Cardiovascular Magnetic Resonance Parametric Mapping Techniques for the Assessment of Chronic Coronary Syndromes
Abstract
1. Introduction
2. T1 Mapping
2.1. Principles of T1 Mapping
2.2. Vasodilator Pharmacological Stressors for Stress CMR
2.3. Clinical Applications of Stress T1 Mapping in Chronic Coronary Syndromes
2.4. Chronic Myocardial Infarction Imaging with T1 Mapping
2.5. Prognostic Role of T1 Mapping in CAD
2.6. Advantages and Limitations of T1 Mapping and Stress T1 Mapping
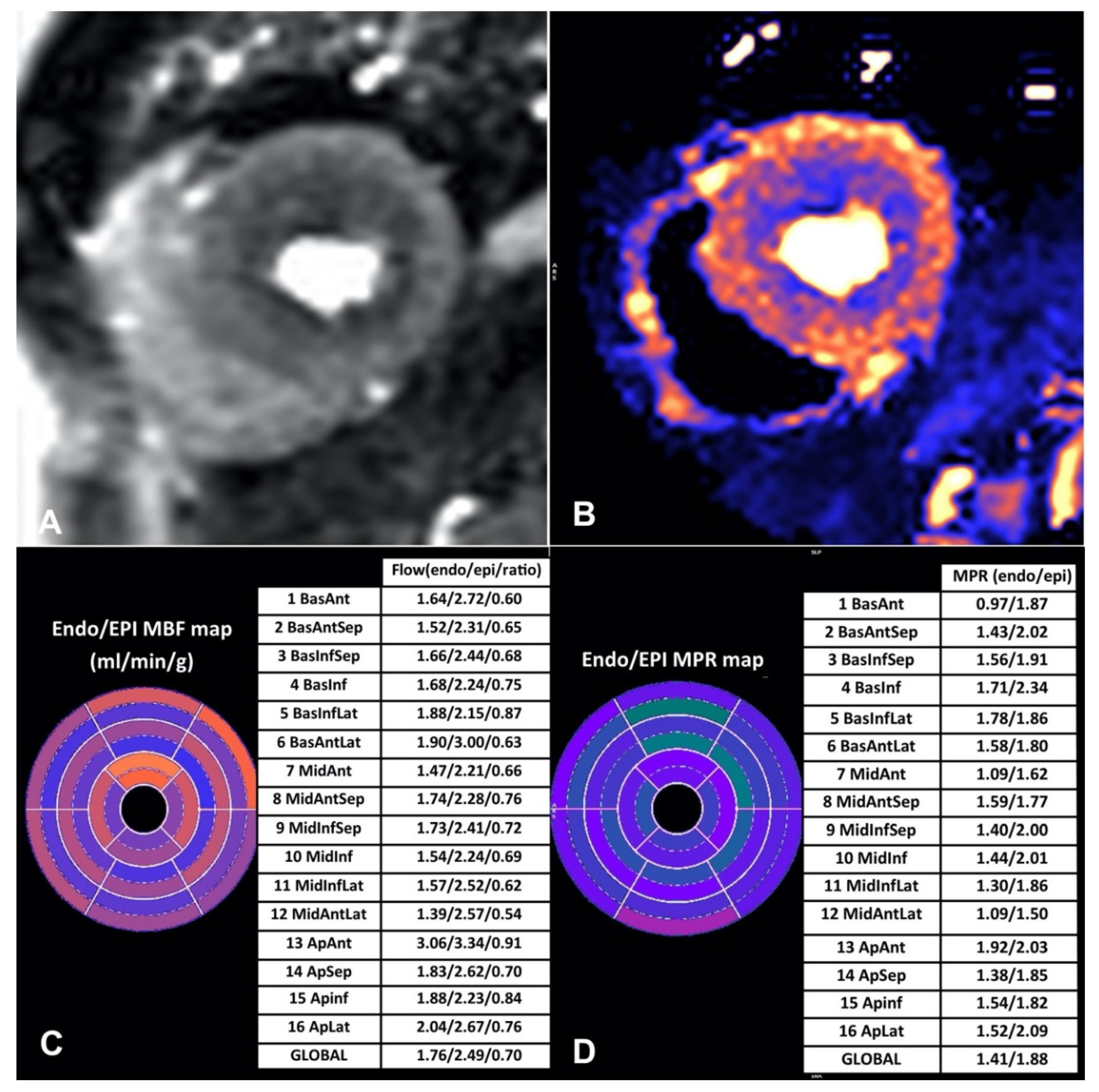
3. Quantitative Myocardial Perfusion Mapping
3.1. Newer Automated Techniques for CMR Perfusion Quantification
3.2. Clinical Applications of Quantitative Stress CMR—What We Know So Far
3.2.1. Validation and Diagnostic Accuracy
3.2.2. Three-Vessel CAD
3.2.3. Microvascular Dysfunction
3.3. Prognostic Significance
3.4. Strengths and Limitations
4. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Fihn, S.D.; Blankenship, J.C.; Alexander, K.P.; Bittl, J.A.; Byrne, J.G.; Fletcher, B.J.; Fonarow, G.C.; Lange, R.A.; Levine, G.N.; Maddox, T.M.; et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS Focused Update of the Guideline for the Diagnosis and Management of Patients with Stable Ischemic Heart Disease. J. Am. Coll. Cardiol. 2014, 64, 1929–1949. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Ballo, H.; Juarez-Orozco, L.E.; Saraste, A.; Kolh, P.; Rutjes, A.W.S.; Jüni, P.; Windecker, S.; Bax, J.J.; Wijns, W. The performance of non-invasive tests to rule-in and rule-out significant coronary artery stenosis in patients with stable angina: A meta-analysis focused on post-test disease probability. Eur. Heart J. 2018, 39, 3322–3330. [Google Scholar] [CrossRef] [PubMed]
- Schwitter, J.; Nanz, D.; Kneifel, S.; Bertschinger, K.; Büchi, M.; Knüsel, P.R.; Marincek, B.; Lüscher, T.F.; von Schulthess, G.K. Assessment of Myocardial Perfusion in Coronary Artery Disease by Magnetic Resonance. Circulation 2001, 103, 2230–2235. [Google Scholar] [CrossRef] [PubMed]
- Hamon, M.; Fau, G.; Née, G.; Ehtisham, J.; Morello, R.; Hamon, M. Meta-analysis of the diagnostic performance of stress perfusion cardiovascular magnetic resonance for detection of coronary artery disease. J. Cardiovasc. Magn. Reson. 2010, 12, 29. [Google Scholar] [CrossRef]
- Takx, R.A.; Blomberg, B.A.; El Aidi, H.; Habets, J.; de Jong, P.A.; Nagel, E.; Hoffmann, U.; Leiner, T. Diagnostic Accuracy of Stress Myocardial Perfusion Imaging Compared to Invasive Coronary Angiography with Fractional Flow Reserve Meta-Analysis. Circ. Cardiovasc. Imaging 2015, 8, e002666. [Google Scholar] [CrossRef]
- Li, M.; Zhou, T.; Yang, L.-F.; Peng, Z.-H.; Ding, J.; Sun, G. Diagnostic Accuracy of Myocardial Magnetic Resonance Perfusion to Diagnose Ischemic Stenosis With Fractional Flow Reserve as Reference. JACC Cardiovasc. Imaging 2014, 7, 1098–1105. [Google Scholar] [CrossRef]
- Ferreira, V.M.; Piechnik, S.K.; Dall’Armellina, E.; Karamitsos, T.D.; Francis, J.M.; Ntusi, N.; Holloway, C.; Choudhury, R.P.; Kardos, A.; Robson, M.D.; et al. Native T1-mapping detects the location, extent and patterns of acute myocarditis without the need for gadolinium contrast agents. J. Cardiovasc. Magn. Reson. 2014, 16, 36. [Google Scholar] [CrossRef]
