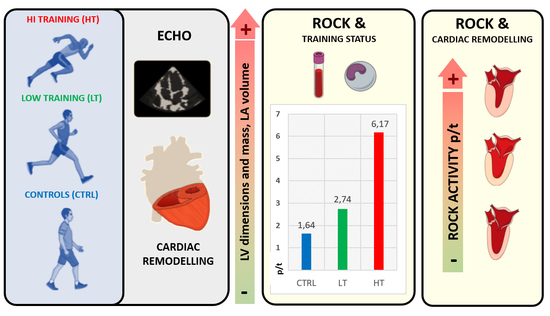
Left Cardiac Remodelling Assessed by Echocardiography Is Associated with Rho-Kinase Activation in Long-Distance Runners
Abstract
1. Introduction
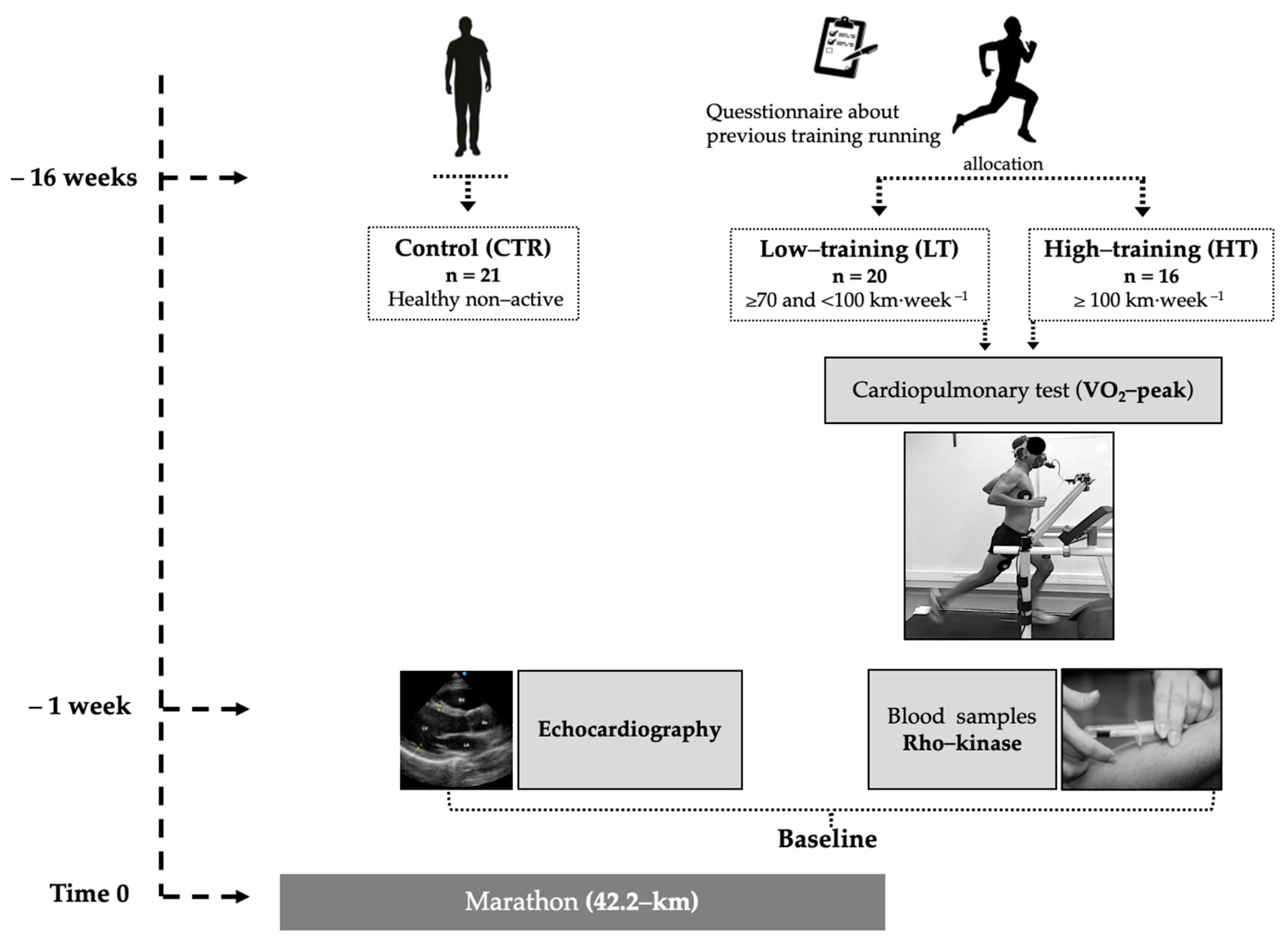
2. Materials and Methods
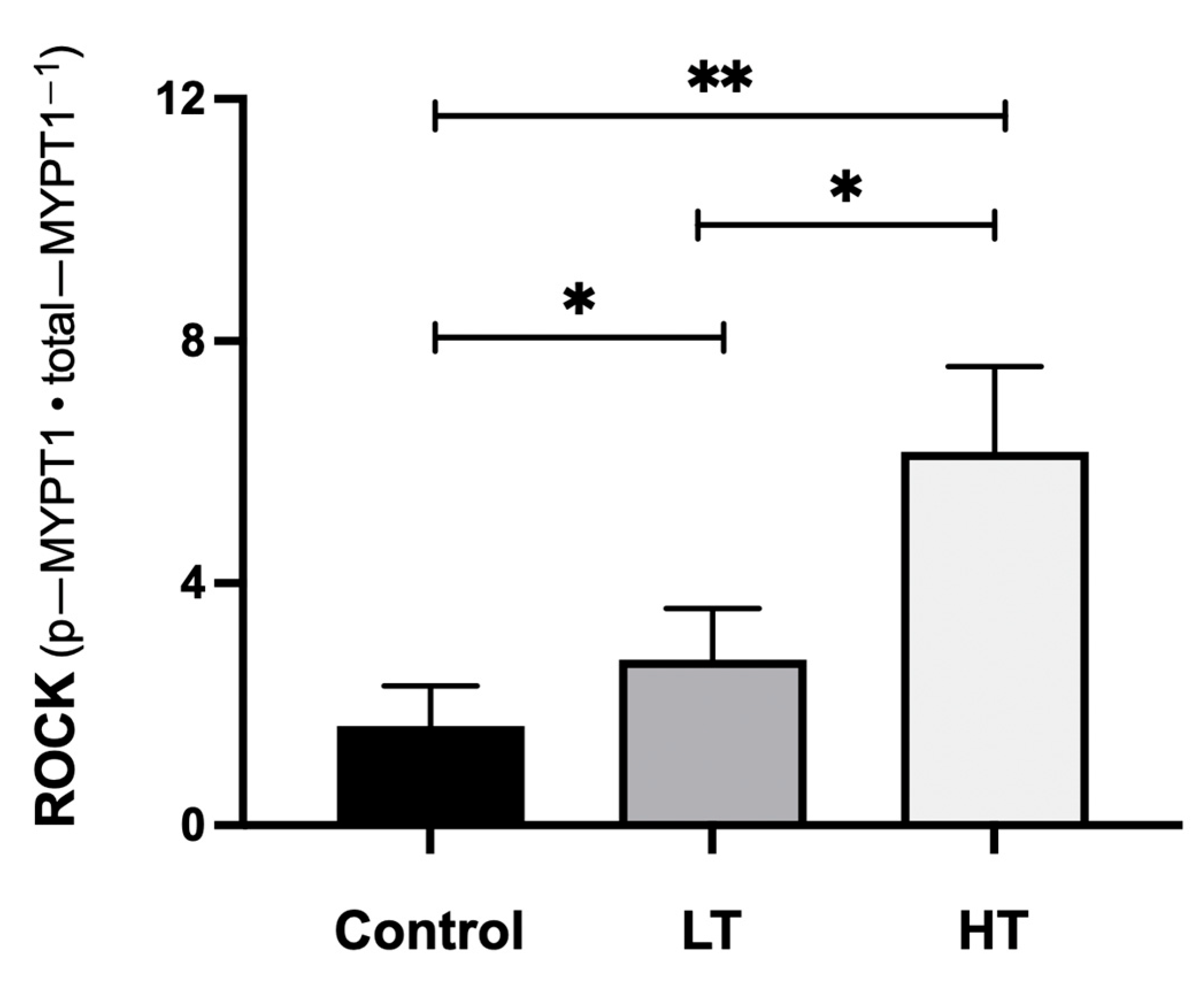
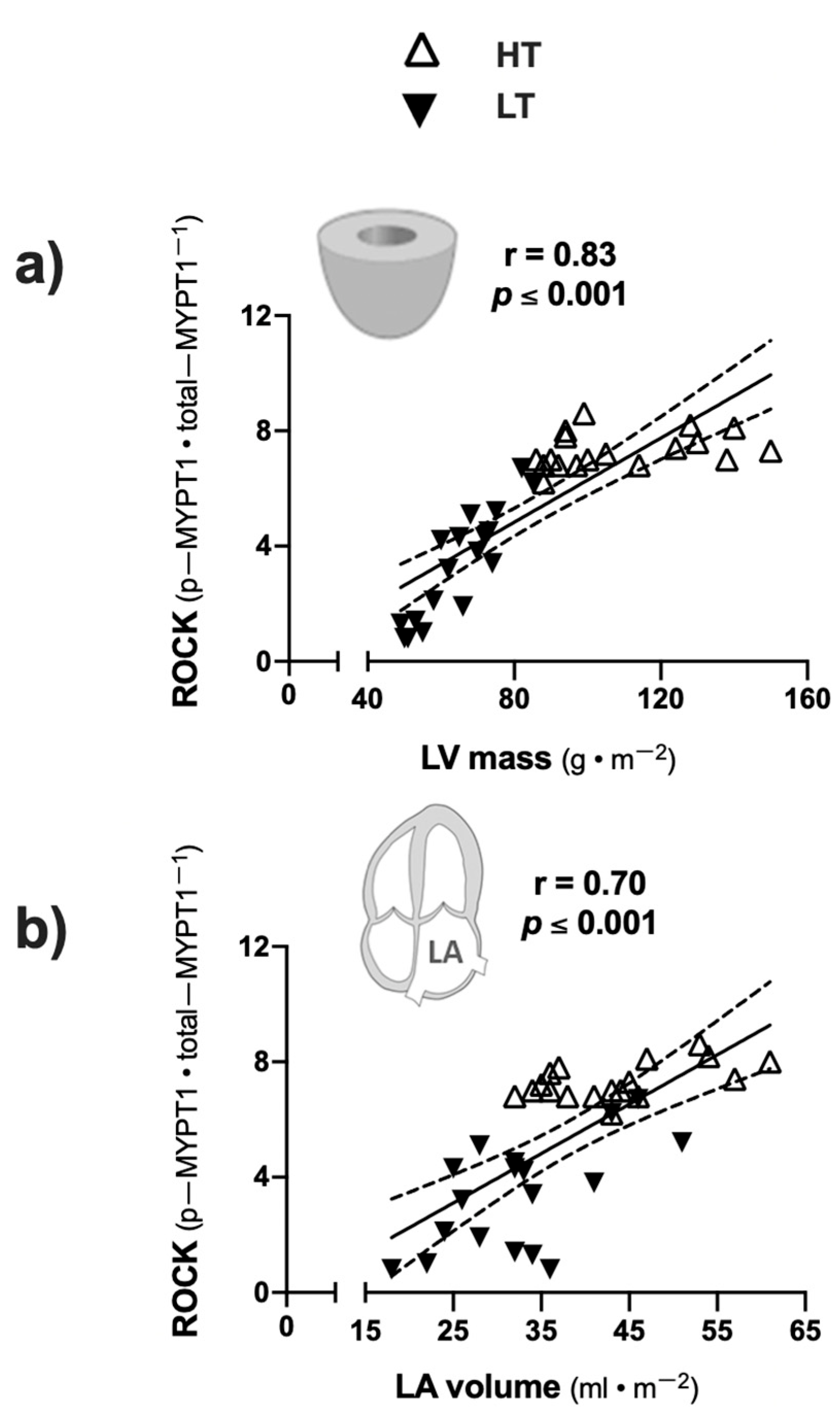
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lear, S.; Hu, W.; Rangarajan, S.; Gasevic, D.; Leong, D.; Iqbal, R.; Casanova, A.; Swaminathan, S.; Anjana, R.; Kumar, R.; et al. The effect of physical activity on mortality and cardiovascular disease in 130 000 people from 17 high-income, middle-income, and low-income countries: The PURE study. Lancet 2017, 390, 2643–2654. [Google Scholar] [CrossRef]
- Sharma, S.; Merghani, A.; Mont, L. Exercise and the heart: The good, the bad, and the ugly. Eur. Heart J. 2015, 36, 1445–1453. [Google Scholar] [CrossRef]
- Cohn, J.; Ferrari, R.; Sharpe, N. Cardiac remodeling-concepts and clinical implications: A consensus paper from an International Forum on Cardiac Remodeling. J. Am. Coll. Cardiol. 2000, 35, 569–582. [Google Scholar] [CrossRef]
- Zdravkovic, M.; Perunicic, J.; Krotin, M.; Ristic, M.; Vukomanovic, V.; Soldatovic, I.; Zdravkovic, D. Echocardiographic study of early left ventricular remodeling in highly trained preadolescent footballers. J. Sci. Med. Sport 2010, 13, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.; Pelliccia, A. The heart of trained athletes: Cardiac remodeling and the risks of sports, including sudden death. Circulation 2006, 114, 1633–1644. [Google Scholar] [CrossRef]
- Gabrielli, L.; Sitges, M.; Chiong, M.; Jalil, J.; Ocaranza, M.; Llevaneras, S.; Herrera, S.; Fernandez, R.; Saavedra, R.; Yañez, F.; et al. Potential adverse cardiac remodelling in highly trained athletes: Still unknown clinical significance. Eur. J. Sport Sci. 2018, 18, 1288–1297. [Google Scholar] [CrossRef] [PubMed]
