Cardiopulmonary Exercise Testing in Repaired Tetralogy of Fallot: Multiparametric Overview and Correlation with Cardiac Magnetic Resonance and Physical Activity Level
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. CPET Protocol
2.3. MRI Imaging
2.4. Image Analysis
2.5. Physical Activity Evaluation
3. Statistical Analysis
4. Results
4.1. Participant Characteristics
4.2. MRI Results
4.3. CPET Results
4.4. Adverse Cardiac Events
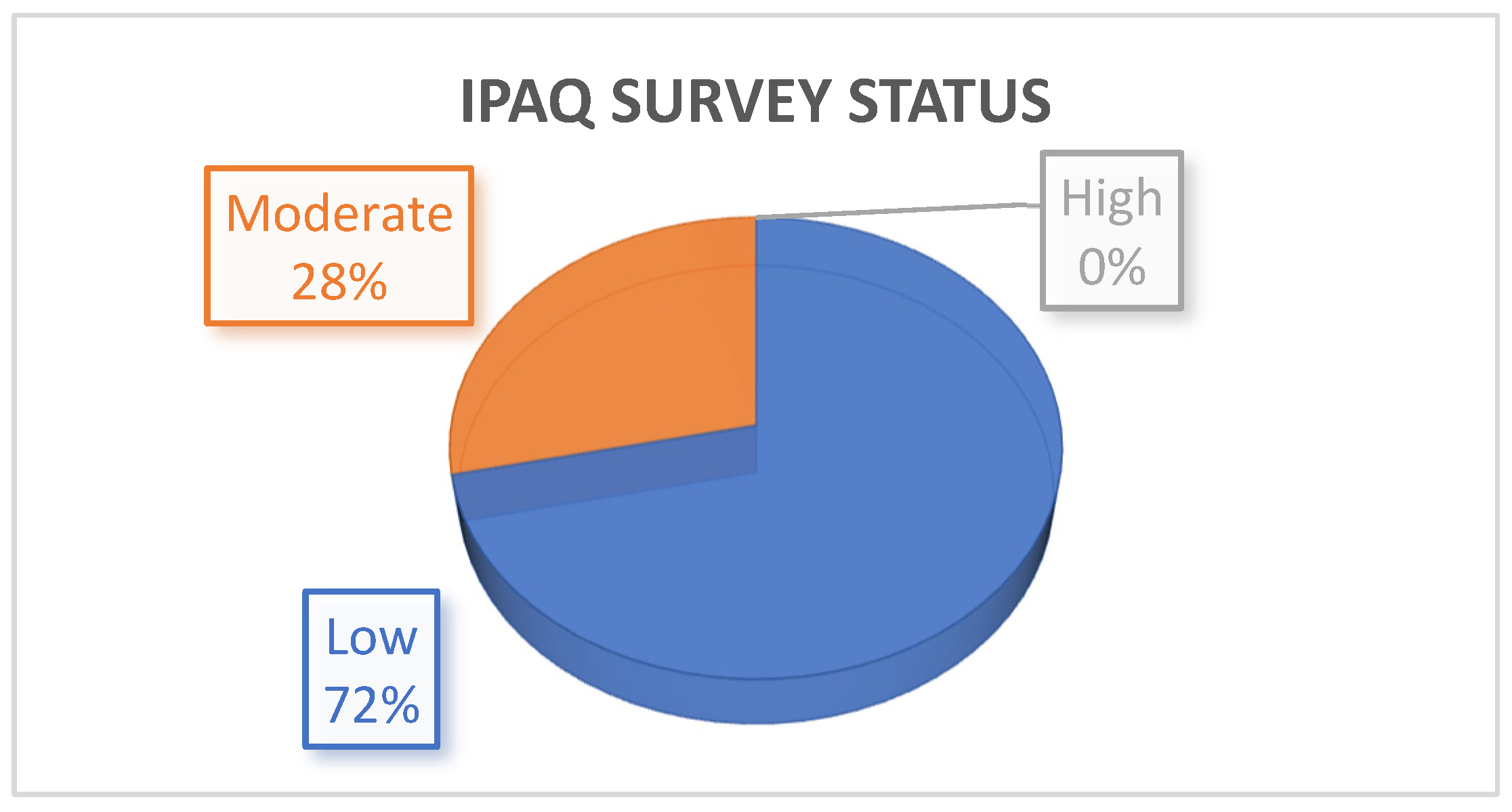
4.5. IPAQ Results
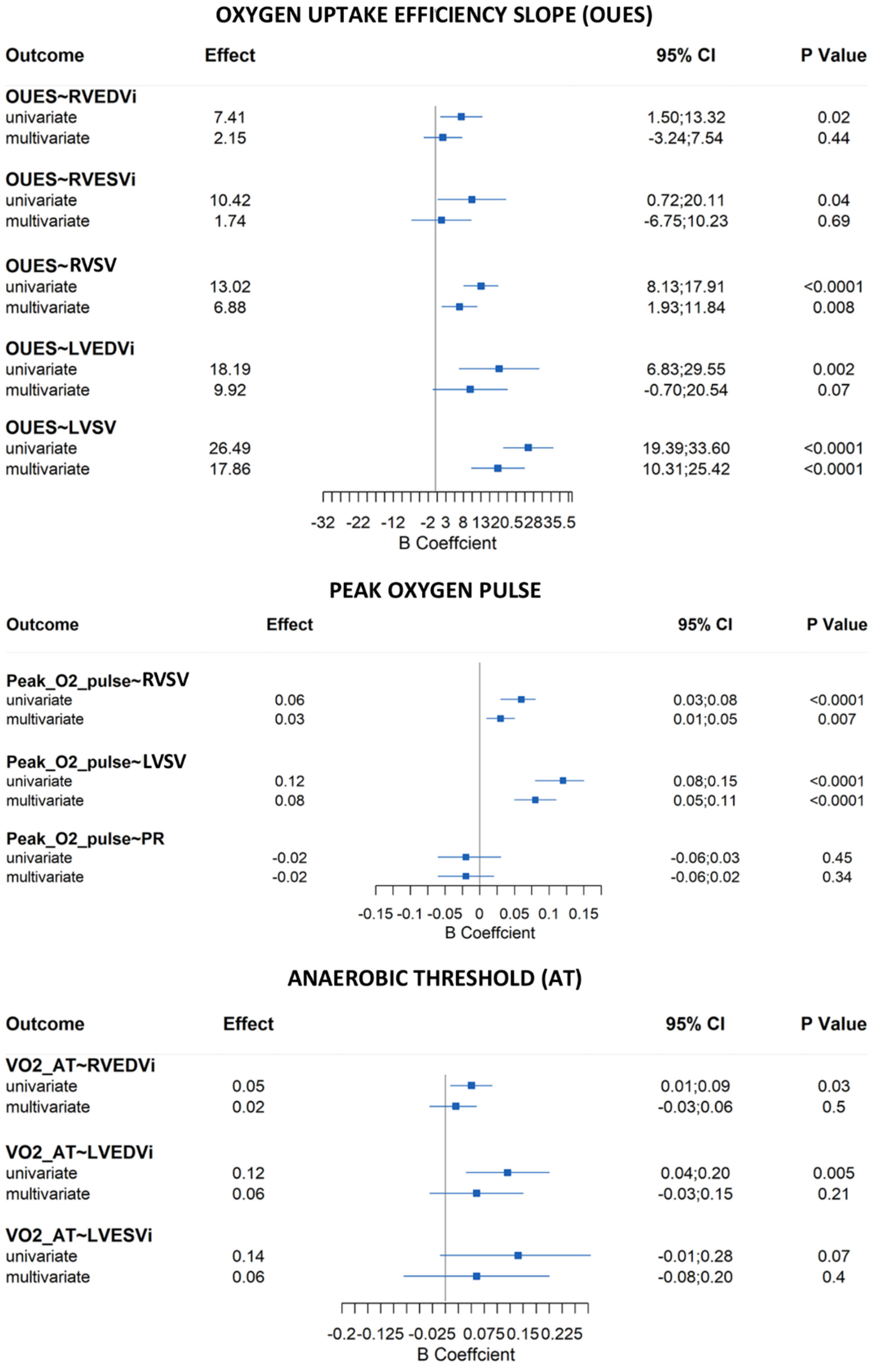
4.6. Correlations—CPET and MRI
4.7. Correlations—IPAQ and CPET/MRI
5. Discussion
5.1. Exercise Capacity in the rToF Population and Its Relationship with RV Size and Dysfunction
5.2. Effectiveness of OUES as an Index of Exercise Performance in rToF Patients
5.3. Usefulness of the IPAQ Survey in rToF Patients
5.4. Ventricular Arrhythmias in the rToF Population
5.5. Study Limitations
5.6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rohit, M.; Rajan, P. Approach to Cyanotic Congenital Heart Disease in Children. Indian J. Pediatr. 2020, 87, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Krieger, E.V.; Valente, A.M. Tetralogy of Fallot. Cardiol. Clin. 2020, 38, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Frias, J.; Guillaume, M. Tetralogy of Fallot; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Geva, T.; Sandweiss, B.M.; Gauvreau, K.; Lock, J.E.; Powell, A.J. Factors associated with impaired clinical status in long-term survivors of tetralogy of Fallot repair evaluated by magnetic resonance imaging. J. Am. Coll. Cardiol. 2004, 43, 1068–1074. [Google Scholar] [CrossRef]
- Frigiola, A.; Hughes, M.; Turner, M.; Taylor, A.; Marek, J.; Giardini, A.; Hsia, T.Y.; Bull, K. Physiological and phenotypic characteristics of late survivors of tetralogy of fallot repair who are free from pulmonary valve replacement. Circulation 2013, 128, 1861–1868. [Google Scholar] [CrossRef][Green Version]
- Knauth, A.L.; Gauvreau, K.; Powell, A.J.; Landzberg, M.J.; Walsh, E.P.; Lock, J.E.; del Nido, P.J.; Geva, T. Ventricular size and function assessed by cardiac MRI predict major adverse clinical outcomes late after tetralogy of Fallot repair. Heart 2008, 94, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, B.; Calvieri, C.; Perrone, M.A.; Di Rocco, A.; Carotti, A.; Caputo, M.; Secinaro, A.; Curione, D.; Gagliardi, M.G.; Guccione, P.; et al. Risk Factors of Right Ventricular Dysfunction and Adverse Cardiac Events in Patients with Repaired Tetralogy of Fallot. Int. J. Environ. Res. Public Health 2021, 18, 10549. [Google Scholar] [CrossRef]
