Ca2+ Signaling in Cardiac Fibroblasts and Fibrosis-Associated Heart Diseases
Abstract
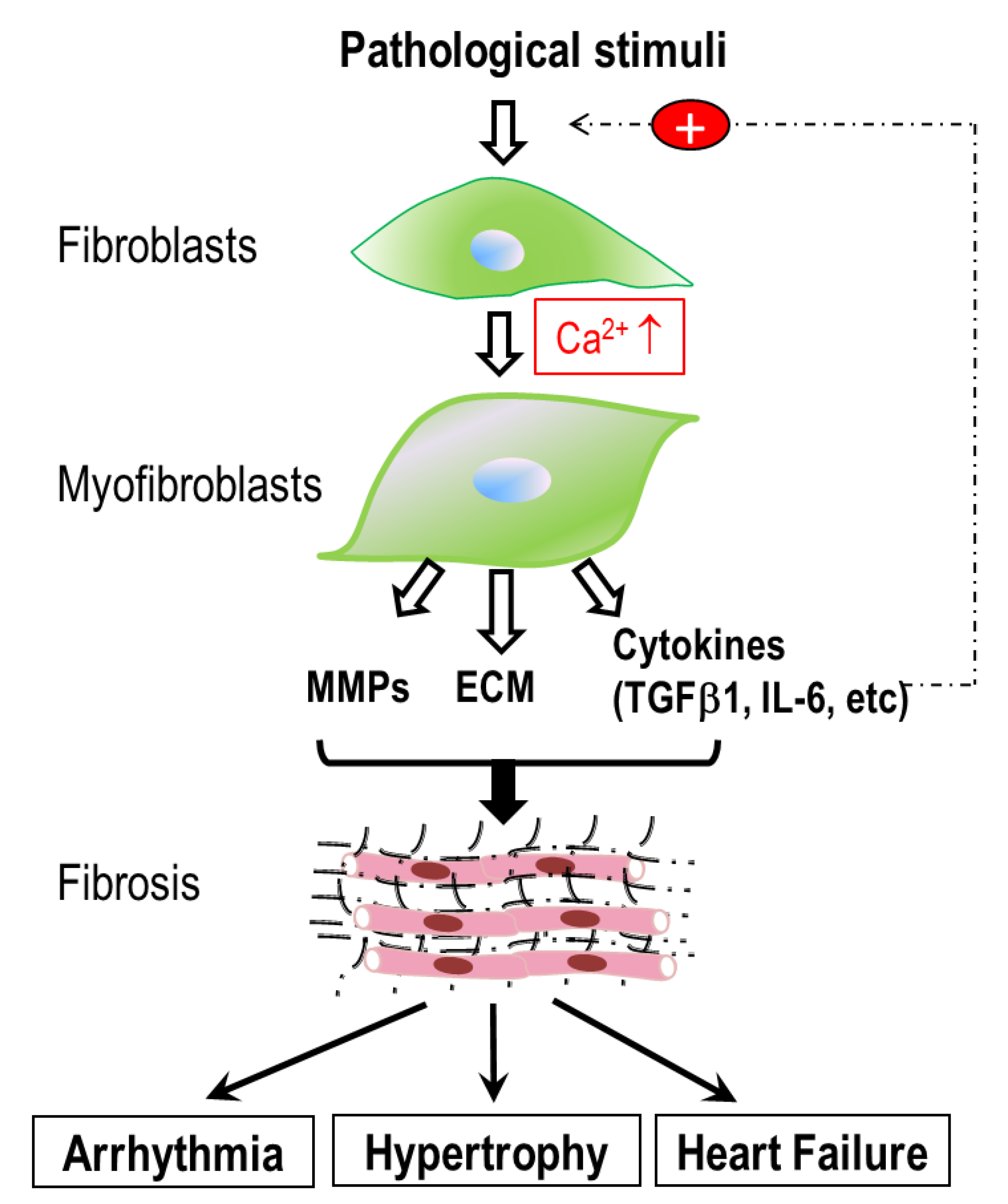
1. Introduction
2. Ion Channels Controlling the Membrane Potential in Cardiac Fibroblasts
2.1. K+ Channels
2.2. Na+ Channels
2.3. Cl− and H+ Channels
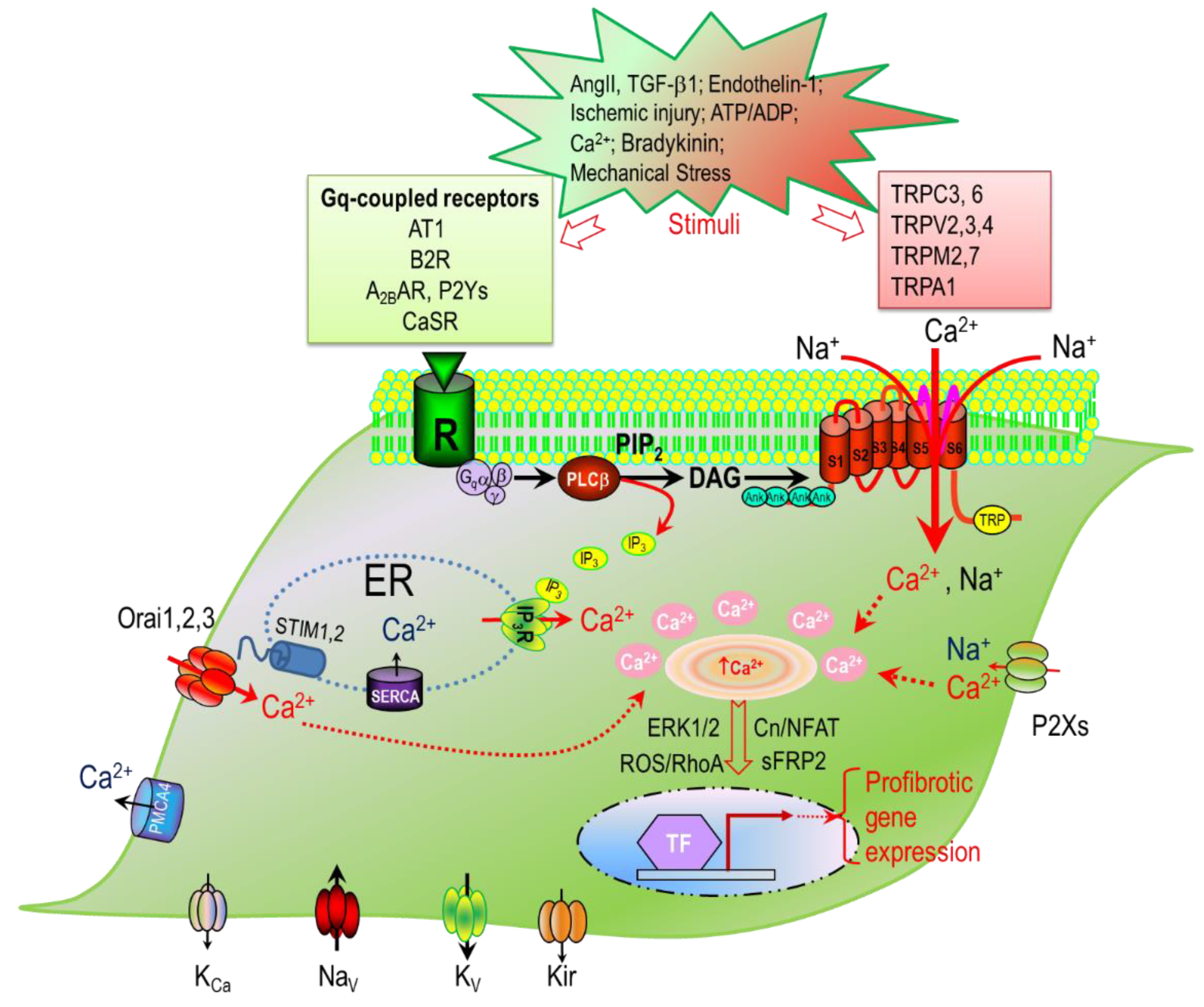
3. Ca2+ Signaling Mechanisms in Cardiac Fibroblasts
3.1. Ca2+ Release Mechanisms in Cardiac Fibroblasts
3.2. Signaling Pathways Mediating Ca2+ Release in Cardiac Fibroblasts
3.3. Ca2+ Entry Mechanisms in Cardiac Fibroblasts
3.3.1. Voltage-gated Ca2+ Channels
3.3.2. Ca2+ Entry Mediated by P2X Receptors
3.3.3. Ca2+ Entry through CRAC (Orai/STIM) Channels in Cardiac Fibroblasts
3.3.4. Ca2+ Entry Mediated by TRP Channels in Cardiac Fibroblasts and Fibrotic Heart Disease
Overview of TRP Channels
TRP Channels are Multifunctional Cellular Sensors
The Functional Expressions of the TRP Channels in Cardiac Fibroblasts
3.4. Ca2+ Signaling Mediated by TRP Channels in Cardiac Fibroblasts and Fibrosis-Associated Heart Diseases
3.4.1. TRPC Channels
3.4.2. TRPV Channels in Fibroblasts and Cardiac Fibrosis
3.4.3. TRPM Channels and Cardiac Fibrosis
3.4.4. TRPA1 in Cardiac Fibroblasts