- Prakash, K.; Sharma, S. Interpretation of the electrocardiogram in athletes. Can. J. Cardiol. 2016, 32, 438–451. [Google Scholar] [CrossRef]
- Tahir, E.; Starekova, J.; Muellerleile, K.; von Stritzky, A.; Münch, J.; Avanesov, M.; Weinrich, J.; Stehning, C.; Bohnen, S.; Radunski, U.; et al. Myocardial fibrosis in competitive triathletes detected by contrast-enhanced CMR correlates with exercise-induced hypertension and competition history. J. Am. Cardiovasc. Coll. Cardiovasc. Imaging 2018, 11, 1260–1270. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, A.; Ghani, S.; Sheikh, N.; Gati, S.; Bastiaenen, R.; Madden, B.; Papadakis, M.; Raju, H.; Reed, M.; Sharma, R.; et al. Clinical significance of electrocardiographic right ventricular hypertrophy in athletes: Comparison with arrhythmogenic right ventricular cardiomyopathy and pulmonary hypertension. Eur. Heart J. 2013, 34, 3649–3656. [Google Scholar] [CrossRef]
- Gabrielli, L.; Herrera, S.; Contreras-Briceño, F.; Vega, J.; Ocaranza, M.; Yáñez, F.; Fernández, R.; Saavedra, R.; Sitges, M.; García, L.; et al. Increased active phase atrial contraction is related to marathon runner performance. Eur. J. Appl. Physiol. 2018, 118, 1931–1939. [Google Scholar] [CrossRef]
- Gabrielli, L.; Bijnens, B.; Butakoff, C.; Duchateau, N.; Montserrat, S.; Merino, B.; Gutierrez, J.; Paré, C.; Mont, L.; Brugada, J.; et al. Atrial functional and geometrical remodeling in highly trained male athletes: For better or worse? Eur. J. Appl. Physiol. 2014, 114, 1143–1152. [Google Scholar] [CrossRef]
- Gabrielli, L.; Bijnens, B.; Brambila, C.; Duchateau, N.; Marin, J.; Sitges-Serra, I.; Mont, L.; Brugada, J.; Sitges, M. Differential atrial performance at rest and exercise in athletes: Potential trigger for developing atrial dysfunction? Scand. J. Med. Sci. Sport. 2016, 26, 1444–1454. [Google Scholar] [CrossRef]