- Ali, L.A.; Gentili, F.; Festa, P.; Perrone, M.A.; Curione, D.; Caputo, M.; Wald, R.; Secinaro, A.; Carotti, A.; Chinali, M.; et al. Long-term assessment of clinical outcomes and disease progression in patients with corrected Tetralogy of Fallot. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 6300–6310. [Google Scholar] [CrossRef] [PubMed]
- Stout, K.K.; Daniels, C.J.; Aboulhosn, J.A.; Bozkurt, B.; Broberg, C.S.; Colman, J.M.; Crumb, S.R.; Dearani, J.A.; Fuller, S.; Gurvitz, M.; et al. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e637–e697. [Google Scholar] [CrossRef]
- Samman, A.; Schwerzmann, M.; Balint, O.H.; Tanous, D.; Redington, A.; Granton, J.; Siu, S.C.; Silversides, C.K. Exercise capacity and biventricular function in adult patients with repaired tetralogy of Fallot. Am. Heart J. 2008, 156, 100–105. [Google Scholar] [CrossRef]
- Takken, T.; Bongers, B.C.; van Brussel, M.; Haapala, E.A.; Hulzebos, E.H.J. Cardiopulmonary Exercise Testing in Pediatrics. Ann. Am. Thorac. Soc. 2017, 14, S123–S128. [Google Scholar] [CrossRef]
- Diller, G.P.; Dimopoulos, K.; Okonko, D.; Li, W.; Babu-Narayan, S.V.; Broberg, C.S.; Johansson, B.; Bouzas, B.; Mullen, M.J.; Poole-Wilson, P.A.; et al. Exercise intolerance in adult congenital heart disease: Comparative severity, correlates, and prognostic implication. Circulation 2005, 112, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.S.; Shinebourne, E.A.; Busst, C.; Rigby, M.L.; Redington, A.N. Exercise capacity after complete repair of tetralogy of Fallot: Deleterious effects of residual pulmonary regurgitation. Br. Heart J. 1992, 67, 470–473. [Google Scholar] [CrossRef]
- Meadows, J.; Powell, A.J.; Geva, T.; Dorfman, A.; Gauvreau, K.; Rhodes, J. Cardiac magnetic resonance imaging correlates of exercise capacity in patients with surgically repaired tetralogy of Fallot. Am. J. Cardiol. 2007, 100, 1446–1450. [Google Scholar] [CrossRef]
- Yang, M.C.; Chen, C.A.; Chiu, H.H.; Wang, J.K.; Lin, M.T.; Chiu, S.N.; Lu, C.W.; Huang, S.C.; Wu, M.H. Assessing utility of exercise test in determining exercise prescription in adolescent and adult patients with repaired tetralogy of fallot. Heart Vessels 2017, 32, 201–207. [Google Scholar] [CrossRef]
- Babu-Narayan, S.V.; Diller, G.P.; Gheta, R.R.; Bastin, A.J.; Karonis, T.; Li, W.; Pennell, D.J.; Uemura, H.; Sethia, B.; Gatzoulis, M.A.; et al. Clinical outcomes of surgical pulmonary valve replacement after repair of tetralogy of Fallot and potential prognostic value of preoperative cardiopulmonary exercise testing. Circulation 2014, 129, 18–27. [Google Scholar] [CrossRef] [PubMed]
- O’Meagher, S.; Munoz, P.A.; Alison, J.A.; Young, I.H.; Tanous, D.J.; Celermajer, D.S.; Puranik, R. Exercise capacity and stroke volume are preserved late after tetralogy repair, despite severe right ventricular dilatation. Heart 2012, 98, 1595–1599. [Google Scholar] [CrossRef]
