Virtual Reality in the Preoperative Planning of Adult Aortic Surgery: A Feasibility Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Computed Tomography
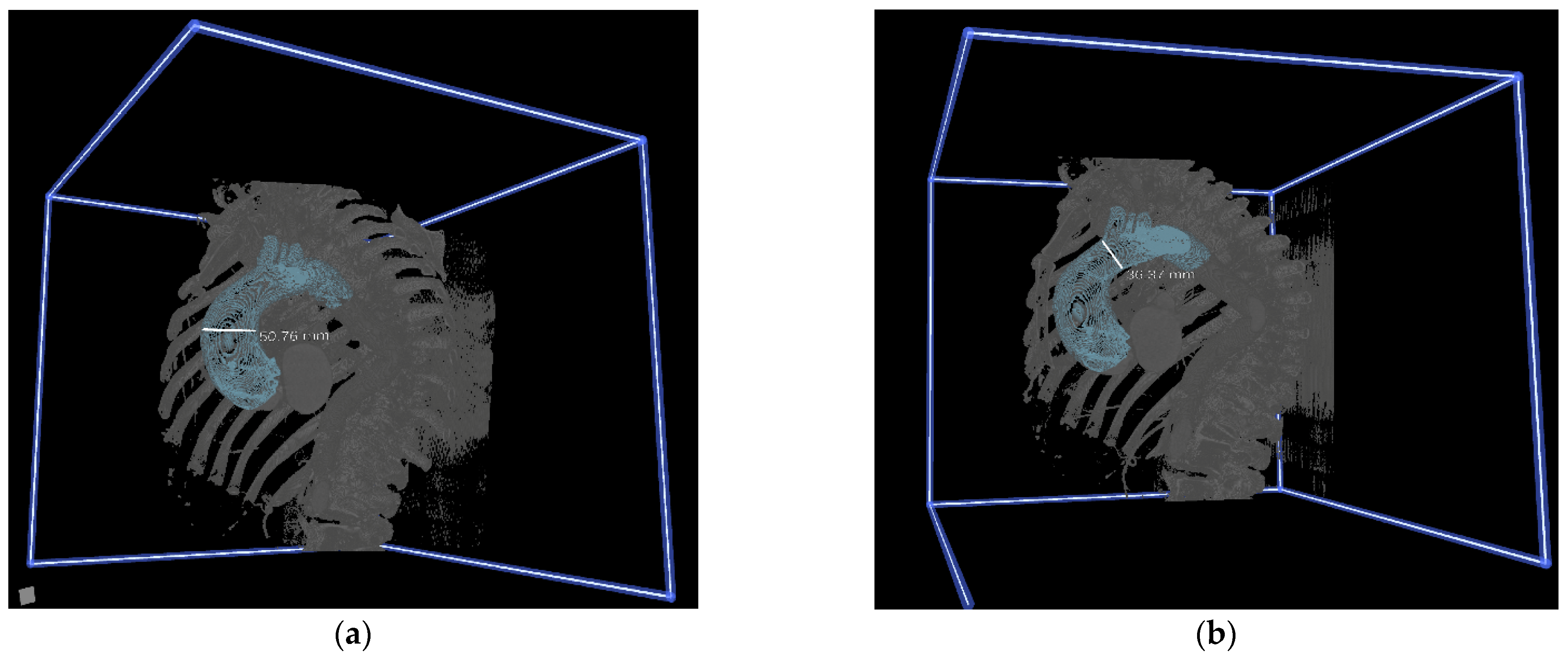
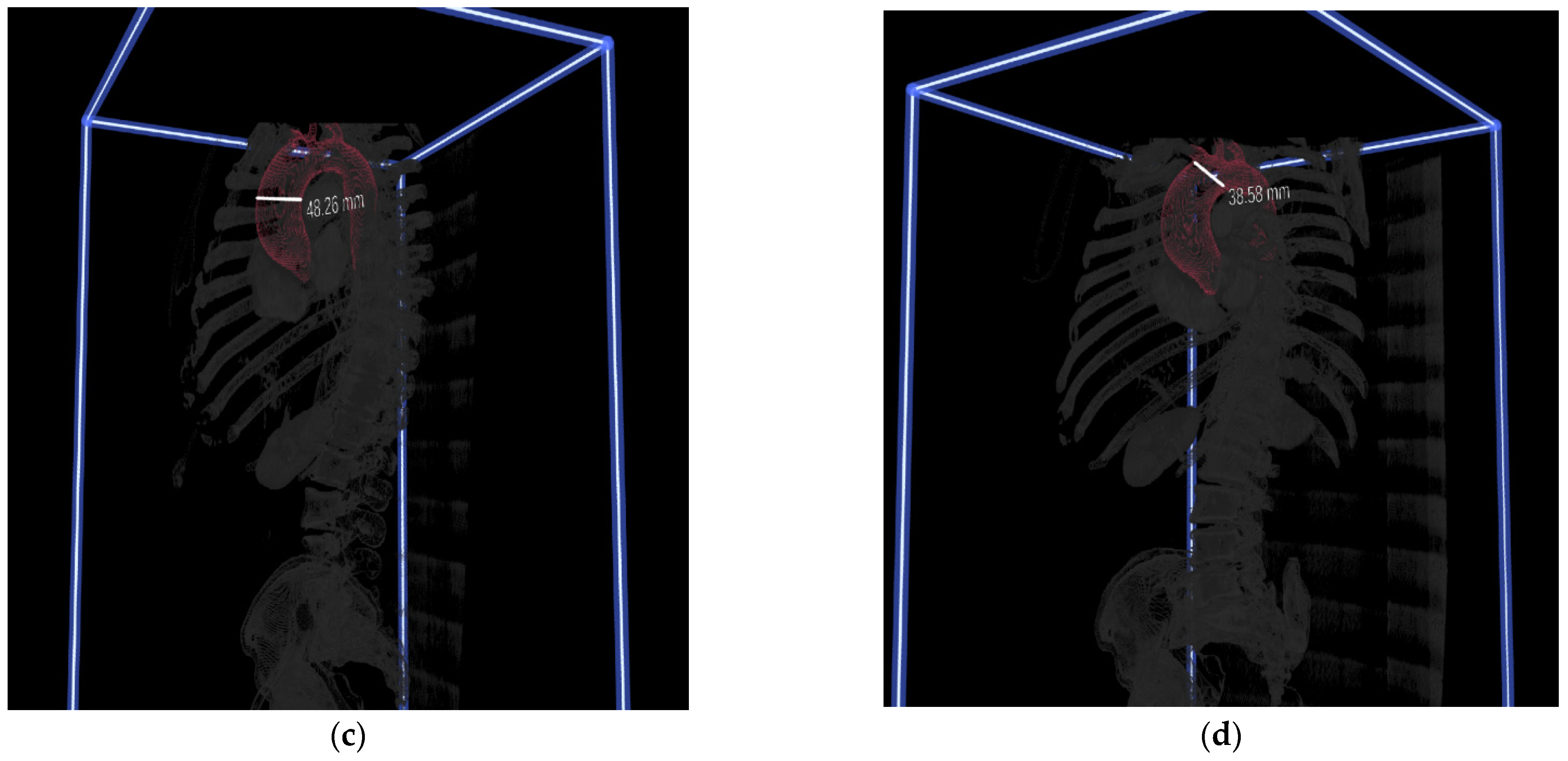
2.3. 3D Image Segmentation
2.4. Immersive 3D VR Rendering and Preoperative Planning
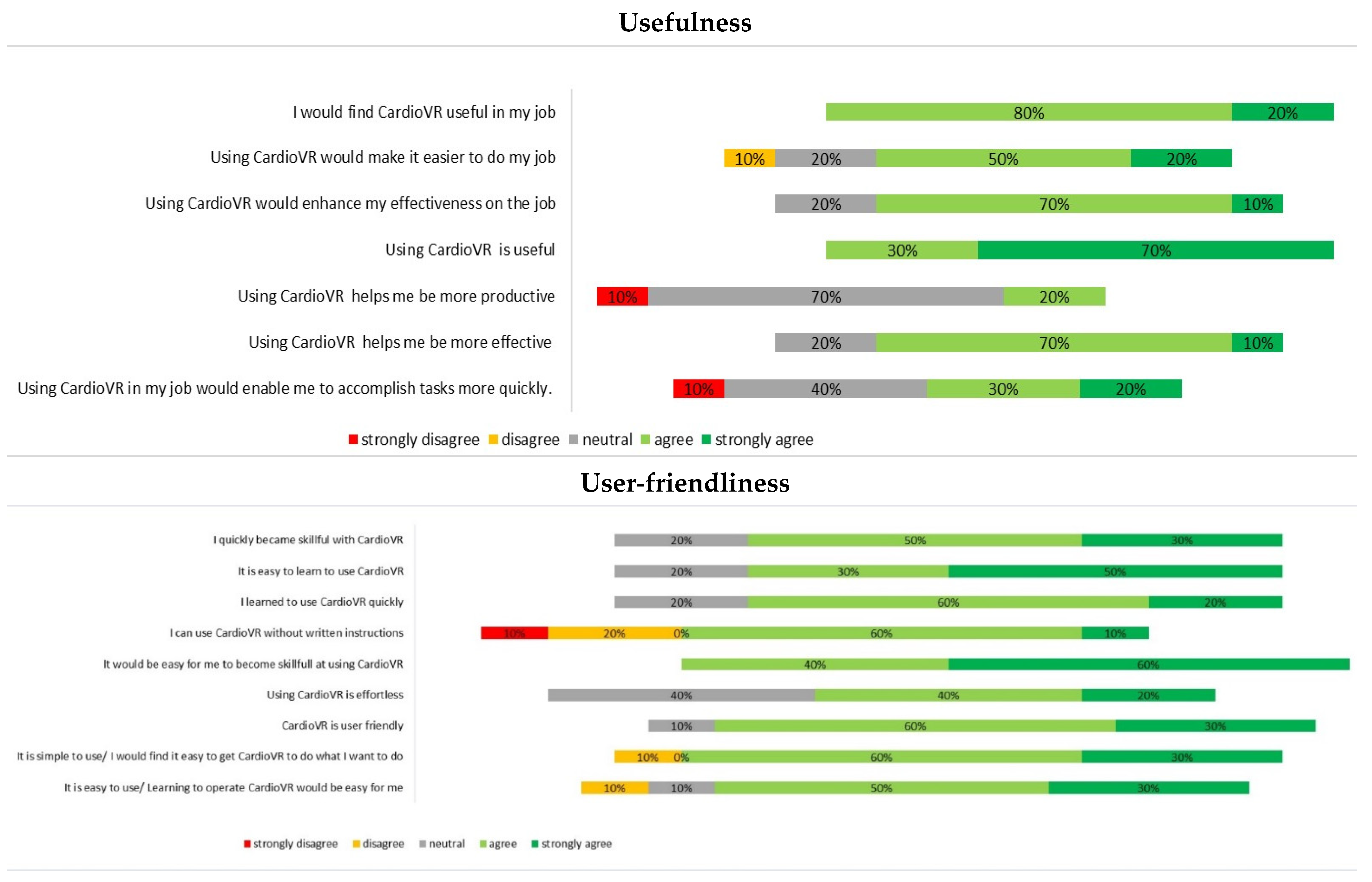
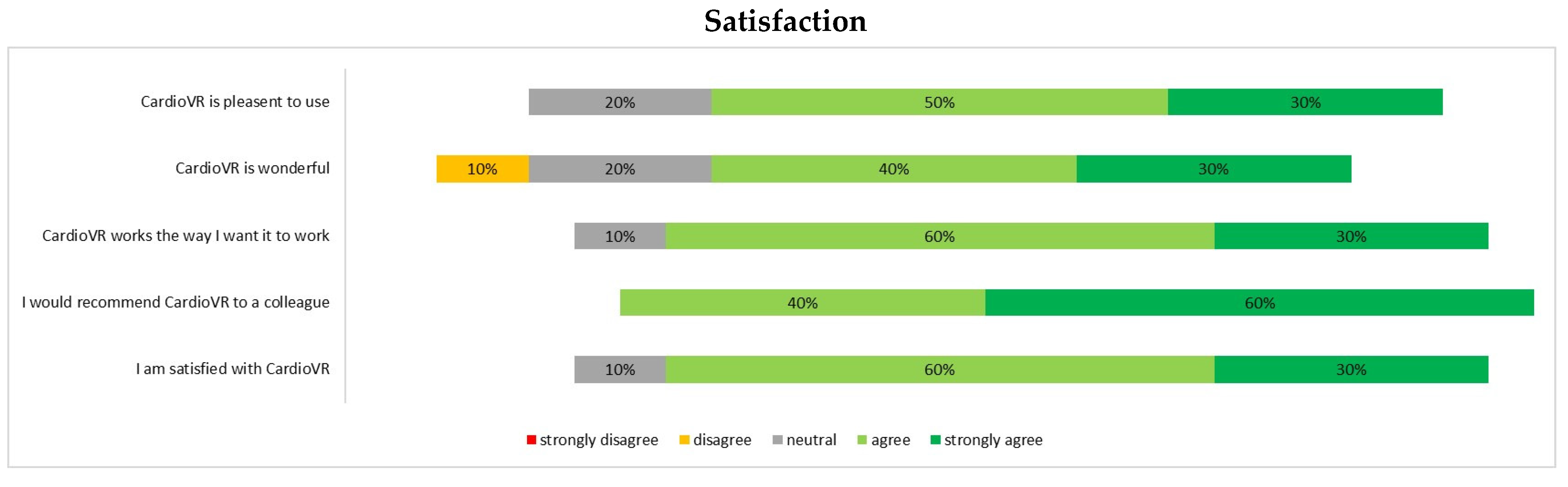
2.5. Data Acquisition and Questionnaire Design
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics and Surgeons’ Attitudes towards VR before the Study
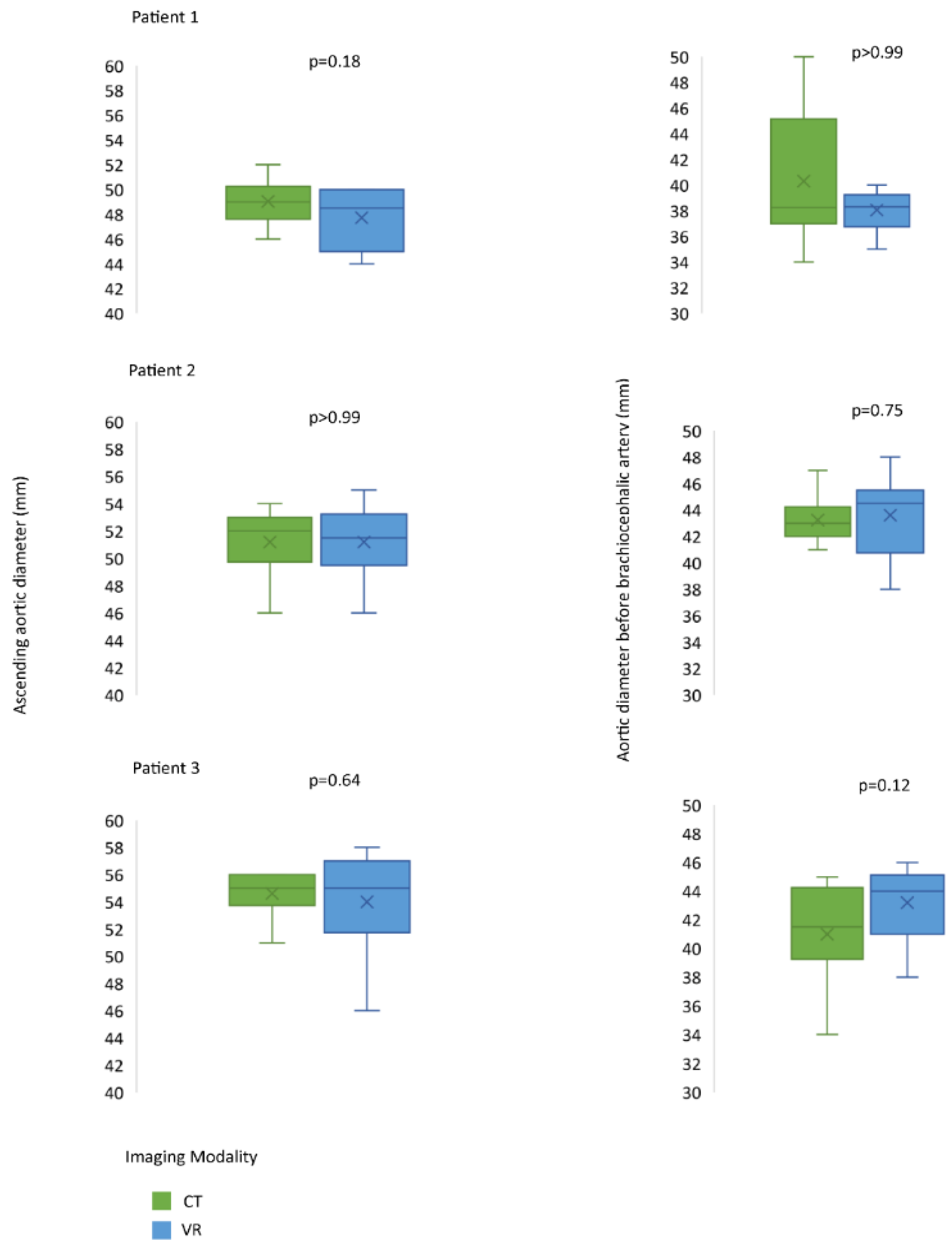
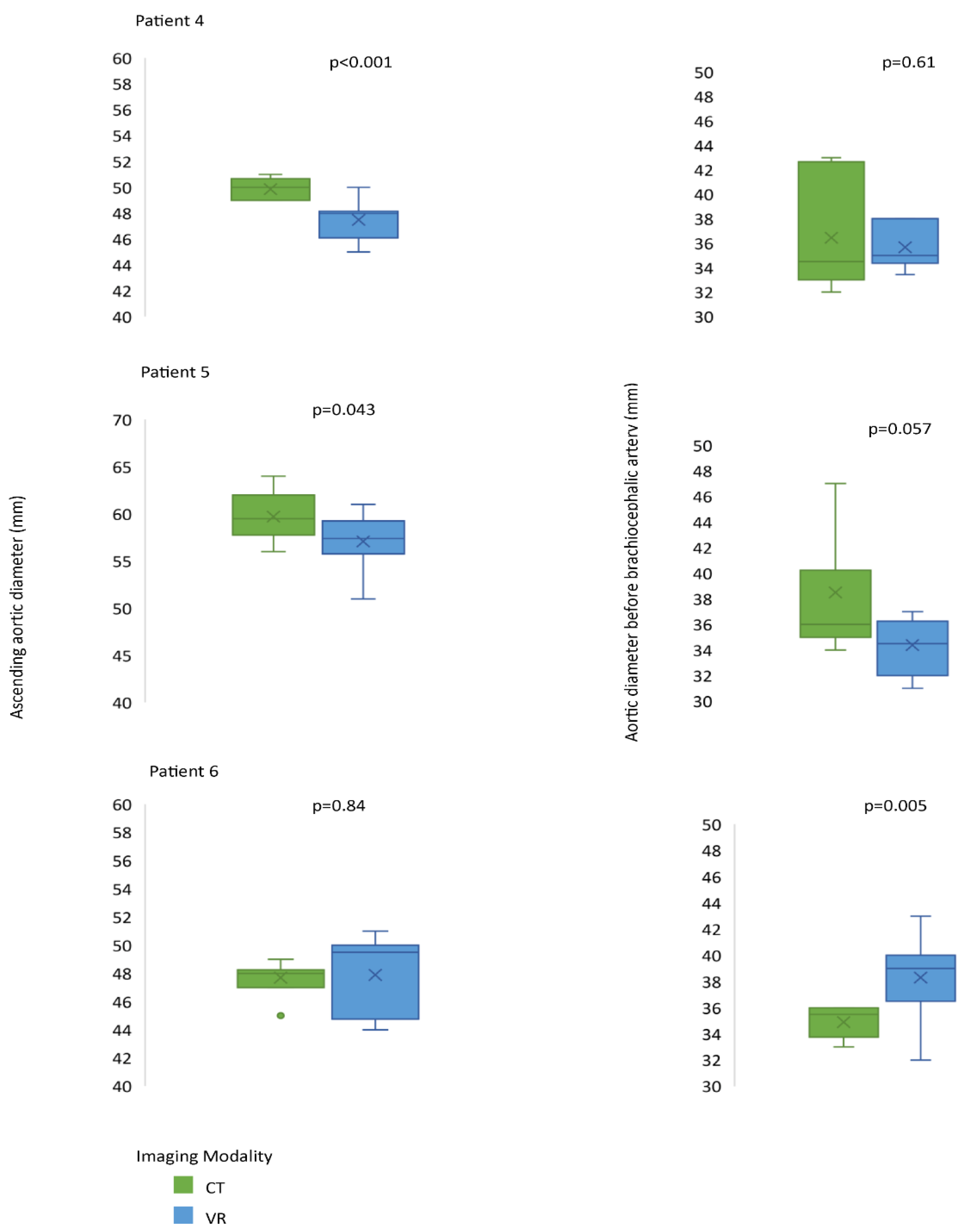
3.2. Assessment of CardioVR in the Planning of Aortic Surgery
3.3. Evaluation of VR-Guided Aortic Surgery Planning
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Narayan, P.; Caputo, M.; Rogers, C.; Alwair, H.; Mahesh, B.; Angelini, G.; Bryan, A. Early and mid-term outcomes of surgery of the ascending aorta/arch: Is there a relationship with caseload? Eur. J. Cardio-Thoracic. Surg. 2004, 25, 676–682. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ehrlich, M.P.; Ergin, M.; McCullough, J.N.; Lansman, S.L.; Galla, J.D.; Bodian, A.C.; Apaydin, A.Z.; Griepp, R.B. Predictors of adverse outcome and transient neurological dysfunction after ascending aorta/hemiarch replacement. Ann. Thorac. Surg. 2000, 69, 1755–1763. [Google Scholar] [CrossRef]
- Englum, B.R.; He, X.; Gulack, B.C.; Ganapathi, A.; Mathew, J.P.; Brennan, J.M.; Reece, T.B.; Keeling, W.B.; Leshnower, B.G.; Chen, E.P.; et al. Hypothermia and cerebral protection strategies in aortic arch surgery: A comparative effectiveness analysis from the STS Adult Cardiac Surgery Database. Eur. J. Cardio-Thoracic. Surg. 2017, 52, 492–498. [Google Scholar] [CrossRef]
- Sadeghi, A.H.; el Mathari, S.; Abjigitova, D.; Maat, A.P.M.; Taverne, Y.J.J.; Bogers, A.J.C.; Mahtab, E.A. Current and Future Applications of Virtual, Augmented, and Mixed Reality in Cardiothoracic Surgery. Ann. Thorac. Surg. 2022, 113, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Napa, S.; Moore, M.; Bardyn, T. Advancing Cardiac Surgery Case Planning and Case Review Conferences