- Currie, K.; Bailey, K.; Jung, M.; McKelvie, R.; MacDonald, M. Effects of resistance training combined with moderate-intensity endurance or low-volume high-intensity interval exercise on cardiovascular risk factors in patients with coronary artery disease. J. Sci. Med. Sport 2015, 18, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Liao, J. Rho kinases and cardiac remodeling. Circ. J. 2016, 80, 1491–1498. [Google Scholar] [CrossRef]
- Ho, T.; Huang, C.; Huang, C.; Lin, W. Fasudil, a Rho-kinase inhibitor, protects against excessive endurance exercise training-induced cardiac hypertrophy, apoptosis and fibrosis in rats. Eur. J. Appl. Physiol. 2012, 112, 2943–2955. [Google Scholar] [CrossRef] [PubMed]
- Ocaranza, M.; Gabrielli, L.; Mora, I.; Garcia, L.; McNab, P.; Godoy, I.; Braun, S.; Córdova, S.; Castro, P.; Novoa, U.; et al. Markedly increased Rho-kinase activity in circulating leukocytes in patients with chronic heart failure. Am. Heart J. 2011, 161, 931–937. [Google Scholar] [CrossRef]
- Gabrielli, L.; Winter, J.; Godoy, I.; McNab, P.; Padilla, I.; Cordova, S.; Rigotti, P.; Novoa, U.; Mora, I.; García, L.; et al. Increased Rho-kinase activity in hypertensive patients with left ventricular hypertrophy. Am. J. Hypertens. 2014, 27, 838–845. [Google Scholar] [CrossRef]
- Muñoz, V.; Gaspar, R.; Minuzzi, L.; dos Santos Canciglieri, R.; da Silva, A.; de Moura, L.; Cintra, D.; Ropelle, E.; Pauli, J. Rho-kinase activity is upregulated in the skeletal muscle of aged exercised rats. Exp. Gerontol. 2019, 128. [Google Scholar] [CrossRef]
- Muñoz, V.; Gaspar, R.; Esteca, M.; Baptista, I.; Vieira, R.; da Silva, A.; de Moura, L.; Cintra, D.; Ropelle, E.; Pauli, J. Physical exercise increases ROCK activity in the skeletal muscle of middle-aged rats. Mech. Ageing Dev. 2020, 186. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Sun, J.; Du, G.; Jiao, F. Association of moderate aerobic exercise & rho-Associated kinase 2 concentration in subjects with dyslipidemia. Arch. Med. Sci. 2017, 13, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Anaruma, C.; Pereira, R.; Cristina da Cruz Rodrigues, K.; Ramos da Silva, A.; Cintra, D.; Ropelle, E.; Pauli, J.; Pereira de Moura, L. Rock protein as cardiac hypertrophy modulator in obesity and physical exercise. Life Sci. 2020, 254. [Google Scholar] [CrossRef]