- Rashid, I.; Mahmood, A.; Ismail, T.F.; O’Meagher, S.; Kutty, S.; Celermajer, D.; Puranik, R. Right ventricular systolic dysfunction but not dilatation correlates with prognostically significant reductions in exercise capacity in repaired Tetralogy of Fallot. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Nes, B.M.; Osthus, I.B.; Welde, B.; Aspenes, S.T.; Wisloff, U. Peak oxygen uptake and physical activity in 13- to 18-year-olds: The Young-HUNT study. Med. Sci. Sports Exerc. 2013, 45, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Rogowski, M.P.; Guilkey, J.P.; Stephens, B.R.; Cole, A.S.; Mahon, A.D. The influence of maturation on the oxygen uptake efficiency slope. Pediatr. Exerc. Sci. 2012, 24, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Dallaire, F.; Wald, R.M.; Marelli, A. The Role of Cardiopulmonary Exercise Testing for Decision Making in Patients with Repaired Tetralogy of Fallot. Pediatr. Cardiol. 2017, 38, 1097–1105. [Google Scholar] [CrossRef]
- Tran, D.L.; Maiorana, A.; Davis, G.M.; Celermajer, D.S.; d’Udekem, Y.; Cordina, R. Exercise Testing and Training in Adults With Congenital Heart Disease: A Surgical Perspective. Ann. Thorac. Surg. 2021, 112, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.; Maiorana, A.; Ayer, J.; Lubans, D.R.; Davis, G.M.; Celermajer, D.S.; d’Udekem, Y.; Cordina, R. Recommendations for exercise in adolescents and adults with congenital heart disease. Prog. Cardiovasc. Dis. 2020, 63, 350–366. [Google Scholar] [CrossRef] [PubMed]
- Koschate, J.; Cettolo, V.; Hoffmann, U.; Francescato, M.P. Breath-by-breath oxygen uptake during running: Effects of different calculation algorithms. Exp. Physiol. 2019, 104, 1829–1840. [Google Scholar] [CrossRef] [PubMed]
- Beaver, W.L.; Lamarra, N.; Wasserman, K. Breath-by-breath measurement of true alveolar gas exchange. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1981, 51, 1662–1675. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.E.; Sue, D.Y.; Wasserman, K. Predicted values for clinical exercise testing. Am. Rev. Respir. Dis. 1984, 129, S49–S55. [Google Scholar] [CrossRef]
- Cooper, D.M.; Weiler-Ravell, D. Gas exchange response to exercise in children. Am. Rev. Respir. Dis. 1984, 129, S47–S48. [Google Scholar] [CrossRef]
- Cooper, D.M.; Leu, S.Y.; Galassetti, P.; Radom-Aizik, S. Dynamic interactions of gas exchange, body mass, and progressive exercise in children. Med. Sci. Sports Exerc. 2014, 46, 877–886. [Google Scholar] [CrossRef]
- Leonardi, B.; Secinaro, A.; Calvieri, C.; Perrone, M.A.; Gimigliano, F.; Muscogiuri, G.; Carotti, A.; Drago, F. The role of 3D imaging in the follow-up of patients with repaired tetralogy of Fallot. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1698–1709. [Google Scholar] [CrossRef]
