remodeling of the valves in the LVNC patients. These data imply that valvular regurgitation is associated with the onset of LVNC and might be a clinical feature for LVNC during diagnosis.
4.2. Reduced Contractility Is a Cause or a Consequence of LVNC
Accumulating pieces of evidence indicate a crucial role of cardiac contraction and the resulting hemodynamic force on ventricular wall compaction [52,55]. Mutations of many genes, including MYH7, MYBPC3, and TTN, that encode sarcomere components cause LVNC [52]. Of the genes whose mutations would affect contraction and develop LVNC, MYH7 was the only sarcomere gene associated with CHD [52]. Therefore, it has been speculated that the contractile function is required for trabecular compaction [56]. The impaired contractile function causes sluggish blood flow, likely resulting in thrombotic formation in deep intertrabecular recesses of the left ventricle, which might be the reason for frequent thrombotic events in LVNC. However, whether the reduced contractile function in LVNC is a cause or consequence is unknown.
In this study, we examined the clinical data and found that 39 out of the 45 LVNC patients display reduced contractility, suggesting that contractile function might be a feature of LVNC. However, 6 out of 45 LVNC patients did not display reduced contractility, suggesting that reduced contractility is not required for the onset of LVNC. We compared the contractile functions between the two age groups and found that 16 out of 23 patients in the young group display a contractile defect, while 22 out of 22 patients in the other group display reduced contractility (Table 1 and Figure 3). The differential contractile defect between the two groups suggests that the onset of the reduced contractility could be an acquired feature in LVNC. A previous study quantified trabeculations in a large cohort of otherwise healthy adults by MRI, which determined the associations of trabeculae with cardiac function and concluded that greater trabeculations are associated with decreased LV function [74]. Consistently, our studies found that the thickness ratio of trabecular layer to compact layer negatively correlates with reduced systolic function. These data suggest that reduced contractility might be a consequence of LVNC and is not necessarily required for the onset of LVNC.
However, this study has some limitations as following monitoring of the contractility changes in LVNC patients in a time-dependent manner will be needed to make a decisive conclusion regarding whether contractility is a cause or consequence of LVNC. Furthermore, due to the small sample size, the limited cases of LVNC do not reflect the etiology of all the LVNC patients and do not include all the causes that onset the LVNC in patients. This study used the ECHO, which is less sensitive than cardiac MRI, to screen LVNC patients, which might be why only 0.48% of subjects display LVNC.
5. Conclusions
In summary, we analyzed the clinical symptoms of 45 LVNC and 1 RVNC patient from 9582 echocardiograms performed on subjects with potential heart diseases. We found that 40 out of 46 LVNC patients showed clinical valvular regurgitation, 39 of the 45 LVNC patients display reduced systolic contraction, and 46 out of the 46 patients display various forms of arrhythmia. This retrospective analysis reveals novel findings that LVNC is associated with regurgitation in most of the patients and arrhythmias in all the patients, aged LVNC patients have a tendency of reduced contractility than young LVNC patients, and thickness ratio of trabecular to compact negatively correlates with the reduced systolic function. The findings suggest that regurgitation might be a clinical feature for LVNC. LVNC is a congenital defect, and the manifestations can be present in the congenital or adult stage depending on the functions of the disrupted genes.
Author Contributions
Conceptualization, M.W. and J.L.; methodology, Q.L. and L.M.; software, L.X.; validation, Q.L., L.M., L.X., Y.L., A.N., M.T., F.Z., J.L. and M.W.; formal analysis, Q.L., L.M. and L.X.; investigation, Q.L., L.M., M.T., L.X., F.Z., Y.L., H.Y.A., A.N., J.L. and M.W.; resources, Q.L., L.X. and J.L.; data curation, Q.L., F.Z., L.X. and J.L.; writing—original draft preparation, M.W.; writing—review and editing, Q.L., L.M., L.X., Y.L., M.T., H.Y.A., A.N., J.L. and M.W.; visualization, Q.L., L.X. and J.L.; supervision, J.L. and M.W.; project administration, J.L. and M.W.; funding acquisition, J.L. and M.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the National Heart, Lung, and Blood Institute grant 2R01HL121700-06A1 and the American Heart Association grant 20TPA35490051 to M.W., and grant 81760065 from the National Natural Science Foundation of China to J. Li.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the Second Affiliated Hospital of Nanchang University.
