Exercise and Escitalopram in the Treatment of Anxiety in Patients with Coronary Heart Disease: One Year Follow-Up of the UNWIND Randomized Clinical Trial
Abstract
:1. Introduction
2. Methods
2.1. Trial Overview
2.2. Participants
2.3. Treatment Conditions
2.4. One-Year Follow-Up Assessments
2.5. Data Analysis
3. Results
3.1. Treatments Received during the One-Year Follow-up Period
3.2. Physical Activity
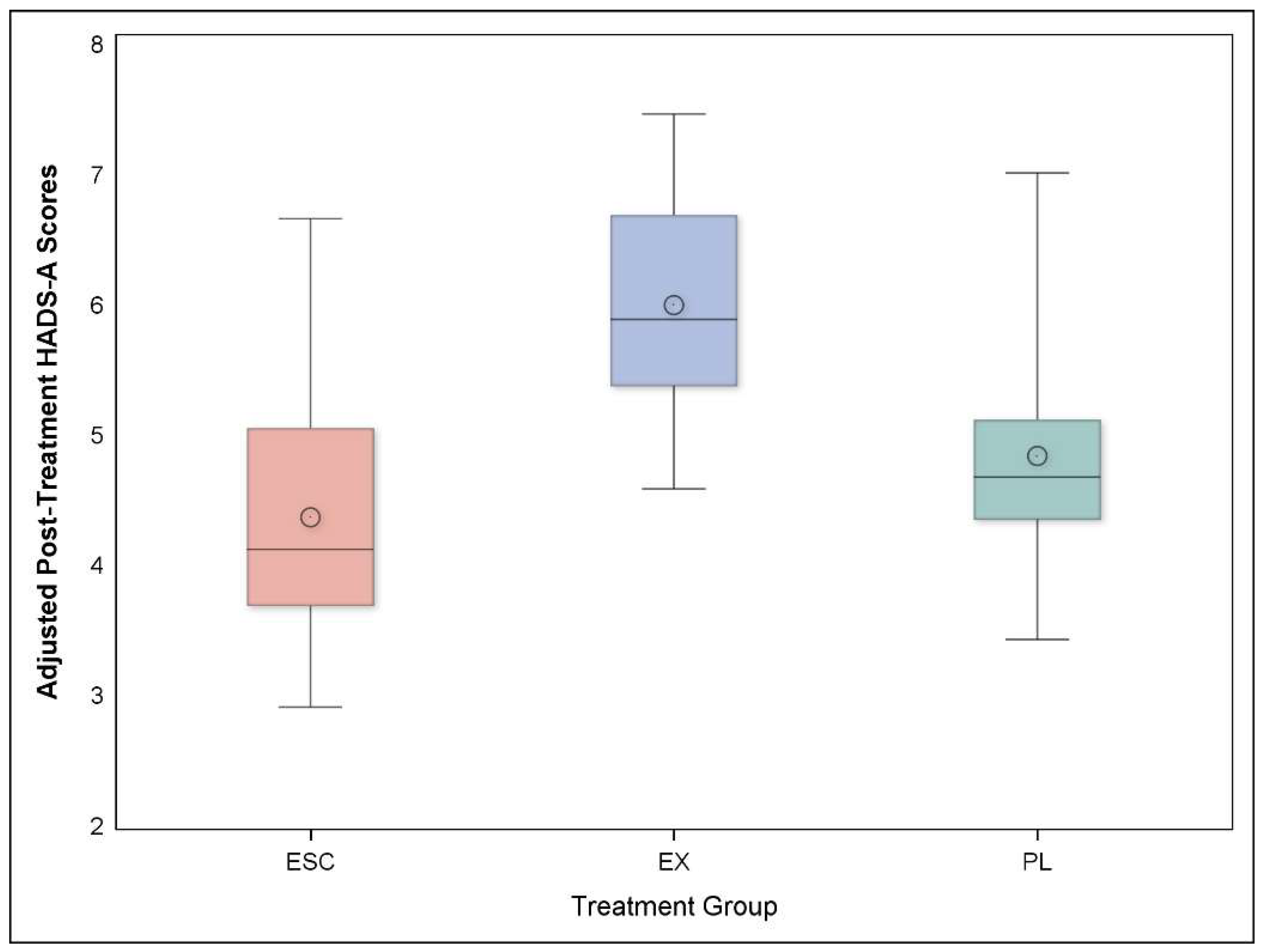
3.3. Anxiety
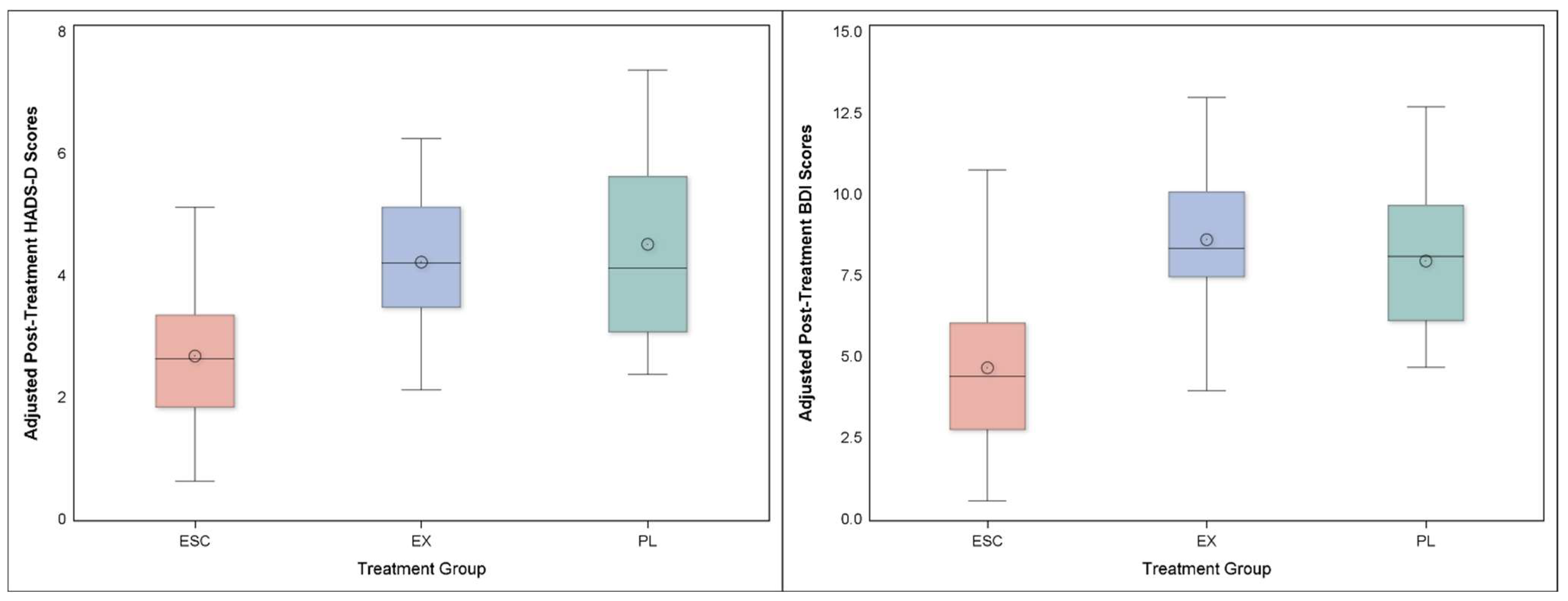
3.4. Depression
3.5. Perceived Stress
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Comorbidity Survey. Data Table 2: 12-Month Prevalence DSM-IV/WMH-CIDI Disorders by Sex and Cohort. Available online: https://www.hcp.med.harvard.edu/ncs/index.php (accessed on 11 July 2022).
- National Comorbidity Survey. Data Table 1: Lifetime prevalence DSM-IV/WMH-CIDI Disorders by Sex and Cohort. Available online: https://www.hcp.med.harvard.edu/ncs/index.php (accessed on 11 July 2022).