- Mooij, C.F.; de Wit, C.J.; Graham, D.A.; Powell, A.J.; Geva, T. Reproducibility of MRI measurements of right ventricular size and function in patients with normal and dilated ventricles. J. Magn. Reson. Imaging 2008, 28, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Winter, M.M.; Bernink, F.J.; Groenink, M.; Bouma, B.J.; van Dijk, A.P.; Helbing, W.A.; Tijssen, J.G.; Mulder, B.J. Evaluating the systemic right ventricle by CMR: The importance of consistent and reproducible delineation of the cavity. J. Cardiovasc. Magn. Reson. 2008, 10, 40. [Google Scholar] [CrossRef]
- Kawel-Boehm, N.; Hetzel, S.J.; Ambale-Venkatesh, B.; Captur, G.; Francois, C.J.; Jerosch-Herold, M.; Salerno, M.; Teague, S.D.; Valsangiacomo-Buechel, E.; van der Geest, R.J.; et al. Reference ranges (“normal values”) for cardiovascular magnetic resonance (CMR) in adults and children: 2020 update. J. Cardiovasc. Magn. Reson. 2020, 22, 87. [Google Scholar] [CrossRef]
- Valente, A.M.; Geva, T. How to Image Repaired Tetralogy of Fallot. Circ. Cardiovasc. Imaging 2017, 10, e004270. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.A.; Avitabile, C.M.; Fu, G.L.; Kim, D.W.; Kim, T.S.; Gillespie, M.J.; Keller, M.S.; Fogel, M.A.; Whitehead, K.K. Accuracy and Internal Consistency of Cardiac Magnetic Resonance Imaging in Measuring Branch Pulmonary Artery Flows in Patients with Conotruncal Anomalies and Branch Pulmonary Artery Stents. Am. J. Cardiol. 2016, 117, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjostrom, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Muller, J.; Hager, A.; Diller, G.P.; Derrick, G.; Buys, R.; Dubowy, K.O.; Takken, T.; Orwat, S.; Inuzuka, R.; Vanhees, L.; et al. Peak oxygen uptake, ventilatory efficiency and QRS-duration predict event free survival in patients late after surgical repair of tetralogy of Fallot. Int. J. Cardiol. 2015, 196, 158–164. [Google Scholar] [CrossRef]
- Kipps, A.K.; Graham, D.A.; Harrild, D.M.; Lewis, E.; Powell, A.J.; Rhodes, J. Longitudinal exercise capacity of patients with repaired tetralogy of fallot. Am. J. Cardiol. 2011, 108, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Giardini, A.; Specchia, S.; Tacy, T.A.; Coutsoumbas, G.; Gargiulo, G.; Donti, A.; Formigari, R.; Bonvicini, M.; Picchio, F.M. Usefulness of cardiopulmonary exercise to predict long-term prognosis in adults with repaired tetralogy of Fallot. Am. J. Cardiol. 2007, 99, 1462–1467. [Google Scholar] [CrossRef] [PubMed]
- Yap, J.; Tan, J.L.; Le, T.T.; Gao, F.; Zhong, L.; Liew, R.; Tan, S.Y.; Tan, R.S. Assessment of left ventricular preload by cardiac magnetic resonance imaging predicts exercise capacity in adult operated tetralogy of Fallot: A retrospective study. BMC Cardiovasc. Disord. 2014, 14, 122. [Google Scholar] [CrossRef]