- Dass, S.; Suttie, J.J.; Piechnik, S.K.; Ferreira, V.M.; Holloway, C.J.; Banerjee, R.; Mahmod, M.; Cochlin, L.; Karamitsos, T.D.; Robson, M.D.; et al. Myocardial Tissue Characterization Using Magnetic Resonance Noncontrast T1 Mapping in Hypertrophic and Dilated Cardiomyopathy. Circ. Cardiovasc. Imaging 2012, 5, 726–733. [Google Scholar] [CrossRef]
- Karamitsos, T.D.; Piechnik, S.K.; Banypersad, S.M.; Fontana, M.; Ntusi, N.B.; Ferreira, V.M.; Whelan, C.J.; Myerson, S.G.; Robson, M.D.; Hawkins, P.N.; et al. Noncontrast T1 Mapping for the Diagnosis of Cardiac Amyloidosis. JACC Cardiovasc. Imaging 2013, 6, 488–497. [Google Scholar] [CrossRef]
- Ntusi, N.A.; Piechnik, S.K.; Francis, J.M.; Ferreira, V.M.; Matthews, P.M.; Robson, M.D.; Wordsworth, P.B.; Neubauer, S.; Karamitsos, T.D. Diffuse Myocardial Fibrosis and Inflammation in Rheumatoid Arthritis. JACC Cardiovasc. Imaging 2015, 8, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, T.; Martinez-Naharro, A.; Boldrini, M.; Knight, D.; Hawkins, P.; Kalra, S.; Patel, D.; Coghlan, G.; Moon, J.; Plein, S.; et al. Automated Pixel-Wise Quantitative Myocardial Perfusion Mapping by CMR to Detect Obstructive Coronary Artery Disease and Coronary Microvascular Dysfunction. JACC Cardiovasc. Imaging 2019, 12, 1958–1969. [Google Scholar] [CrossRef]
- Brown, L.A.E.; Onciul, S.C.; Broadbent, D.A.; Johnson, K.; Fent, G.J.; Foley, J.R.J.; Garg, P.; Chew, P.G.; Knott, K.; Dall’Armellina, E.; et al. Fully automated, inline quantification of myocardial blood flow with cardiovascular magnetic resonance: Repeatability of measurements in healthy subjects. J. Cardiovasc. Magn. Reson. 2018, 20, 48. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.-Y.; Jacobs, M.; Benovoy, M.; Ta, A.D.; Conn, H.M.; Winkler, S.; Greve, A.M.; Chen, M.Y.; Shanbhag, S.M.; Bandettini, W.P.; et al. Diagnostic Performance of Fully Automated Pixel-Wise Quantitative Myocardial Perfusion Imaging by Cardiovascular Magnetic Resonance. JACC Cardiovasc. Imaging 2018, 11, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Levelt, E.; Piechnik, S.K.; Liu, A.; Wijesurendra, R.S.; Mahmod, M.; Ariga, R.; Francis, J.M.; Greiser, A.; Clarke, K.; Neubauer, S.; et al. Adenosine stress CMR T1-mapping detects early microvascular dysfunction in patients with type 2 diabetes mellitus without obstructive coronary artery disease. J. Cardiovasc. Magn. Reson. 2017, 19, 81. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.C.; Messroghli, D.R.; Kellman, P.; Piechnik, S.K.; Robson, M.D.; Ugander, M.; Gatehouse, P.D.; Arai, A.; Friedrich, M.G.; Neubauer, S.; et al. Myocardial T1 mapping and extracellular volume quantification: A Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J. Cardiovasc. Magn. Reson. 2013, 15, 92. [Google Scholar] [CrossRef]
- Messroghli, D.R.; Radjenovic, A.; Kozerke, S.; Higgins, D.M.; Sivananthan, M.U.; Ridgway, J.P. Modified Look-Locker inversion recovery (MOLLI) for high-resolutionT1 mapping of the heart. Magn. Reson. Med. 2004, 52, 141–146. [Google Scholar] [CrossRef]