- Fierro, C.; Novoa, U.; González, V.; Ocaranza, M.; Jalil, J. Simultaneous Rho kinase inhibition in circulating leukocytes and in cardiovascular tissue in rats with high angiotensin converting enzyme levels. Int. J. Cardiol. 2016, 215, 309–317. [Google Scholar] [CrossRef]
- Lang, R.; Badano, L.; Victor, M.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.; Foster, E.; Goldstein, S.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 01–39. [Google Scholar] [CrossRef] [PubMed]
- Rosner, B. Fundamental of Biostatistics, 7th ed.; Brooks/Cole: Boston, MA, USA, 2011. [Google Scholar]
- Middleton, N.; Shave, R.; George, K.; Whyte, G.; Hart, E.; Atkinson, G. Left ventricular function immediately following prolonged exercise: A meta-analysis. Med. Sci. Sports Exerc. 2006, 38, 681–687. [Google Scholar] [CrossRef]
- Shave, R.; Baggish, A.; George, K.; Wood, M.; Scharhag, J.; Whyte, G.; Gaze, D.; Thompson, P. Exercise-induced cardiac troponin elevation: Evidence, mechanisms, and implications. J. Am. Coll. Cardiol. 2010, 56, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Zhang, H.; Kong, Z.; Wang, C.; Liu, Y.; Shi, Q.; George, K. The impact of exercise modality and menstrual cycle phase on circulating cardiac troponin T. J. Sci. Med. Sport 2020, 23, 309–314. [Google Scholar] [CrossRef]
- Calvo, N.; Brugada, J.; Sitges, M.; Mont, L. Atrial fibrillation and atrial flutter in athletes. Br. J. Sports Med. 2012, 46, 37–43. [Google Scholar] [CrossRef]
- Chen, Y.; Su, F.; Han, J.; Jiao, P.; Guo, W. Expression of rho kinase and its mechanism in the left atrial appendage in patients with atrial fibrillation. Heart Surg. Forum 2018, 21, 44–48. [Google Scholar] [CrossRef]