- Lavie, C.J.; Milani, R.V. Prevalence of anxiety in coronary patients with improvement following cardiac rehabilitation and exercise training. Am. J. Cardiol. 2004, 93, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Roest, A.M.; Martens, E.J.; de Jonge, P.; Denollet, J. Anxiety and risk of incident coronary heart disease: A meta-analysis. J. Am. Coll. Cardiol. 2010, 56, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Albert, C.M.; Chae, C.U.; Rexrode, K.M.; Manson, J.E.; Kawachi, I. Phobic anxiety and risk of coronary heart disease and sudden cardiac death among women. Circulation 2005, 111, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Fan, A.Z.; Strine, T.W.; Jiles, R.; Mokdad, A.H. Depression and anxiety associated with cardiovascular disease among persons aged 45 years and older in 38 states of the United States, 2006. Prev. Med. 2008, 46, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Frasure-Smith, N.; Lesperance, F. Depression and anxiety as predictors of 2-year cardiac events in patients with stable coronary artery disease. Arch. Gen. Psychiatry 2008, 65, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Kubzansky, L.D.; Kawachi, I.; Spiro, A.; Weiss, S.T., 3rd; Vokonas, P.S.; Sparrow, D. Is worrying bad for your heart? A prospective study of worry and coronary heart disease in the Normative Aging Study. Circulation 1997, 95, 818–824. [Google Scholar] [CrossRef]
- Scherrer, J.F.; Chrusciel, T.; Zeringue, A.; Garfield, L.D.; Hauptman, P.J.; Lustman, P.J.; Freedland, K.E.; Carney, R.M.; Bucholz, K.K.; Owen, R.; et al. Anxiety disorders increase risk for incident myocardial infarction in depressed and nondepressed Veterans Administration patients. Am. Heart J. 2010, 159, 772–779. [Google Scholar] [CrossRef]
- Phillips, A.C.; Batty, G.D.; Gale, C.R.; Deary, I.J.; Osborn, D.; MacIntyre, K.; Carroll, D. Generalized anxiety disorder, major depressive disorder, and their comorbidity as predictors of all-cause and cardiovascular mortality: The Vietnam experience study. Psychosom. Med. 2009, 71, 395–403. [Google Scholar] [CrossRef]
- Roest, A.M.; Martens, E.J.; Denollet, J.; de Jonge, P. Prognostic association of anxiety post myocardial infarction with mortality and new cardiac events: A meta-analysis. Psychosom. Med. 2010, 72, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Grace, S.L.; Abbey, S.E.; Irvine, J.; Shnek, Z.M.; Stewart, D.E. Prospective examination of anxiety persistence and its relationship to cardiac symptoms and recurrent cardiac events. Psychother. Psychosom. 2004, 73, 344–352. [Google Scholar] [CrossRef]
- Strik, J.J.; Denollet, J.; Lousberg, R.; Honig, A. Comparing symptoms of depression and anxiety as predictors of cardiac events and increased health care consumption after myocardial infarction. J. Am. Coll. Cardiol. 2003, 42, 1801–1807. [Google Scholar] [CrossRef] [PubMed]
- Berkman, L.F.; Blumenthal, J.; Burg, M.; Carney, R.M.; Catellier, D.; Cowan, M.J.; Czajkowski, S.M.; DeBusk, R.; Hosking, J.; Jaffe, A.; et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: The Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA 2003, 289, 3106–3116. [Google Scholar] [CrossRef] [PubMed]
- Glassman, A.H.; O’Connor, C.M.; Califf, R.M.; Swedberg, K.; Schwartz, P.; Bigger Jr, J.T.; Krishnan, K.R.; Van Zyl, L.T.; Swenson, J.R.; Finkel, M.S.; et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA 2002, 288, 701–709. [Google Scholar] [CrossRef]