- Bull, S.; White, S.K.; Piechnik, S.K.; Flett, A.S.; Ferreira, V.; Loudon, M.; Francis, J.M.; Karamitsos, T.; Prendergast, B.D.; Robson, M.D.; et al. Human non-contrast T1 values and correlation with histology in diffuse fibrosis. Heart 2013, 99, 932–937. [Google Scholar] [CrossRef]
- Sado, D.M.; White, S.K.; Piechnik, S.K.; Banypersad, S.M.; Treibel, T.; Captur, G.; Fontana, M.; Maestrini, V.; Flett, A.S.; Robson, M.D.; et al. Identification and Assessment of Anderson-Fabry Disease by Cardiovascular Magnetic Resonance Noncontrast Myocardial T1 Mapping. Circ. Cardiovasc. Imaging 2013, 6, 392–398. [Google Scholar] [CrossRef]
- Ferreira, V.M.; Piechnik, S.K.; Dall’Armellina, E.; Karamitsos, T.D.; Francis, J.M.; Choudhury, R.P.; Friedrich, M.G.; Robson, M.D.; Neubauer, S. Non-contrast T1-mapping detects acute myocardial edema with high diagnostic accuracy: A comparison to T2-weighted cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2012, 14, 42. [Google Scholar] [CrossRef]
- Ugander, M.; Bagi, P.S.; Oki, A.J.; Chen, B.; Hsu, L.-Y.; Aletras, A.H.; Shah, S.; Greiser, A.; Kellman, P.; Arai, A.E. Myocardial Edema as Detected by Pre-Contrast T1 and T2 CMR Delineates Area at Risk Associated with Acute Myocardial Infarction. JACC Cardiovasc. Imaging 2012, 5, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Piechnik, S.K.; Ferreira, V.M.; Lewandowski, A.J.; Ab Ntusi, N.; Banerjee, R.; Holloway, C.; Hofman, M.B.; Sado, D.M.; Maestrini, V.; White, S.K.; et al. Normal variation of magnetic resonance T1 relaxation times in the human population at 1.5 T using ShMOLLI. J. Cardiovasc. Magn. Reson. 2013, 15, 13. [Google Scholar] [CrossRef] [PubMed]
- Messroghli, D.R.; Walters, K.; Plein, S.; Sparrow, P.; Friedrich, M.G.; Ridgway, J.P.; Sivananthan, M.U. Myocardial T 1 mapping: Application to patients with acute and chronic myocardial infarction. Magn. Reson. Med. 2007, 58, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Piechnik, S.K.; Ferreira, V.M.; Dall’Armellina, E.; Cochlin, L.E.; Greiser, A.; Neubauer, S.; Robson, M.D. Shortened Modified Look-Locker Inversion recovery (ShMOLLI) for clinical myocardial T1-mapping at 1.5 and 3 T within a 9 heartbeat breathhold. J. Cardiovasc. Magn. Reson. 2010, 12, 69. [Google Scholar] [CrossRef]
- Ferreira, V.M.; Wijesurendra, R.S.; Liu, A.; Greiser, A.; Casadei, B.; Robson, M.D.; Neubauer, S.; Piechnik, S.K. Systolic ShMOLLI myocardial T1-mapping for improved robustness to partial-volume effects and applications in tachyarrhythmias. J. Cardiovasc. Magn. Reson. 2015, 17, 77. [Google Scholar] [CrossRef]
- Zhao, L.; Li, S.; Ma, X.; Greiser, A.; Zhang, T.; An, J.; Bai, R.; Dong, J.; Fan, Z. Systolic MOLLI T1 mapping with heart-rate-dependent pulse sequence sampling scheme is feasible in patients with atrial fibrillation. J. Cardiovasc. Magn. Reson. 2016, 18, 13. [Google Scholar] [CrossRef]
- Chow, K.; Flewitt, J.A.; Green, J.D.; Pagano, J.J.; Friedrich, M.G.; Thompson, R.B. Saturation recovery single-shot acquisition (SASHA) for myocardial T 1 mapping. Magn. Reson. Med. 2013, 71, 2082–2095. [Google Scholar] [CrossRef]