Informed Consent Statement
Patient consent was waived due to the retrospective nature of this study.
Data Availability Statement
All data supporting reported results in this paper are provided in the figures and tables in this paper.
Acknowledgments
We thank Wu’s and Liu’s lab members for the scientific discussion.
Conflicts of Interest
The authors declare no conflict of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Pignatelli, R.H.; McMahon, C.J.; Dreyer, W.J.; Denfield, S.W.; Price, J.; Belmont, J.W.; Craigen, W.J.; Wu, J.; El Said, H.; Bezold, L.I.; et al. Clinical characterization of left ventricular noncompaction in children: A relatively common form of cardiomyopathy. Circulation 2003, 108, 2672–2678. [Google Scholar] [CrossRef] [PubMed]
- Towbin, J.A.; Jefferies, J.L. Cardiomyopathies Due to Left Ventricular Noncompaction, Mitochondrial and Storage Diseases, and Inborn Errors of Metabolism. Circ. Res. 2017, 121, 838–854. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Towbin, J.A.; Thiene, G.; Antzelevitch, C.; Corrado, D.; Arnett, D.; Moss, A.J.; Seidman, C.E.; Young, J.B.; American Heart, A.; et al. Contemporary definitions and classification of the cardiomyopathies: An American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 2006, 113, 1807–1816. [Google Scholar] [CrossRef]
- Feldt, R.H.; Rahimtoola, S.H.; Davis, G.D.; Swan, H.J.; Titus, J.L. Anomalous ventricular myocardial patterns in a child with complex congenital heart disease. Am. J. Cardiol. 1969, 23, 732–734. [Google Scholar] [CrossRef]
- Stahli, B.E.; Gebhard, C.; Biaggi, P.; Klaassen, S.; Valsangiacomo Buechel, E.; Attenhofer Jost, C.H.; Jenni, R.; Tanner, F.C.; Greutmann, M. Left ventricular non-compaction: Prevalence in congenital heart disease. Int. J. Cardiol. 2013, 167, 2477–2481. [Google Scholar] [CrossRef]
- Finsterer, J.; Stollberger, C.; Towbin, J.A. Left ventricular noncompaction cardiomyopathy: Cardiac, neuromuscular, and genetic factors. Nat. Rev.. Cardiol. 2017, 14, 224–237. [Google Scholar] [CrossRef]
- Camuglia, A.C.; Younger, J.F.; McGaughran, J.; Lo, A.; Atherton, J.J. Cardiac myosin-binding protein C gene mutation expressed as hypertrophic cardiomyopathy and left ventricular noncompaction within two families: Insights from cardiac magnetic resonance in clinical screening: Camuglia MYBPC3 gene mutation and MRI. Int. J. Cardiol. 2013, 168, 2950–2952. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, H.; Shou, W. Potential Common Pathogenic Pathways for the Left Ventricular Noncompaction Cardiomyopathy (LVNC). Pediatr. Cardiol. 2018, 39, 1099–1106. [Google Scholar] [CrossRef]
- Arbustini, E.; Weidemann, F.; Hall, J.L. Left ventricular noncompaction: A distinct cardiomyopathy or a trait shared by different cardiac diseases? J. Am. Coll. Cardiol. 2014, 64, 1840–1850. [Google Scholar] [CrossRef]