- Kitano, K.; Usui, S.; Ootsuji, H.; Takashima, S.; Kobayashi, D.; Murai, H.; Furusho, H.; Nomura, A.; Kaneko, S.; Takamura, M. Rho-kinase activation in leukocytes plays a pivotal role in myocardial ischemia/reperfusion injury. PLoS ONE 2014, 9, 01–10. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, J.; Su, T.; Zhang, S.; Lin, X. Fasudil, a rho-kinase inhibitor, exerts cardioprotective function in animal models of myocardial ischemia/reperfusion injury: A meta-analysis and review of preclinical evidence and possible mechanisms. Front. Pharmacol. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Gresslien, T.; Agewall, S. Troponin and exercise. Int. J. Cardiol. 2016, 221, 609–621. [Google Scholar] [CrossRef]
- Schild, M.; Eichner, G.; Beiter, T.; Zügel, M.; Krumholz-Wagner, I.; Hudemann, J.; Pilat, C.; Krüger, K.; Niess, A.; Steinacker, J.; et al. Effects of acute endurance exercise on plasma protein profiles of endurance-trained and untrained individuals over time. Mediators Inflamm. 2016, 2016, 4851935. [Google Scholar] [CrossRef] [PubMed]
- Benito, B.; Gay-Jordi, G.; Serrano-Mollar, A.; Guasch, E.; Shi, Y.; Tardif, J.; Brugada, J.; Nattel, S.; Mont, L. Cardiac arrhythmogenic remodeling in a rat model of long-term intensive exercise training. Circulation 2011, 123, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Ocaranza, M.; Díaz-Araya, G.; Carreño, J.; Muñoz, D.; Riveros, J.; Jalil, J.; Lavandero, S. Polymorphism in gene coding for ACE determines different development of myocardial fibrosis in rats. Am. J. Physiol.—Hear. Circ. Physiol. 2004, 286, 498–506. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meleleo, D.; Bartolomeo, N.; Cassano, L.; Nitti, A.; Susca, G.; Mastrototaro, G.; Armenise, U.; Zito, A.; Devito, F.; Scicchitano, P.; et al. Evaluation of body composition with bioimpedence. A comparison between athletic and non-athletic children. Eur. J. Sport Sci. 2017, 17, 710–719. [Google Scholar] [CrossRef] [PubMed]

| Groups | ||||
|---|---|---|---|---|
| Variables | Control (n = 21) | LT (n = 20) | HT (n = 16) | p-Value |