- Chow, K.; Yang, Y.; Shaw, P.; Kramer, C.M.; Salerno, M. Robust free-breathing SASHA T1 mapping with high-contrast image registration. J. Cardiovasc. Magn. Reson. 2016, 18, 1–14. [Google Scholar] [CrossRef]
- Weingärtner, S.; Akçakaya, M.; Basha, T.; Kissinger, K.V.; Goddu, B.; Berg, S.; Manning, W.J.; Nezafat, R. Combined saturation/inversion recovery sequences for improved evaluation of scar and diffuse fibrosis in patients with arrhythmia or heart rate variability. Magn. Reson. Med. 2013, 71, 1024–1034. [Google Scholar] [CrossRef]
- Karamitsos, T.D.; Ab Ntusi, N.; Francis, J.M.; Holloway, C.J.; Myerson, S.G.; Neubauer, S. Feasibility and safety of high-dose adenosine perfusion cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2010, 12, 66–68. [Google Scholar] [CrossRef]
- Cerqueira, M.D.; Verani, M.S.; Schwaiger, M.; Heo, J.; Iskandrian, A.S. Safety profile of adenosine stress perfusion imaging: Results from the adenoscan multicenter trial registry. J. Am. Coll. Cardiol. 1994, 23, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Menadas, J.V.M.; Gonzalez, M.P.G.; Lopez-Lereu, M.P.; Ortega, L.H.; Gonzalez, A.M.M. Safety and tolerability of regadenoson in comparison with adenosine stress cardiovascular magnetic resonance: Data from a multicentre prospective registry. Int. J. Cardiovasc. Imaging 2021, 38, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Prenner, B.M.; Bukofzer, S.; Behm, S.; Feaheny, K.; McNutt, B.E. A randomized, double-blind, placebo-controlled study assessing the safety and tolerability of regadenoson in subjects with asthma or chronic obstructive pulmonary disease. J. Nucl. Cardiol. 2012, 19, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Manisty, C.; Ripley, D.P.; Herrey, A.S.; Captur, G.; Wong, T.C.; Petersen, S.; Plein, S.; Peebles, C.; Schelbert, E.; Greenwood, J.P.; et al. Splenic Switch-off: A Tool to Assess Stress Adequacy in Adenosine Perfusion Cardiac MR Imaging. Radiology 2015, 276, 732–740. [Google Scholar] [CrossRef]
- Bettencourt, N.; Nagel, E. Diagnosing ischemia with vasodilatatory stress cardiac magnetic resonance: The benefit of a comprehensive approach. Rev. Española De Cardiol. Engl. Ed. 2009, 62, 350–353. [Google Scholar] [CrossRef]
- van Dijk, R.; Kuijpers, D.; Kaandorp, T.A.M.; van Dijkman, P.R.M.; Vliegenthart, R.; van der Harst, P.; Oudkerk, M. Effects of caffeine intake prior to stress cardiac magnetic resonance perfusion imaging on regadenoson- versus adenosine-induced hyperemia as measured by T1 mapping. Int. J. Cardiovasc. Imaging 2017, 33, 1753–1759. [Google Scholar] [CrossRef]
- Piechnik, S.K.; Neubauer, S.; Ferreira, V.M. State-of-the-art review: Stress T1 mapping—Technical considerations, pitfalls and emerging clinical applications. Magn. Reson. Mater. Phys. Biol. Med. 2017, 31, 131–141. [Google Scholar] [CrossRef]
- McCommis, K.S.; Goldstein, T.A.; Abendschein, D.R.; Misselwitz, B.; Pilgram, T.; Gropler, R.J.; Zheng, J. Roles of myocardial blood volume and flow in coronary artery disease: An experimental MRI study at rest and during hyperemia. Eur. Radiol. 2010, 20, 2005–2012. [Google Scholar] [CrossRef]