3.5. Ca2+ Efflux and Fibroblast Function
4. Conclusions and Future Perspectives
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Weber, K. Cardiac interstitium. In Heart Failure; Poole-Wilson, P., Colucci, W., Massie, B., Chatterjee, K., Coats, A., Eds.; Churchill Livingstone: New York, NY, USA, 1997; pp. 13–31. [Google Scholar]
- Gourdie, R.G.; Dimmeler, S.; Kohl, P. Novel therapeutic strategies targeting fibroblasts and fibrosis in heart disease. Nat. Rev. Drug Discov. 2016, 15, 620–638. [Google Scholar] [CrossRef] [PubMed]
- Travers, J.G.; Kamal, F.A.; Robbins, J.; Yutzey, K.E.; Blaxall, B.C. Cardiac Fibrosis: The Fibroblast Awakens. Circ. Res. 2016, 118, 1021–1040. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.G.; Yuan, Y.P.; Wu, H.M.; Zhang, X.; Tang, Q.Z. Cardiac fibrosis: New insights into the pathogenesis. Int. J. Biol. Sci. 2018, 14, 1645–1657. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.N.; Kiriazis, H.; Gao, X.M.; Du, X.J. Cardiac Fibrosis and Arrhythmogenesis. Compr. Physiol. 2017, 7, 1009–1049. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.T.; Sun, Y.; Diez, J. Fibrosis: A Living Tissue and the Infarcted Heart. J. Am. Coll. Cardiol. 2008, 52, 2029–2031. [Google Scholar] [CrossRef] [PubMed]