Using Virtual Reality in Medical Libraries: Evaluation of the Usability of Two Virtual Reality Apps. JMIR Hum. Factors 2019, 6, e12008. [Google Scholar] [CrossRef]
- Sadeghi, A.H.; Bakhuis, W.; Van Schaagen, F.; Oei, F.B.S.; Bekkers, A.J.; Maat, A.P.W.M.; Mahtab, E.A.F.; Bogers, A.J.J.C.; Taverne, Y.J.H.J. Immersive 3D virtual reality imaging in planning minimally invasive and complex adult cardiac surgery. Eur. Hear. J.-Digit. Health 2020, 1, 62–70. [Google Scholar] [CrossRef]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. NeuroImage 2006, 31, 1116–1128. [Google Scholar] [CrossRef]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.-C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef]
- Fred, D.D. Perceived usefulness, perceived ease of use, and user acceptance of information technology. Mis. Q 1989, 13, 319–340. [Google Scholar]
- Schijven, M.P.; Jakimowicz, J.J. Validation of virtual reality simulators: Key to the successful integration of a novel teaching technology into minimal access surgery. Minim. Invasive Ther. Allied Technol. 2005, 14, 244–246. [Google Scholar] [CrossRef]
- Reich, D.L.; Uysal, S.; Sliwinski, M.; Ergin, M.; Kahn, R.A.; Konstadt, S.N.; McCullough, J.; Hibbard, M.R.; Gordon, W.A.; Griepp, R.B. Neuropsychologic outcome after deep hypothermic circulatory arrest in adults. J. Thorac. Cardiovasc. Surg. 1999, 117, 156–163. [Google Scholar] [CrossRef]
- Wilde, J.T. Hematological consequences of profound hypothermic circulatory arrest and aortic dissection. J. Card. Surg. 1997, 12, 201–206. [Google Scholar] [PubMed]
- Gupta, P.; Harky, A.; Jahangeer, S.; Adams, B.; Bashir, M. Varying Evidence on Deep Hypothermic Circulatory Arrest in Thoracic Aortic Aneurysm Surgery. Tex. Hear. Inst. J. 2018, 45, 70–75. [Google Scholar] [CrossRef]
- Hemminger, B.M.; Molina, P.L.; Egan, T.; Detterbeck, F.C.; Muller, K.E.; Coffey, C.S.; Lee, J.K.T. Assessment of Real-Time 3D Visualization for Cardiothoracic Diagnostic Evaluation and Surgery Planning. J. Digit. Imaging 2005, 18, 145–153. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Batteux, C.; Haidar, M.A.; Bonnet, D. 3D-Printed Models for Surgical Planning in Complex Congenital Heart Diseases: A Systematic Review. Front. Pediatr. 2019, 7, 23. [Google Scholar] [CrossRef]