- Liu, A.; Wijesurendra, R.S.; Francis, J.M.; Robson, M.D.; Neubauer, S.; Piechnik, S.K.; Ferreira, V.M. Adenosine Stress and Rest T1 Mapping Can Differentiate Between Ischemic, Infarcted, Remote, and Normal Myocardium Without the Need for Gadolinium Contrast Agents. JACC Cardiovasc. Imaging 2015, 9, 27–36. [Google Scholar] [CrossRef]
- Burrage, M.K.; Shanmuganathan, M.; Masi, A.; Hann, E.; Zhang, Q.; Popescu, I.A.; Soundarajan, R.; Pelado, J.L.; Chow, K.; Neubauer, S.; et al. Cardiovascular magnetic resonance stress and rest T1-mapping using regadenoson for detection of ischemic heart disease compared to healthy controls. Int. J. Cardiol. 2021, 333, 239–245. [Google Scholar] [CrossRef]
- Bohnen, S.; Prüßner, L.; Vettorazzi, E.; Radunski, U.K.; Tahir, E.; Schneider, J.; Cavus, E.; Avanesov, M.; Stehning, C.; Adam, G.; et al. Stress T1-mapping cardiovascular magnetic resonance imaging and inducible myocardial ischemia. Clin. Res. Cardiol. 2019, 108, 909–920. [Google Scholar] [CrossRef] [PubMed]
- van Assen, M.; van Dijk, R.; Kuijpers, D.; Vliegenthart, R.; Oudkerk, M. T1 reactivity as an imaging biomarker in myocardial tissue characterization discriminating normal, ischemic and infarcted myocardium. Int. J. Cardiovasc. Imaging 2019, 35, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Yimcharoen, S.; Zhang, S.; Kaolawanich, Y.; Tanapibunpon, P.; Krittayaphong, R. Clinical assessment of adenosine stress and rest cardiac magnetic resonance T1 mapping for detecting ischemic and infarcted myocardium. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Burrage, M.K.; Shanmuganathan, M.; Gonzales, R.; Lukaschuk, E.; Thomas, K.E.; Mills, R.; Pelado, J.L.; Nikolaidou, C.; Popescu, I.A.; et al. Artificial Intelligence for Contrast-free MRI: Scar Assessment in Myocardial Infarction Using Deep Learning-based Virtual Native Enhancement (VNE). Circulation 2022, 146, 1492–1503. [Google Scholar] [CrossRef] [PubMed]
- Puntmann, V.O.; Carr-White, G.; Jabbour, A.; Yu, C.-Y.; Gebker, R.; Kelle, S.; Rolf, A.; Zitzmann, S.; Peker, E.; D’Angelo, T.; et al. Native T1 and ECV of Noninfarcted Myocardium and Outcome in Patients with Coronary Artery Disease. J. Am. Coll. Cardiol. 2018, 71, 766–778. [Google Scholar] [CrossRef]
- Kellman, P.; Hansen, M.S. T1-mapping in the heart: Accuracy and precision. J. Cardiovasc. Magn. Reson. 2014, 16, 2. [Google Scholar] [CrossRef]
- Roujol, S.; Weingärtner, S.; Foppa, M.; Chow, K.; Kawaji, K.; Ngo, L.H.; Kellman, P.; Manning, W.J.; Thompson, R.; Nezafat, R. Accuracy, Precision, and Reproducibility of Four T1 Mapping Sequences: A Head-to-Head Comparison of MOLLI, ShMOLLI, SASHA, and SAPPHIRE. Radiology 2014, 272, 683–689. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Winau, L.; Heinke, R.; Schnoes, K.; Wichmann, J.L.; Vogl, T.; Zeiher, A.M.; Greiser, A.; Nagel, E.P.E. T1 mapping at rest and adenosine stress—Comparison of T1 mapping sequences for feasibility and effect size. In Proceedings of the 20th SCMR Annual Scientific Sessions, Washington, DC, USA, 1–4 February 2017. [Google Scholar]