- Samsa, L.A.; Yang, B.; Liu, J. Embryonic cardiac chamber maturation: Trabeculation, conduction, and cardiomyocyte proliferation. Am. J. Med. Genet. C Semin. Med. Genet. 2013, 163, 157–168. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, H.; Qu, X.; Chang, C.P.; Shou, W. Molecular mechanism of ventricular trabeculation/compaction and the pathogenesis of the left ventricular noncompaction cardiomyopathy (LVNC). Am. J. Med. Genet. C Semin. Med. Genet. 2013, 163, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Choquet, C.; Kelly, R.G.; Miquerol, L. Defects in Trabecular Development Contribute to Left Ventricular Noncompaction. Pediatr. Cardiol. 2019, 40, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Guo, H.; Li, J.; Myint, T.; Pittman, W.; Yang, L.; Zhong, W.; Schwartz, R.J.; Schwarz, J.J.; Singer, H.A.; et al. Numb family proteins are essential for cardiac morphogenesis and progenitor differentiation. Development 2014, 141, 281–295. [Google Scholar] [CrossRef]
- Sedmera, D.; Thomas, P.S. Trabeculation in the embryonic heart. Bioessays 1996, 18, 607. [Google Scholar] [CrossRef] [PubMed]
- Jenni, R.; Rojas, J.; Oechslin, E. Isolated noncompaction of the myocardium. N. Engl. J. Med. 1999, 340, 966–967. [Google Scholar] [CrossRef]
- Weiford, B.C.; Subbarao, V.D.; Mulhern, K.M. Noncompaction of the ventricular myocardium. Circulation 2004, 109, 2965–2971. [Google Scholar] [CrossRef] [PubMed]
- Choquet, C.; Nguyen, T.H.M.; Sicard, P.; Buttigieg, E.; Tran, T.T.; Kober, F.; Varlet, I.; Sturny, R.; Costa, M.W.; Harvey, R.P.; et al. Deletion of Nkx2-5 in trabecular myocardium reveals the developmental origins of pathological heterogeneity associated with ventricular non-compaction cardiomyopathy. PLoS Genet. 2018, 14, e1007502. [Google Scholar] [CrossRef]
- Towbin, J.A.; Lorts, A.; Jefferies, J.L. Left ventricular non-compaction cardiomyopathy. Lancet 2015, 386, 813–825. [Google Scholar] [CrossRef]
- Parent, J.J.; Towbin, J.A.; Jefferies, J.L. Left ventricular noncompaction in a family with lamin A/C gene mutation. Tex. Heart Inst. J. 2015, 42, 73–76. [Google Scholar] [CrossRef]
- Jefferies, J.L.; Wilkinson, J.D.; Sleeper, L.A.; Colan, S.D.; Lu, M.; Pahl, E.; Kantor, P.F.; Everitt, M.D.; Webber, S.A.; Kaufman, B.D.; et al. Cardiomyopathy Phenotypes and Outcomes for Children With Left Ventricular Myocardial Noncompaction: Results From the Pediatric Cardiomyopathy Registry. J. Card. Fail 2015, 21, 877–884. [Google Scholar] [CrossRef]
- Ikeda, U.; Minamisawa, M.; Koyama, J. Isolated left ventricular non-compaction cardiomyopathy in adults. J. Cardiol. 2015, 65, 91–97. [Google Scholar] [CrossRef]
- Shieh, J.T.; Jefferies, J.L.; Chin, A.J. Disorders of left ventricular trabeculation/compaction or right ventricular wall formation. Am. J. Med. Genet. C Semin. Med. Genet. 2013, 163, 141–143. [Google Scholar] [CrossRef][Green Version]