- Gnanappa, G.K.; Celermajer, D.S.; Zhu, D.; Puranik, R.; Ayer, J. Severe right ventricular dilatation after repair of Tetralogy of Fallot is associated with increased left ventricular preload and stroke volume. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 1020–1026. [Google Scholar] [CrossRef]
- Tsai, Y.J.; Li, M.H.; Tsai, W.J.; Tuan, S.H.; Liao, T.Y.; Lin, K.L. Oxygen uptake efficiency slope and peak oxygen consumption predict prognosis in children with tetralogy of Fallot. Eur. J. Prev. Cardiol. 2016, 23, 1045–1050. [Google Scholar] [CrossRef]
- Gavotto, A.; Vandenberghe, D.; Abassi, H.; Huguet, H.; Macioce, V.; Picot, M.C.; Guillaumont, S.; Matecki, S.; Amedro, P. Oxygen uptake efficiency slope: A reliable surrogate parameter for exercise capacity in healthy and cardiac children? Arch. Dis. Child. 2020, 105, 1167–1174. [Google Scholar] [CrossRef]
- Myers, J.; Arena, R.; Dewey, F.; Bensimhon, D.; Abella, J.; Hsu, L.; Chase, P.; Guazzi, M.; Peberdy, M.A. A cardiopulmonary exercise testing score for predicting outcomes in patients with heart failure. Am. Heart J. 2008, 156, 1177–1183. [Google Scholar] [CrossRef]
- Davies, L.C.; Wensel, R.; Georgiadou, P.; Cicoira, M.; Coats, A.J.; Piepoli, M.F.; Francis, D.P. Enhanced prognostic value from cardiopulmonary exercise testing in chronic heart failure by non-linear analysis: Oxygen uptake efficiency slope. Eur. Heart J. 2006, 27, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Pardaens, K.; Van Cleemput, J.; Vanhaecke, J.; Fagard, R.H. Peak oxygen uptake better predicts outcome than submaximal respiratory data in heart transplant candidates. Circulation 2000, 101, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.A.; Chen, S.Y.; Chiu, H.H.; Wang, J.K.; Chang, C.I.; Chiu, I.S.; Chen, Y.S.; Lu, C.W.; Lin, M.T.; Lue, H.C.; et al. Prognostic value of submaximal exercise data for cardiac morbidity in Fontan patients. Med. Sci. Sports Exerc. 2014, 46, 10–15. [Google Scholar] [CrossRef]
- Li, X.; Chen, N.; Zhou, X.; Yang, Y.; Chen, S.; Song, Y.; Sun, K.; Du, Q. Exercise Training in Adults With Congenital Heart Disease: A SYSTEMATIC REVIEW AND META-ANALYSIS. J. Cardiopulm. Rehabil. Prev. 2019, 39, 299–307. [Google Scholar] [CrossRef]
- Thaulow, E.; Fredriksen, P.M. Exercise and training in adults with congenital heart disease. Int. J. Cardiol. 2004, 97 (Suppl. 1), 35–38. [Google Scholar] [CrossRef] [PubMed]
- Shafer, K.M.; Opotowsky, A.R.; Rhodes, J. Exercise testing and spirometry as predictors of mortality in congenital heart disease: Contrasting Fontan physiology with repaired tetralogy of Fallot. Congenit. Heart Dis. 2018, 13, 903–910. [Google Scholar] [CrossRef]