- Davidson, K.W.; Rieckmann, N.; Clemow, L.; Schwartz, J.E.; Shimbo, D.; Medina, V.; Albanese, G.; Kronish, I.; Hegel, M.; Burg, M.M. Enhanced depression care for patients with acute coronary syndrome and persistent depressive symptoms: Coronary psychosocial evaluation studies randomized controlled trial. Arch. Intern. Med. 2010, 170, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, J.A.; Smith, P.J.; Jiang, W.; Hinderliter, A.; Watkins, L.L.; Hoffman, B.M.; Kraus, W.E.; Liao, L.; Davidson, J.; Sherwood, A. Effect of Exercise, Escitalopram, or Placebo on Anxiety in Patients With Coronary Heart Disease: The Understanding the Benefits of Exercise and Escitalopram in Anxious Patients With Coronary Heart Disease (UNWIND) Randomized Clinical Trial. JAMA Psychiatry 2021, 78, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, J.A.; Smith, P.J.; Jiang, W.; Hinderliter, A.; Watkins, L.L.; Hoffman, B.M.; Kraus, W.E.; Mabe, S.; Liao, L.; Davidson, J.; et al. Longer term benefits of exercise and escitalopram in the treatment of anxiety in patients with coronary heart disease: Six month follow-up of the UNWIND randomized clinical trial. Am. Heart J. 2022, 251, 91–100. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Godin, G.; Shephard, R.J. A simple method to assess exercise behavior in the community. Can. J. Appl. Sport Sci. 1985, 10, 141–146. [Google Scholar]
- Spielberger, C.E.G.R. Manual for the State-Trait Anxiety Inventory; Consulting Psychologists Press: Palo Alto, CA, USA, 1970. [Google Scholar]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.; Lowe, B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar] [CrossRef]
- Beck, A.T.S.R.; Brown, G.K. Beck Depression Inventory Manual, 2nd ed.; The Psychological Corporation: New York, NY, USA, 1996. [Google Scholar]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef]
- Herring, M.P.; O’Connor, P.J.; Dishman, R.K. The effect of exercise training on anxiety symptoms among patients: A systematic review. Arch. Intern. Med. 2010, 170, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Stonerock, G.L.; Hoffman, B.M.; Smith, P.J.; Blumenthal, J.A. Exercise as Treatment for Anxiety: Systematic Review and Analysis. Ann. Behav. Med. 2015, 49, 542–556. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, B.; Vancampfort, D.; Rosenbaum, S.; Firth, J.; Cosco, T.; Veronese, N.; Salum, G.A.; Schuch, F.B. An examination of the anxiolytic effects of exercise for people with anxiety and stress-related disorders: A meta-analysis. Psychiatry Res. 2017, 249, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Sanchez, C.P.; Schuch, F.B.; Seedat, S.; Louw, Q.A.; Stubbs, B.; Rosenbaum, S.; Firth, J.; van Winkel, R.; Vancampfort, D. The anxiolytic effects of exercise for people with anxiety and related disorders: An update of the available meta-analytic evidence. Psychiatry Res. 2021, 302, 114046. [Google Scholar] [CrossRef]
- Gokal, K.; Wallis, D.; Ahmed, S.; Boiangiu, I.; Kancherla, K.; Munir, F. Effects of a self-managed home-based walking intervention on psychosocial health outcomes for breast cancer patients receiving chemotherapy: A randomised controlled trial. Supportive Care Cancer 2016, 24, 1139–1166. [Google Scholar] [CrossRef] [PubMed]
- Kwok, J.Y.; Kwan, J.C.; Auyeung, M.; Mok, V.C.; Lau, C.K.; Choi, K.C.; Chan, H.Y. Effects of Mindfulness Yoga vs Stretching and Resistance Training Exercises on Anxiety and Depression for People With Parkinson Disease: A Randomized Clinical Trial. JAMA Neurol. 2019, 76, 755–763. [Google Scholar] [CrossRef]
- Hall, K.S.; Morey, M.C.; Bosworth, H.B.; Beckham, J.C.; Pebole, M.M.; Sloane, R.; Pieper, C.F. Pilot randomized controlled trial of exercise training for older veterans with PTSD. J. Behav. Med. 2020, 43, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Cooney, G.M.; Dwan, K.; Greig, C.A.; Lawlor, D.A.; Rimer, J.; Waugh, F.R.; McMurdo, M.; Mead, G.E. Exercise for depression. Cochrane Database Syst. Rev. 2013, 9, CD004366. [Google Scholar] [CrossRef]
- Blumenthal, J.A.; Sherwood, A.; Babyak, M.A.; Watkins, L.L.; Smith, P.J.; Hoffman, B.M.; O’Hayer, C.V.; Mabe, S.; Johnson, J.; Doraiswamy, P.M.; et al. Exercise and pharmacological treatment of depressive symptoms in patients with coronary heart disease: Results from the UPBEAT (Understanding the Prognostic Benefits of Exercise and Antidepressant Therapy) study. J. Am. Coll. Cardiol. 2012, 60, 1053–1063. [Google Scholar] [CrossRef]