- Kiaos, A.; Tziatzios, I.; Hadjimiltiades, S.; Karvounis, C.; Karamitsos, T.D. Diagnostic performance of stress perfusion cardiac magnetic resonance for the detection of coronary artery disease. Int. J. Cardiol. 2018, 252, 229–233. [Google Scholar] [CrossRef]
- Nagel, E.; Greenwood, J.P.; McCann, G.P.; Bettencourt, N.; Shah, A.M.; Hussain, S.T.; Perera, D.; Plein, S.; Bucciarelli-Ducci, C.; Paul, M.; et al. Magnetic Resonance Perfusion or Fractional Flow Reserve in Coronary Disease. N. Engl. J. Med. 2019, 380, 2418–2428. [Google Scholar] [CrossRef]
- Korosoglou, G.; Elhmidi, Y.; Steen, H.; Schellberg, D.; Riedle, N.; Ahrens, J.; Lehrke, S.; Merten, C.; Lossnitzer, D.; Radeleff, J.; et al. Prognostic Value of High-Dose Dobutamine Stress Magnetic Resonance Imaging in 1,493 Consecutive Patients: Assessment of Myocardial Wall Motion and Perfusion. J. Am. Coll. Cardiol. 2010, 56, 1225–1234. [Google Scholar] [CrossRef]
- Jahnke, C.; Nagel, E.; Gebker, R.; Kokocinski, T.; Kelle, S.; Manka, R.; Fleck, E.; Paetsch, I. Prognostic Value of Cardiac Magnetic Resonance Stress Tests. Circulation 2007, 115, 1769–1776. [Google Scholar] [CrossRef] [PubMed]
- Ingkanisorn, W.P.; Kwong, R.Y.; Bohme, N.S.; Geller, N.L.; Rhoads, K.L.; Dyke, C.K.; Paterson, D.I.; Syed, M.A.; Aletras, A.; Arai, A.E. Prognosis of Negative Adenosine Stress Magnetic Resonance in Patients Presenting to an Emergency Department with Chest Pain. J. Am. Coll. Cardiol. 2006, 47, 1427–1432. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, R.; Van Assen, M.; Vliegenthart, R.; De Bock, G.H.; Van Der Harst, P.; Oudkerk, M. Diagnostic performance of semi-quantitative and quantitative stress CMR perfusion analysis: A meta-analysis. J. Cardiovasc. Magn. Reson. 2017, 19, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Knott, K.D.; Fernandes, J.L.; Moon, J.C. Automated Quantitative Stress Perfusion in a Clinical Routine. Magn. Reson. Imaging Clin. N. Am. 2019, 27, 507–520. [Google Scholar] [CrossRef]
- Seraphim, A.; Knott, K.D.; Augusto, J.; Bhuva, A.N.; Manisty, C.; Moon, J.C. Quantitative cardiac MRI. J. Magn. Reson. Imaging 2019, 51, 693–711. [Google Scholar] [CrossRef]
- Kellman, P.; Hansen, M.S.; Nielles-Vallespin, S.; Nickander, J.; Themudo, R.; Ugander, M.; Xue, H. Myocardial perfusion cardiovascular magnetic resonance: Optimized dual sequence and reconstruction for quantification. J. Cardiovasc. Magn. Reson. 2017, 19, 1–14. [Google Scholar] [CrossRef]
- Lockie, T.; Ishida, M.; Perera, D.; Chiribiri, A.; De Silva, K.; Kozerke, S.; Marber, M.; Nagel, E.; Rezavi, R.; Redwood, S.; et al. High-Resolution Magnetic Resonance Myocardial Perfusion Imaging at 3.0-Tesla to Detect Hemodynamically Significant Coronary Stenoses as Determined by Fractional Flow Reserve. J. Am. Coll. Cardiol. 2011, 57, 70–75. [Google Scholar] [CrossRef]
- Mordini, F.E.; Haddad, T.; Hsu, L.-Y.; Kellman, P.; Lowrey, T.B.; Aletras, A.H.; Bandettini, W.P.; Arai, A.E. Diagnostic Accuracy of Stress Perfusion CMR in Comparison with Quantitative Coronary Angiography. JACC Cardiovasc. Imaging 2014, 7, 14–22. [Google Scholar] [CrossRef]