- Dua, J.S.; Cooper, A.R.; Fox, K.R.; Graham Stuart, A. Exercise training in adults with congenital heart disease: Feasibility and benefits. Int. J. Cardiol. 2010, 138, 196–205. [Google Scholar] [CrossRef]
- Therrien, J.; Fredriksen, P.; Walker, M.; Granton, J.; Reid, G.J.; Webb, G. A pilot study of exercise training in adult patients with repaired tetralogy of Fallot. Can. J. Cardiol. 2003, 19, 685–689. [Google Scholar]
- Muller, J.; Amberger, T.; Berg, A.; Goeder, D.; Remmele, J.; Oberhoffer, R.; Ewert, P.; Hager, A. Physical activity in adults with congenital heart disease and associations with functional outcomes. Heart 2017, 103, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.; Johansson, B.; Wadell, K.; Thilen, U.; Sandberg, C. Adults with congenital heart disease overestimate their physical activity level. Int. J. Cardiol. Heart Vasc. 2019, 22, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Gentili, F.; Cafiero, G.; Perrone, M.A.; Bianco, M.; Salvati, A.; Giordano, U.; Silva Kikina, S.; Guccione, P.; De Zorzi, A.; Galletti, L.; et al. The Effects of Physical Inactivity and Exercise at Home in Young Patients with Congenital Heart Disease during the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2021, 18, 10065. [Google Scholar] [CrossRef]
- Perrone, M.A.; Feola, A.; Pieri, M.; Donatucci, B.; Salimei, C.; Lombardo, M.; Perrone, A.; Parisi, A. The Effects of Reduced Physical Activity on the Lipid Profile in Patients with High Cardiovascular Risk during COVID-19 Lockdown. Int. J. Environ. Res. Public Health 2021, 18, 8858. [Google Scholar] [CrossRef] [PubMed]
- Perrone, M.; Donatucci, B.; Salvati, A.; Gualtieri, P.; De Lorenzo, A.; Romeo, F.; Bernardini, S. Inflammation, oxidative stress and gene expression: The postprandial approach in professional soccer players to reduce the risk of muscle injuries and early atherosclerosis. Med. Sport 2019, 72, 234–243. [Google Scholar] [CrossRef]
- Perrone, M.A.; Santilli, A.; De Zorzi, A.; Turchetta, A.; Bevilacqua, M.; Carotti, A.; Rinelli, G.; Guccione, P. The effects of physical activity in children with hypoplastic left heart syndrome after complete palliation with Fontan procedure. Med. Sport 2020, 73, 526–533. [Google Scholar] [CrossRef]
- Sirichand, S.; Killu, A.M.; Padmanabhan, D.; Hodge, D.O.; Chamberlain, A.M.; Brady, P.A.; Kapa, S.; Noseworthy, P.A.; Packer, D.L.; Munger, T.M.; et al. Incidence of Idiopathic Ventricular Arrhythmias: A Population-Based Study. Circ. Arrhythm. Electrophysiol. 2017, 10, e004662. [Google Scholar] [CrossRef]

| Overall (N = 84) | ||
|---|---|---|
| Demographic features | Male sex, n (%) | 41 (48.8%) |
| Age at repair (months) (median, IQR) | 13.4 (0.0–87.0) | |
| Age at CPET (yrs) (median, IQR) | 21.1 (15.0–30.0) | |
| Time between surgery and CPET (yrs) (median, IQR) | 20.2 (10.2–28.9) | |