- Blumenthal, J.A.; Babyak, M.A.; Craighead, W.E.; Davidson, J.; Hinderliter, A.; Hoffman, B.; Doraiswamy, P.M.; Sherwood, A. The role of comorbid anxiety in exercise and depression trials: Secondary analysis of the SMILE-II randomized clinical trial. Depress. Anxiety 2021, 38, 124–133. [Google Scholar] [CrossRef]
| Treatment Group | ||||
|---|---|---|---|---|
| Variable | Aerobic Exercise | Escitalopram | Placebo | Total Sample |
| EX | ESC | PL | ||
| (N = 52) | (N = 53) | (N = 23) | (N = 128) | |
| Demographic Characteristics | ||||
| Age | 65.2 (10.1) | 63.9 (8.6) | 65.2 (10.8) | 64.6 (9.6) |
| Sex (Female) | 17 (33%) | 14 (26%) | 6 (26%) | 37 (29%) |
| Race | ||||
| Caucasian | 39 (75%) | 36 (68%) | 18 (78%) | 92 (72%) |
| African-American | 8 (15%) | 12 (23%) | 5 (22%) | 25 (20%) |
| Other | 5 (10%) | 5 (9%) | 0 (%) | 11 (8%) |
| Marital Status | ||||
| Married/ Co-Habiting | 43 (83%) | 41 (77%) | 17 (74%) | 98 (77%) |
| Single, Never Married | 5 (10%) | 3 (6%) | 2 (9%) | 10 (8%) |
| Divorced/Separated | 4 (8%) | 6 (11%) | 2 (9%) | 12 (9%) |
| Widowed | 0 (0%) | 3 (6%) | 2 (9%) | 5 (4%) |
| Clinical Characteristics | ||||
| Prior Myocardial Infarction | 29 (56%) | 31 (60%) | 13 (57%) | 73 (57%) |
| Prior Stent | 37 (71%) | 38 (73%) | 16 (70%) | 91 (71%) |
| Prior Cabg | 12 (23%) | 12 (23%) | 6 (26%) | 30 (23%) |
| Smoker, N (%) | 2 (4%) | 4 (8%) | 4 (17%) | 10 (8%) |
| Diabetic, N (%) | 16 (31%) | 19 (36%) | 11 (48%) | 46 (36%) |
| Clinic SBP, MM HG | 127 (17) | 126 (17) | 128 (16) | 127 (17) |
| Clinic DBP, MM HG | 74 (9) | 73 (9) | 74 (11) | 73 (9) |
| Total Cholesterol, MG/DL | 157 (38) | 152 (43) | 150 (48) | 154 (42) |
| Low Density (LDL), MG/DL | 81 (33) | 83 (37) | 77 (39) | 81 (36) |
| High Density (HDL), MG/DL | 48 (14) | 47 (12) | 45 (14) | 47 (13) |
| Very Low Density, MG/DL | 27 (11) | 23 (11) | 28 (15) | 25 (12) |
| Triglycerides | 143 (74) | 113 (56) | 138 (74) | 130 (68) |
| DSM 5 Diagnosis of Anxiety Disorder (Y/N), N (%) | 37 (71) | 37 (70) | 19 (83) | 93 (73) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blumenthal, J.A.; Smith, P.J.; Jiang, W.; Hinderliter, A.; Watkins, L.L.; Hoffman, B.M.; Kraus, W.E.; Mabe, S.; Liao, L.; Davidson, J.; et al. Exercise and Escitalopram in the Treatment of Anxiety in Patients with Coronary Heart Disease: One Year Follow-Up of the UNWIND Randomized Clinical Trial. J. Cardiovasc. Dev. Dis. 2022, 9, 320. https://doi.org/10.3390/jcdd9100320
Blumenthal JA, Smith PJ, Jiang W, Hinderliter A, Watkins LL, Hoffman BM, Kraus WE, Mabe S, Liao L, Davidson J, et al. Exercise and Escitalopram in the Treatment of Anxiety in Patients with Coronary Heart Disease: One Year Follow-Up of the UNWIND Randomized Clinical Trial. Journal of Cardiovascular Development and Disease. 2022; 9(10):320. https://doi.org/10.3390/jcdd9100320
Chicago/Turabian StyleBlumenthal, James A., Patrick J. Smith, Wei Jiang, Alan Hinderliter, Lana L. Watkins, Benson M. Hoffman, William E. Kraus, Stephanie Mabe, Lawrence Liao, Jonathan Davidson, and et al. 2022. "Exercise and Escitalopram in the Treatment of Anxiety in Patients with Coronary Heart Disease: One Year Follow-Up of the UNWIND Randomized Clinical Trial" Journal of Cardiovascular Development and Disease 9, no. 10: 320. https://doi.org/10.3390/jcdd9100320
APA StyleBlumenthal, J. A., Smith, P. J., Jiang, W., Hinderliter, A., Watkins, L. L., Hoffman, B. M., Kraus, W. E., Mabe, S., Liao, L., Davidson, J., & Sherwood, A. (2022). Exercise and Escitalopram in the Treatment of Anxiety in Patients with Coronary Heart Disease: One Year Follow-Up of the UNWIND Randomized Clinical Trial. Journal of Cardiovascular Development and Disease, 9(10), 320. https://doi.org/10.3390/jcdd9100320