- Karkucinska-Wieckowska, A.; Trubicka, J.; Werner, B.; Kokoszynska, K.; Pajdowska, M.; Pronicki, M.; Czarnowska, E.; Lebiedzinska, M.; Sykut-Cegielska, J.; Ziolkowska, L.; et al. Left ventricular noncompaction (LVNC) and low mitochondrial membrane potential are specific for Barth syndrome. J. Inherit. Metab. Dis. 2013, 36, 929–937. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, W.; Sun, X.; Yoshimoto, M.; Chen, Z.; Zhu, W.; Liu, J.; Shen, Y.; Yong, W.; Li, D.; et al. Fkbp1a controls ventricular myocardium trabeculation and compaction by regulating endocardial Notch1 activity. Development 2013, 140, 1946–1957. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Bucker, S.; Jungblut, B.; Bottger, T.; Cinnamon, Y.; Tchorz, J.; Muller, M.; Bettler, B.; Harvey, R.; Sun, Q.Y.; et al. Inhibition of Notch2 by Numb/Numblike controls myocardial compaction in the heart. Cardiovasc. Res. 2012, 96, 276–285. [Google Scholar] [CrossRef]
- Ryan, T.D.; Ware, S.M.; Lucky, A.W.; Towbin, J.A.; Jefferies, J.L.; Hinton, R.B. Left ventricular noncompaction cardiomyopathy and aortopathy in a patient with recessive dystrophic epidermolysis bullosa. Circ. Heart Fail. 2012, 5, e81–e82. [Google Scholar] [CrossRef] [PubMed]
- Towbin, J.A. Left ventricular noncompaction: A new form of heart failure. Heart Fail. Clin. 2010, 6, 453–469. [Google Scholar] [CrossRef] [PubMed]
- Breckenridge, R.A.; Anderson, R.H.; Elliott, P.M. Isolated left ventricular non-compaction: The case for abnormal myocardial development. Cardiol. Young 2007, 17, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Stollberger, C.; Finsterer, J. Left ventricular hypertrabeculation/noncompaction. J. Am. Soc. Echocardiogr. 2004, 17, 91–100. [Google Scholar] [CrossRef]
- Rasouli, S.J.; Stainier, D.Y.R. Regulation of cardiomyocyte behavior in zebrafish trabeculation by Neuregulin 2a signaling. Nat. Commun. 2017, 8, 15281. [Google Scholar] [CrossRef]
- Passer, D.; van de Vrugt, A.; Atmanli, A.; Domian, I.J. Atypical Protein Kinase C-Dependent Polarized Cell Division Is Required for Myocardial Trabeculation. Cell Rep. 2016, 14, 1662–1672. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Amilburu, V.; Rasouli, S.J.; Staudt, D.W.; Nakajima, H.; Chiba, A.; Mochizuki, N.; Stainier, D.Y.R. In Vivo Visualization of Cardiomyocyte Apicobasal Polarity Reveals Epithelial to Mesenchymal-like Transition during Cardiac Trabeculation. Cell Rep. 2016, 17, 2687–2699. [Google Scholar] [CrossRef] [PubMed]
- Cherian, A.V.; Fukuda, R.; Augustine, S.M.; Maischein, H.M.; Stainier, D.Y. N-cadherin relocalization during cardiac trabeculation. Proc. Natl. Acad. Sci. USA 2016, 113, 7569–7574. [Google Scholar] [CrossRef]
- Liu, J.; Bressan, M.; Hassel, D.; Huisken, J.; Staudt, D.; Kikuchi, K.; Poss, K.D.; Mikawa, T.; Stainier, D.Y. A dual role for ErbB2 signaling in cardiac trabeculation. Development 2010, 137, 3867–3875. [Google Scholar] [CrossRef]
- Finsterer, J.; Stollberger, C.; Wegmann, R.; Janssen, L.A. Acquired left ventricular hypertrabeculation/noncompaction in myotonic dystrophy type 1. Int. J. Cardiol. 2009, 137, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Wu, M. Mechanisms of Trabecular Formation and Specification During Cardiogenesis. Pediatr Cardiol. 2018, 39, 1082–1089. [Google Scholar] [CrossRef]