| BSA (m2) (median, IQR) | 1.7 (1.1–2.2) | |
| BMI (median, IQR) | 22.6 (15.6–33.3) | |
| MRI parameters | RVEDV (mL) (median, IQR) | 205.0 (106.0–327.9) |
| RVEDVi (mL/m2) (median, IQR) | 122.1 (63.3–174.3) | |
| RVESV (mL) (median, IQR) | 92.2 (43.0–152.0) | |
| RVESVi (mL/m2) (median, IQR) | 54.9 (25.7–84.6) | |
| RVEF (%) (median, IQR) | 55.0 (45–69.0) | |
| RVSV (mL/beat) (median, IQR) | 109.7 (6.0–182.5) | |
| LVEDV (mL) (median, IQR) | 125.0 (69.0–206.5) | |
| LVEDVi (mL/m2) (median, IQR) | 75.4 (48.8–113.7) | |
| LVESV (mL) (median, IQR) | 54.1 (27.0–86.0) | |
| LVEDVi (mL/m2) (median, IQR) | 33.5 (19.9–52.9) | |
| LVEF, (%) (median, IQR) | 57.0 (45.5–66.0) | |
| LVSV (mL/beat) (median, IQR) | 69.8 (42.0–108.3) | |
| PR (%) (median, IQR) | 37.4 (27.0–60.0) | |
| RVOTO and/or PAs branches stenosis, n (%) | 8 (9.5) | |
| CPET values | Total test duration (s) (median, IQR) | 607 (551–724) |
| Peak HR (bpm) (median, IQR) | 176 (167–182) | |
| Peak HR (%) (median, IQR) | 88 (85–92) | |
| HR at AT (bpm) (median, IQR) | 128 (119–138) | |
| Peak VO2 (mL/min) (median, IQR) | 1885 (1530–2166) | |
| Peak VO2/Kg (mL/min/Kg) (median, IQR) | 31 (25–33) | |
| Peak VO2/Kg (% of predicted) (median, IQR) | 68 (61–78) | |
| VO2 at AT (mL/min/Kg) (median, IQR) | 19.7 (18.3–23.7) | |
| Peak RER (median, IQR) | 1.09 (1.02–1.15) | |
| Peak O2 pulse (mL/bpm) (median, IQR) | 10.4 (8.81; 12.28) | |
| Peak O2 pulse (% of predicted) (median, IQR) | 81 (71; 93) | |
| O2 pulse trend n (%) | ||
| 7 (10.3%) 58 (85.3%) 3 (4.4%) | |
| OUES (mL/min/L/min) (median, IQR) | 1908 (1665–2538) | |
| OUES (% of predicted) (median, IQR) | 79 (68–85) | |
| VE/VCO2 at AT (median, IQR) | 27.5 (25.0–29.9) | |
| VE/VCO2 slope (AT) (median, IQR) | 25.4 (23.6–28.9) | |
| Peak VE/VCO2 (median, IQR) | 31.1 (27.3–34.3) | |
| VE/VCO2 slope (VCP) (median, IQR) | 28.3 (25.5–31.5) | |
| VE/VCO2 slope (stop) (median, IQR) | 30.7 (27.5–34.5) | |
| FVC (l) (median, IQR) | 3.6 (2.97–4.26) | |
| FEV1 (l) (median, IQR) | 3.1 (2.77–3.79) | |
| Peak VE (l/min) (median, IQR) | 61 (54–76) | |
| Peak VE/VO2 (median, IQR) | 33.0 (28.9–38.2) | |
| BR (%) (median, IQR) | 50 (40–57) | |
| RVEF ≤ 51% | RVEF > 51% | p Value | |
|---|---|---|---|
| n | 28 | 56 | |
| age at CPET (mean (SD)) | 26.14 (8.85) | 21.01 (7.61) | 0.007 |
| IPAQ (%) | 0.303 | ||
| 0 | 6 (20.7) | 20 (36.4) | |
| 1 | 21 (72.4) | 33 (60.0) | |
| 2 | 2 (6.9) | 2 (3.6) | |
| male (%) | 16 (55.2) | 25 (45.5) | 0.537 |
| BMI (mean (SD)) | 22.00 (2.41) | 22.82 (4.03) | 0.321 |
| RVEDVi (mean (SD)) | 128.50 (21.21) | 121.46 (21.48) | 0.155 |
| RVESVi (mean (SD)) | 65.23 (11.18) | 51.22 (11.43) | <0.001 |
| LVSV (mean (SD)) | 73.05 (16.27) | 72.66 (14.50) | 0.913 |