- Shirk, J.D.; Thiel, D.D.; Wallen, E.M.; Linehan, J.M.; White, W.M.; Badani, K.K.; Porter, J.R. Effect of 3-Dimensional Virtual Reality Models for Surgical Planning of Robotic-Assisted Partial Nephrectomy on Surgical Outcomes: A Randomized Clinical Trial. JAMA Netw. Open 2019, 2, e1911598. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.C.; Ensing, G.J.; Ohye, R.G.; Romano, J.C.; Sassalos, P.; Owens, S.T.; Thorsson, T.; Yu, S.; Lowery, R.; Si, M.-S. Stereoscopic Three-Dimensional Visualization for Congenital Heart Surgery Planning: Surgeons’ Perspectives. J. Am. Soc. Echocardiogr. 2020, 33, 775–777. [Google Scholar] [CrossRef]
- Mert, A.; Buehler, K.; Sutherland, G.R.; Tomanek, B.; Widhalm, G.; Kasprian, G.; Knosp, E.; Wolfsberger, S. Brain Tumor Surgery With 3-Dimensional Surface Navigation. Oper. Neurosurg. 2012, 71, ons286–ons295. [Google Scholar] [CrossRef]
- Sadeghi, A.H.; Maat, A.P.M.; Taverne, Y.J.J.; Cornelissen, R.; Dingemans, A.-M.C.; Bogers, A.J.C.; Mahtab, E.A. Virtual reality and artificial intelligence for 3-dimensional planning of lung segmentectomies. JTCVS Tech. 2021, 7, 309–321. [Google Scholar] [CrossRef]
- Uppot, R.N.; Laguna, B.; McCarthy, C.J.; De Novi, G.; Phelps, A.; Siegel, E.; Courtier, J. Implementing Virtual and Augmented Reality Tools for Radiology Education and Training, Communication, and Clinical Care. Radiology 2019, 291, 570–580. [Google Scholar] [CrossRef]
- Pasta, S.; Gentile, G.; Raffa, G.M.; Scardulla, F.; Bellavia, D.; Luca, A.; Pilato, M.; Scardulla, C. Three-dimensional parametric modeling of bicuspid aortopathy and comparison with computational flow predictions. Artif. Organs 2017, 41, E92–E102. [Google Scholar] [CrossRef] [PubMed]
- Pasta, S.; Cannata, S.; Gentile, G.; Di Giuseppe, M.; Cosentino, F.; Pasta, F.; Agnese, V.; Bellavia, D.; Raffa, G.M.; Pilato, M.; et al. Simulation study of transcatheter heart valve implantation in patients with stenotic bicuspid aortic valve. Med. Biol. Eng. Comput. 2020, 58, 815–829. [Google Scholar] [CrossRef] [PubMed]