- Li, J.; Miao, L.; Shieh, D.; Spiotto, E.; Li, J.; Zhou, B.; Paul, A.; Schwartz, R.J.; Firulli, A.B.; Singer, H.A.; et al. Single-Cell Lineage Tracing Reveals that Oriented Cell Division Contributes to Trabecular Morphogenesis and Regional Specification. Cell Rep. 2016, 15, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Li, J. Numb family proteins: Novel players in cardiac morphogenesis and cardiac progenitor cell differentiation. Biomol. Concepts 2015, 6, 137–148. [Google Scholar] [CrossRef]
- Miao, L.; Li, J.; Li, J.; Tian, X.; Lu, Y.; Hu, S.; Shieh, D.; Kanai, R.; Zhou, B.Y.; Zhou, B.; et al. Notch signaling regulates Hey2 expression in a spatiotemporal dependent manner during cardiac morphogenesis and trabecular specification. Sci. Rep. 2018, 8, 2678. [Google Scholar] [CrossRef]
- Jimenez-Amilburu, V.; Stainier, D.Y.R. The transmembrane protein Crb2a regulates cardiomyocyte apicobasal polarity and adhesion in zebrafish. Development 2019, 146, dev171207. [Google Scholar] [CrossRef]
- Miao, L.; Li, J.; Li, J.; Lu, Y.; Shieh, D.; Mazurkiewicz, J.E.; Barroso, M.; Schwarz, J.J.; Xin, H.B.; Singer, H.A.; et al. Cardiomyocyte orientation modulated by the Numb family proteins-N-cadherin axis is essential for ventricular wall morphogenesis. Proc. Natl. Acad. Sci. USA 2019, 116, 15560–15569. [Google Scholar] [CrossRef] [PubMed]
- Del Monte-Nieto, G.; Ramialison, M.; Adam, A.A.S.; Wu, B.; Aharonov, A.; D’Uva, G.; Bourke, L.M.; Pitulescu, M.E.; Chen, H.; de la Pompa, J.L.; et al. Control of cardiac jelly dynamics by NOTCH1 and NRG1 defines the building plan for trabeculation. Nature 2018, 557, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Harmelink, C.; Baldwin, H.S. Tie2 regulates endocardial sprouting and myocardial trabeculation. JCI Insight 2019, 5, e96002. [Google Scholar] [CrossRef]
- Rhee, S.; Chung, J.I.; King, D.A.; D’Amato, G.; Paik, D.T.; Duan, A.; Chang, A.; Nagelberg, D.; Sharma, B.; Jeong, Y.; et al. Endothelial deletion of Ino80 disrupts coronary angiogenesis and causes congenital heart disease. Nat. Commun. 2018, 9, 368. [Google Scholar] [CrossRef]
- Sandireddy, R.; Cibi, D.M.; Gupta, P.; Singh, A.; Tee, N.; Uemura, A.; Epstein, J.A.; Singh, M.K. Semaphorin 3E/PlexinD1 signaling is required for cardiac ventricular compaction. JCI Insight 2019, 4, e125908. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Li, Y.; He, L.; Zhang, H.; Huang, X.; Liu, Q.; Pu, W.; Zhang, L.; Li, Y.; Zhao, H.; et al. Identification of a hybrid myocardial zone in the mammalian heart after birth. Nat. Commun. 2017, 8, 87. [Google Scholar] [CrossRef]
- Shemisa, K.; Li, J.; Tam, M.; Barcena, J. Left ventricular noncompaction cardiomyopathy. Cardiovasc Diagn Ther. 2013, 3, 170–175. [Google Scholar] [CrossRef]
- Chin, T.K.; Perloff, J.K.; Williams, R.G.; Jue, K.; Mohrmann, R. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation 1990, 82, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef]
- Stollberger, C.; Finsterer, J.; Blazek, G. Left ventricular hypertrabeculation/noncompaction and association with additional cardiac abnormalities and neuromuscular disorders. Am. J. Cardiol. 2002, 90, 899–902. [Google Scholar] [CrossRef]
- Oechslin, E.; Klaassen, S. Left Ventricular Noncompaction: Phenotype in an Integrated Model of Cardiomyopathy? J. Am. Coll. Cardiol. 2019, 73, 1612–1615. [Google Scholar] [CrossRef] [PubMed]