| Age (years) | 35 ± 4 | 39 ± 5 | 37 ± 6 | 0.32 |
| Height (cm) | 175 ± 6 | 174 ± 6 | 172 ± 7 | 0.47 |
| Weight (kg) | 72 ± 9 | 73 ± 8 | 69 ± 8 | 0.09 |
| Body surface (m2) | 1.89 ± 0.13 | 1.88 ± 0.12 | 1.82 ± 0.13 | 0.08 |
| Creatinine (mg·dL−1) | 0.99 ± 0.11 | 0.97 ± 0.10 | 0.98 ± 0.09 | 0.63 |
| Hematocrit (%) | 42 ± 3 | 43 ± 3 | 43 ± 2 | 0.87 |
| Sodium (mEq/L) | 142 ± 2 | 142 ± 2 | 142 ± 3 | 0.44 |
| AST (U/L) | 26 ± 7 | 28 ± 8 | 29 ± 9 | 0.67 |
| Uric acid (mg/dL) | 5.2 ± 0.8 | 5.0 ± 0.9 | 5.6 ± 0.9 | 0.17 |
| O2-peak (mL·kg−1·min−1) | - | 52.5 ± 8.1 | 58.5 ± 5.3 | 0.02 * |
| Running experience (years) | - | 15 ± 3 | 17 ± 3 | 0.81 |
| Time training per week (h) | - | 14 ± 2 | 19 ± 2 | 0.01 * |
| Training intensity (%HR máx., 220-age) | - | 82 ± 2 | 81 ± 3 | 0.78 |
| Groups | ||||
|---|---|---|---|---|
| Control (n = 21) | LT (n = 20) | HT (n = 16) | p-Value | |
| Left Cardiac Cavities | ||||
| Interventricular septum (mm) | 7.6 ± 0.8; (10.5) | 9.0 ± 1.6; (17.7) | 10.2 ± 1.2 *; (11.89 | <0.001 |
| Posterior wall (mm) | 7.6 ± 0.8; (10.5) | 8.5 ± 1.2; (14.1) | 9.3 ± 2.1 *; (22.6) | 0.01 |
| LVEDD (mm) | 46 ± 4; (8) | 50 ± 5; (10) | 58 ± 4 *; (6) | 0.04 |
| LVESD (mm) | 30 ± 3; (10) | 30 ± 4; (13) | 33 ± 5; (15) | 0.40 |
| Ejection fraction (%) | 57 ± 4; (7) | 55 ± 6; (10) | 54 ± 3; (5) | 0.11 |
| LV mas index (g·m−2) | 58 ± 11; (18) | 78 ± 18; (23) | 106 ± 27 *; (25) | <0.001 |
| LA diameter (mm) | 33 ± 4; (12) | 34 ± 3; (8) | 36 ± 4; (11) | 0.22 |
| LA area (cm2) | 19 ± 5; (26) | 22 ± 4; (18) | 25 ± 3 *; (12) | <0.001 |
| LA volumen index (mL·m−2) | 25 ± 9; (36) | 30 ± 11; (36) | 42 ± 8 *; (19) | <0.001 |
| Global LV longitudinal strain (%) | −21.0 ± −2.0; (9.5) | −19.6 ± −1.6; (8.2) | −19.5 ± −2.4; (12.3) | 0.11 |
| E wave (cm·s−1) | 77 ± 15; (19) | 84 ± 12; (14) | 78 ± 13; (17) | 0.21 |
| A wave (cm·s−1) | 48 ± 16; (33) | 53 ± 10; (19) | 50 ± 12; (24) | 0.43 |
| Deceleration time (ms) | 200 ± 66; (33) | 229 ± 65; (28) | 233 ± 65; (28) | 0.18 |