- Boussel, L.; Rayz, V.; Martin, A.; Acevedo-Bolton, G.; Lawton, M.T.; Higashida, R.; Smith, W.S.; Young, W.L.; Saloner, D. Phase-contrast magnetic resonance imaging measurements in intracranial aneurysms in vivo of flow patterns, velocity fields, and wall shear stress: Comparison with computational fluid dynamics. Magn. Reson. Med. 2009, 61, 409–417. [Google Scholar] [CrossRef] [PubMed]

| Preference for Open Approach n (%) | Final Preference | Operation Performed Initially | |
|---|---|---|---|
| Patient 1 | |||
| CT1 | 10 (100) | Open | Open |
| VR1 | 8 (80) | Open | |
| Patient 2 | |||
| CT2 | 8 (80) | Open | Open |
| VR2 | 8 (80) | Open | |
| Patient 3 | |||
| CT3 | 10 (100) | Open | Open |
| VR3 | 9 (90) | Open | |
| Patient 4 | |||
| CT4 | 7 (70) | Open | Open |
| VR4 | 4 (40) | Clamp | |
| Patient 5 | |||
| CT5 | 5 (50) | 50–50 | Open |
| VR5 | 2 (20) | Clamp | |
| Patient 6 | |||
| CT6 | 1 (10) | Clamp | Open |