- van Waning, J.I.; Caliskan, K.; Hoedemaekers, Y.M.; van Spaendonck-Zwarts, K.Y.; Baas, A.F.; Boekholdt, S.M.; van Melle, J.P.; Teske, A.J.; Asselbergs, F.W.; Backx, A.; et al. Genetics, Clinical Features, and Long-Term Outcome of Noncompaction Cardiomyopathy. J. Am. Coll. Cardiol. 2018, 71, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Xu, R.; Li, X.; Xu, H.Y.; Yang, Z.G.; Wang, Y.N.; Fan, H.M.; Guo, Y.K. The mitral regurgitation effects of cardiac structure and function in left ventricular noncompaction. Sci. Rep. 2021, 11, 4616. [Google Scholar] [CrossRef] [PubMed]
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of valvular heart diseases: A population-based study. Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef]
- Kolokotronis, K.; Kuhnisch, J.; Klopocki, E.; Dartsch, J.; Rost, S.; Huculak, C.; Mearini, G.; Stork, S.; Carrier, L.; Klaassen, S.; et al. Biallelic mutation in MYH7 and MYBPC3 leads to severe cardiomyopathy with left ventricular noncompaction phenotype. Hum. Mutat. 2019, 40, 1101–1104. [Google Scholar] [CrossRef] [PubMed]
- Kayvanpour, E.; Sedaghat-Hamedani, F.; Gi, W.T.; Tugrul, O.F.; Amr, A.; Haas, J.; Zhu, F.; Ehlermann, P.; Uhlmann, L.; Katus, H.A.; et al. Clinical and genetic insights into non-compaction: A meta-analysis and systematic review on 7598 individuals. Clin. Res. Cardiol. 2019, 108, 1297–1308. [Google Scholar] [CrossRef]
- Steffel, J.; Duru, F. Rhythm disorders in isolated left ventricular noncompaction. Ann. Med. 2012, 44, 101–108. [Google Scholar] [CrossRef]
- Miyake, C.Y.; Kim, J.J. Arrhythmias in left ventricular noncompaction. Card. Electrophysiol Clin. 2015, 7, 319–330. [Google Scholar] [CrossRef]
- Szentpali, Z.; Szili-Torok, T.; Caliskan, K. Primary electrical disorder or primary cardiomyopathy? A case with a unique association of noncompaction cardiomyopathy and cathecolaminergic polymorphic ventricular tachycardia caused by ryanodine receptor mutation. Circulation 2013, 127, 1165–1166. [Google Scholar] [CrossRef]
- Nakashima, K.; Kusakawa, I.; Yamamoto, T.; Hirabayashi, S.; Hosoya, R.; Shimizu, W.; Sumitomo, N. A left ventricular noncompaction in a patient with long QT syndrome caused by a KCNQ1 mutation: A case report. Heart Vessel. 2013, 28, 126–129. [Google Scholar] [CrossRef]
- Ichida, F.; Hamamichi, Y.; Miyawaki, T.; Ono, Y.; Kamiya, T.; Akagi, T.; Hamada, H.; Hirose, O.; Isobe, T.; Yamada, K.; et al. Clinical features of isolated noncompaction of the ventricular myocardium: Long-term clinical course, hemodynamic properties, and genetic background. J. Am. Coll. Coll. Cardiol. 1999, 34, 233–240. [Google Scholar] [CrossRef]
- Ritter, M.; Oechslin, E.; Sutsch, G.; Attenhofer, C.; Schneider, J.; Jenni, R. Isolated noncompaction of the myocardium in adults. Mayo Clin. Proc. 1997, 72, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Brescia, S.T.; Rossano, J.W.; Pignatelli, R.; Jefferies, J.L.; Price, J.F.; Decker, J.A.; Denfield, S.W.; Dreyer, W.J.; Smith, O.; Towbin, J.A.; et al. Mortality and sudden death in pediatric left ventricular noncompaction in a tertiary referral center. Circulation 2013, 127, 2202–2208. [Google Scholar] [CrossRef] [PubMed]