- Morton, G.; Chiribiri, A.; Ishida, M.; Hussain, S.T.; Schuster, A.; Indermuehle, A.; Perera, D.; Knuuti, J.; Baker, S.; Hedström, E.; et al. Quantification of Absolute Myocardial Perfusion in Patients with Coronary Artery Disease. J. Am. Coll. Cardiol. 2012, 60, 1546–1555. [Google Scholar] [CrossRef]
- Engblom, H.; Xue, H.; Akil, S.; Carlsson, M.; Hindorf, C.; Oddstig, J.; Hedeer, F.; Hansen, M.S.; Aletras, A.H.; Kellman, P.; et al. Fully quantitative cardiovascular magnetic resonance myocardial perfusion ready for clinical use: A comparison between cardiovascular magnetic resonance imaging and positron emission tomography. J. Cardiovasc. Magn. Reson. 2017, 19, 78. [Google Scholar] [CrossRef]
- Nazir, M.S.; Milidonis, X.; McElroy, S.; Ryan, M.; Yazdani, M.; Kunze, K.; Hajhosseiny, R.; Vergani, V.; Stäb, D.; Speier, P.; et al. Quantitative Myocardial Perfusion with Simultaneous-Multislice Stress CMR for Detection of Significant Coronary Artery Disease. JACC Cardiovasc. Imaging 2022, 15, 1672–1674. [Google Scholar] [CrossRef] [PubMed]
- Biglands, J.D.; Ibraheem, M.; Magee, D.R.; Radjenovic, A.; Plein, S.; Greenwood, J.P. Quantitative Myocardial Perfusion Imaging Versus Visual Analysis in Diagnosing Myocardial Ischemia. JACC Cardiovasc. Imaging 2018, 11, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Foley, J.R.J.; Kidambi, A.; Biglands, J.D.; Maredia, N.; Dickinson, C.J.; Plein, S.; Greenwood, J.P. A comparison of cardiovascular magnetic resonance and single photon emission computed tomography (SPECT) perfusion imaging in left main stem or equivalent coronary artery disease: A CE-MARC substudy. J. Cardiovasc. Magn. Reson. 2017, 19, 84. [Google Scholar] [CrossRef] [PubMed]
- Desai, D.; Kozeski, G.; Akinboboye, O. Detection of multivessel coronary artery disease: Looking beyond the extent of perfusion abnormalities. J. Nucl. Cardiol. 2009, 16, 4–5. [Google Scholar] [CrossRef]
- Kotecha, T.; Chacko, L.; Chehab, O.; O’Reilly, N.; Martinez-Naharro, A.; Lazari, J.; Knott, K.D.; Brown, J.; Knight, D.; Muthurangu, V.; et al. Assessment of Multivessel Coronary Artery Disease Using Cardiovascular Magnetic Resonance Pixelwise Quantitative Perfusion Mapping. JACC Cardiovasc. Imaging 2020, 13, 2546–2557. [Google Scholar] [CrossRef]
- Mathew, R.C.; Bourque, J.M.; Salerno, M.; Kramer, C.M. Cardiovascular Imaging Techniques to Assess Microvascular Dysfunction. JACC Cardiovasc. Imaging 2019, 13, 1577–1590. [Google Scholar] [CrossRef]
- Zorach, B.; Shaw, P.W.; Bourque, J.; Kuruvilla, S., Jr.; Balfour, P.C.; Yang, Y.; Mathew, R.; Pan, J.; Gonzalez, J.A.; Taylor, A.M.; et al. Quantitative cardiovascular magnetic resonance perfusion imaging identifies reduced flow reserve in microvascular coronary artery disease. J. Cardiovasc. Magn. Reson. 2018, 20, 1–8. [Google Scholar] [CrossRef]
- Rahman, H.; Scannell, C.M.; Demir, O.M.; Ryan, M.; McConkey, H.; Ellis, H.; Masci, P.G.; Perera, D.; Chiribiri, A. High-Resolution Cardiac Magnetic Resonance Imaging Techniques for the Identification of Coronary Microvascular Dysfunction. JACC Cardiovasc. Imaging 2020, 14, 978–986. [Google Scholar] [CrossRef]