| VR6 | 2 (20) | Clamp | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abjigitova, D.; Sadeghi, A.H.; Peek, J.J.; Bekkers, J.A.; Bogers, A.J.J.C.; Mahtab, E.A.F. Virtual Reality in the Preoperative Planning of Adult Aortic Surgery: A Feasibility Study. J. Cardiovasc. Dev. Dis. 2022, 9, 31. https://doi.org/10.3390/jcdd9020031
Abjigitova D, Sadeghi AH, Peek JJ, Bekkers JA, Bogers AJJC, Mahtab EAF. Virtual Reality in the Preoperative Planning of Adult Aortic Surgery: A Feasibility Study. Journal of Cardiovascular Development and Disease. 2022; 9(2):31. https://doi.org/10.3390/jcdd9020031
Chicago/Turabian StyleAbjigitova, Djamila, Amir H. Sadeghi, Jette J. Peek, Jos A. Bekkers, Ad J. J. C. Bogers, and Edris A. F. Mahtab. 2022. "Virtual Reality in the Preoperative Planning of Adult Aortic Surgery: A Feasibility Study" Journal of Cardiovascular Development and Disease 9, no. 2: 31. https://doi.org/10.3390/jcdd9020031
APA StyleAbjigitova, D., Sadeghi, A. H., Peek, J. J., Bekkers, J. A., Bogers, A. J. J. C., & Mahtab, E. A. F. (2022). Virtual Reality in the Preoperative Planning of Adult Aortic Surgery: A Feasibility Study. Journal of Cardiovascular Development and Disease, 9(2), 31. https://doi.org/10.3390/jcdd9020031