- Oechslin, E.N.; Attenhofer Jost, C.H.; Rojas, J.R.; Kaufmann, P.A.; Jenni, R. Long-term follow-up of 34 adults with isolated left ventricular noncompaction: A distinct cardiomyopathy with poor prognosis. J. Am. Coll. Cardiol. 2000, 36, 493–500. [Google Scholar] [CrossRef]
- Murphy, R.T.; Thaman, R.; Blanes, J.G.; Ward, D.; Sevdalis, E.; Papra, E.; Kiotsekoglou, A.; Tome, M.T.; Pellerin, D.; McKenna, W.J.; et al. Natural history and familial characteristics of isolated left ventricular non-compaction. Eur. Heart J. 2005, 26, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Aras, D.; Tufekcioglu, O.; Ergun, K.; Ozeke, O.; Yildiz, A.; Topaloglu, S.; Deveci, B.; Sahin, O.; Kisacik, H.L.; Korkmaz, S. Clinical features of isolated ventricular noncompaction in adults long-term clinical course, echocardiographic properties, and predictors of left ventricular failure. J. Card. Fail. 2006, 12, 726–733. [Google Scholar] [CrossRef]
- Stollberger, C.; Finsterer, J. Arrhythmias and left ventricular hypertrabeculation/noncompaction. Curr. Pharm. Pharm. Des. 2010, 16, 2880–2894. [Google Scholar] [CrossRef]
- Saglam, M.; Saygin, H.; Kozan, H.; Ozturk, E.; Mutlu, H. Noncompaction of Ventricular Myocardium Involving the Right Ventricle. Korean Circ. J. 2015, 45, 439–441. [Google Scholar] [CrossRef]
- Wengrofsky, P.; Armenia, C.; Oleszak, F.; Kupferstein, E.; Rednam, C.; Mitre, C.A.; McFarlane, S.I. Left Ventricular Trabeculation and Noncompaction Cardiomyopathy: A Review. EC Clin. Exp. Anat. 2019, 2, 267–283. [Google Scholar]
- Dusek, J.; Ostadal, B.; Duskova, M. Postnatal persistence of spongy myocardium with embryonic blood supply. Arch. Pathol. 1975, 99, 312–317. [Google Scholar]
- Luxan, G.; Casanova, J.C.; Martinez-Poveda, B.; Prados, B.; D’Amato, G.; MacGrogan, D.; Gonzalez-Rajal, A.; Dobarro, D.; Torroja, C.; Martinez, F.; et al. Mutations in the NOTCH pathway regulator MIB1 cause left ventricular noncompaction cardiomyopathy. Nat. Med. 2013, 19, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Hallett, M.A.; Zhu, W.; Rubart, M.; Liu, Y.; Yang, Z.; Chen, H.; Haneline, L.S.; Chan, R.J.; Schwartz, R.J.; et al. Dishevelled-associated activator of morphogenesis 1 (Daam1) is required for heart morphogenesis. Development 2011, 138, 303–315. [Google Scholar] [CrossRef] [PubMed]
- de Soysa, T.Y.; Ranade, S.S.; Okawa, S.; Ravichandran, S.; Huang, Y.; Salunga, H.T.; Schricker, A.; Del Sol, A.; Gifford, C.A.; Srivastava, D. Single-cell analysis of cardiogenesis reveals basis for organ-level developmental defects. Nature 2019, 572, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Tizon-Marcos, H.; de la Paz Ricapito, M.; Pibarot, P.; Bertrand, O.; Bibeau, K.; Le Ven, F.; Sinha, S.; Engert, J.; Bedard, E.; Pasian, S.; et al. Characteristics of trabeculated myocardium burden in young and apparently healthy adults. Am. J. Cardiol. 2014, 114, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).