- Sammut, E.C.; Villa, A.D.; Di Giovine, G.; Dancy, L.; Bosio, F.; Gibbs, T.; Jeyabraba, S.; Schwenke, S.; Williams, S.E.; Marber, M.; et al. Prognostic Value of Quantitative Stress Perfusion Cardiac Magnetic Resonance. JACC Cardiovasc. Imaging 2017, 11, 686–694. [Google Scholar] [CrossRef]
- Knott, K.D.; Seraphim, A.; Augusto, J.B.; Xue, H.; Chacko, L.; Aung, N.; Petersen, S.E.; Cooper, J.A.; Manisty, C.; Bhuva, A.N.; et al. The Prognostic Significance of Quantitative Myocardial Perfusion: An Artificial Intelligence-Based Approach Using Perfusion Mapping. Circulation 2020, 16, 1282–1291. [Google Scholar] [CrossRef]
- Seraphim, A.; Dowsing, B.; Rathod, K.S.; Shiwani, H.; Patel, K.; Knott, K.D.; Zaman, S.; Johns, I.; Razvi, Y.; Patel, R.; et al. Quantitative Myocardial Perfusion Predicts Outcomes in Patients with Prior Surgical Revascularization. J. Am. Coll. Cardiol. 2022, 79, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Scannell, C.; Veta, M.; Villa, A.; Sammut, E.C.; Lee, J.; Breeuwer, M.; Chiribiri, A. Deep-Learning-Based Preprocessing for Quantitative Myocardial Perfusion MRI. J. Magn. Reson. Imaging 2019, 51, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.W.; Soto, J.T.; Glicksberg, B.S.; Shameer, K.; Miotto, R.; Ali, M.; Ashley, E.; Dudley, J.T. Artificial Intelligence in Cardiology. J. Am. Coll. Cardiol. 2018, 71, 2668–2679. [Google Scholar] [CrossRef] [PubMed]
- Dey, D.; Slomka, P.J.; Leeson, P.; Comaniciu, D.; Shrestha, S.; Sengupta, P.P.; Marwick, T.H. Artificial Intelligence in Cardiovascular Imaging. J. Am. Coll. Cardiol. 2019, 73, 1317–1335. [Google Scholar] [CrossRef]

| Indications | Contraindications | |
|---|---|---|
| T1 mapping |
|
|
| Quantitative myocardial perfusion mapping |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazmpani, M.A.; Nikolaidou, C.; Papanastasiou, C.A.; Ziakas, A.; Karamitsos, T.D. Cardiovascular Magnetic Resonance Parametric Mapping Techniques for the Assessment of Chronic Coronary Syndromes. J. Cardiovasc. Dev. Dis. 2022, 9, 443. https://doi.org/10.3390/jcdd9120443
Bazmpani MA, Nikolaidou C, Papanastasiou CA, Ziakas A, Karamitsos TD. Cardiovascular Magnetic Resonance Parametric Mapping Techniques for the Assessment of Chronic Coronary Syndromes. Journal of Cardiovascular Development and Disease. 2022; 9(12):443. https://doi.org/10.3390/jcdd9120443
Chicago/Turabian StyleBazmpani, Maria Anna, Chrysovalantou Nikolaidou, Christos A. Papanastasiou, Antonios Ziakas, and Theodoros D. Karamitsos. 2022. "Cardiovascular Magnetic Resonance Parametric Mapping Techniques for the Assessment of Chronic Coronary Syndromes" Journal of Cardiovascular Development and Disease 9, no. 12: 443. https://doi.org/10.3390/jcdd9120443
APA StyleBazmpani, M. A., Nikolaidou, C., Papanastasiou, C. A., Ziakas, A., & Karamitsos, T. D. (2022). Cardiovascular Magnetic Resonance Parametric Mapping Techniques for the Assessment of Chronic Coronary Syndromes. Journal of Cardiovascular Development and Disease, 9(12), 443. https://doi.org/10.3390/jcdd9120443