| e’ lateral (cm·s−1) | 15.0 ± 1.8; (12.0) | 15.0 ± 2.5; (16.7) | 15.0 ± 2.3; (15.3) | 0.70 |
| e’ medial (cm·s−1) | 11.0 ± 1.8; (16.4) | 10.0 ± 2.0; (20.0) | 10.0 ± 2.0; (20.0) | 0.75 |
| Right Ventricle | ||||
| TAPSE (mm) | 25.4 ± 3.3; (12.9) | 25.6 ± 4.7; (18.3) | 25.8 ± 3.0; (11.6) | 0.16 |
| FAC (%) | 52.5 ± 3.9; (7.4) | 57.3 ± 4.6; (8.0) | 56.4 ± 3.7; (6.6) | 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contreras-Briceño, F.; Vega, J.; Mandiola, J.; Ocaranza, M.P.; Herrera, S.; Salinas, M.; Fernández, R.; Jalil, J.E.; Lavandero, S.; Chiong, M.; et al. Left Cardiac Remodelling Assessed by Echocardiography Is Associated with Rho-Kinase Activation in Long-Distance Runners. J. Cardiovasc. Dev. Dis. 2021, 8, 118. https://doi.org/10.3390/jcdd8100118
Contreras-Briceño F, Vega J, Mandiola J, Ocaranza MP, Herrera S, Salinas M, Fernández R, Jalil JE, Lavandero S, Chiong M, et al. Left Cardiac Remodelling Assessed by Echocardiography Is Associated with Rho-Kinase Activation in Long-Distance Runners. Journal of Cardiovascular Development and Disease. 2021; 8(10):118. https://doi.org/10.3390/jcdd8100118
Chicago/Turabian StyleContreras-Briceño, Felipe, Julián Vega, Jorge Mandiola, María Paz Ocaranza, Sebastián Herrera, Manuel Salinas, Rodrigo Fernández, Jorge E. Jalil, Sergio Lavandero, Mario Chiong, and et al. 2021. "Left Cardiac Remodelling Assessed by Echocardiography Is Associated with Rho-Kinase Activation in Long-Distance Runners" Journal of Cardiovascular Development and Disease 8, no. 10: 118. https://doi.org/10.3390/jcdd8100118
APA StyleContreras-Briceño, F., Vega, J., Mandiola, J., Ocaranza, M. P., Herrera, S., Salinas, M., Fernández, R., Jalil, J. E., Lavandero, S., Chiong, M., Godoy, P., Castro, P. F., Sitges, M., & Gabrielli, L. (2021). Left Cardiac Remodelling Assessed by Echocardiography Is Associated with Rho-Kinase Activation in Long-Distance Runners. Journal of Cardiovascular Development and Disease, 8(10), 118. https://doi.org/10.3390/jcdd8100118