| RVSV (mean (SD)) | 106.45 (19.02) | 116.94 (26.13) | 0.061 |
| PR (mean (SD)) | 31.97 (17.45) | 34.45 (13.06) | 0.463 |
| Peak RER (mean (SD)) | 1.07 (0.10) | 1.10 (0.10) | 0.160 |
| VE/VCO2 slope (stop) (mean (SD)) | 30.74 (4.69) | 30.68 (5.13) | 0.958 |
| VE/VCO2 slope (VCP) (mean (SD)) | 28.52 (3.99) | 28.94 (4.74) | 0.724 |
| peak VE/VCO2 (mean (SD)) | 31.01 (4.50) | 31.32 (5.77) | 0.806 |
| VE/VCO2 slope (AT) (mean (SD)) | 25.75 (3.47) | 26.73 (4.44) | 0.316 |
| VE/VCO2 AT (mean (SD)) | 27.39 (3.47) | 28.55 (4.68) | 0.258 |
| OUES (mean (SD)) | 2107.36 (579.95) | 2083.61 (617.28) | 0.869 |
| Trend VO2 HR (mean (SD)) | 1.96 (0.36) | 1.93 (0.39) | 0.796 |
| Peak oxygen pulse (mean (SD)) | 11.26 (2.64) | 10.46 (3.20) | 0.249 |
| VO2 AT (mean (SD)) | 21.00 (4.23) | 21.14 (4.41) | 0.889 |
| Peak VO2/kg (mean (SD)) | 29.61 (7.11) | 30.42 (6.87) | 0.612 |
| Peak VO2 (mean (SD)) | 1896.24 (458.10) | 1884.59 (522.23) | 0.920 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leonardi, B.; Gentili, F.; Perrone, M.A.; Sollazzo, F.; Cocomello, L.; Silva Kikina, S.; Wald, R.M.; Palmieri, V.; Secinaro, A.; Gagliardi, M.G.; et al. Cardiopulmonary Exercise Testing in Repaired Tetralogy of Fallot: Multiparametric Overview and Correlation with Cardiac Magnetic Resonance and Physical Activity Level. J. Cardiovasc. Dev. Dis. 2022, 9, 26. https://doi.org/10.3390/jcdd9010026
Leonardi B, Gentili F, Perrone MA, Sollazzo F, Cocomello L, Silva Kikina S, Wald RM, Palmieri V, Secinaro A, Gagliardi MG, et al. Cardiopulmonary Exercise Testing in Repaired Tetralogy of Fallot: Multiparametric Overview and Correlation with Cardiac Magnetic Resonance and Physical Activity Level. Journal of Cardiovascular Development and Disease. 2022; 9(1):26. https://doi.org/10.3390/jcdd9010026
Chicago/Turabian StyleLeonardi, Benedetta, Federica Gentili, Marco Alfonso Perrone, Fabrizio Sollazzo, Lucia Cocomello, Stefani Silva Kikina, Rachel M. Wald, Vincenzo Palmieri, Aurelio Secinaro, Maria Giulia Gagliardi, and et al. 2022. "Cardiopulmonary Exercise Testing in Repaired Tetralogy of Fallot: Multiparametric Overview and Correlation with Cardiac Magnetic Resonance and Physical Activity Level" Journal of Cardiovascular Development and Disease 9, no. 1: 26. https://doi.org/10.3390/jcdd9010026
APA StyleLeonardi, B., Gentili, F., Perrone, M. A., Sollazzo, F., Cocomello, L., Silva Kikina, S., Wald, R. M., Palmieri, V., Secinaro, A., Gagliardi, M. G., Parisi, A., Turchetta, A., Galletti, L., Bianco, M., & Drago, F. (2022). Cardiopulmonary Exercise Testing in Repaired Tetralogy of Fallot: Multiparametric Overview and Correlation with Cardiac Magnetic Resonance and Physical Activity Level. Journal of Cardiovascular Development and Disease, 9(1), 26. https://doi.org/10.3390/jcdd9010026