- de Bakker, J.M.; van Capelle, F.J.; Janse, M.J.; Tasseron, S.; Vermeulen, J.T.; de Jonge, N.; Lahpor, J.R. Fractionated electrograms in dilated cardiomyopathy: Origin and relation to abnormal conduction. J. Am. Coll. Cardiol. 1996, 27, 1071–1078. [Google Scholar] [CrossRef]
- Burstein, B.; Nattel, S. Atrial Fibrosis: Mechanisms and Clinical Relevance in Atrial Fibrillation. J. Am. Coll. Cardiol. 2008, 51, 802–809. [Google Scholar] [CrossRef]
- Yue, L.; Xie, J.; Nattel, S. Molecular determinants of cardiac fibroblast electrical function and therapeutic implications for atrial fibrillation. Cardiovasc Res. 2011, 89, 744–753. [Google Scholar] [CrossRef]
- Khan, R.; Sheppard, R. Fibrosis in heart disease: Understanding the role of transforming growth factor-beta in cardiomyopathy, valvular disease and arrhythmia. Immunology 2006, 118, 10–24. [Google Scholar] [CrossRef]
- Weber, K.T. Are myocardial fibrosis and diastolic dysfunction reversible in hypertensive heart disease? Congest. Heart Fail. 2005, 11, 322–324. [Google Scholar] [CrossRef]
- Kania, G.; Blyszczuk, P.; Eriksson, U. Mechanisms of cardiac fibrosis in inflammatory heart disease. Trends Cardiovasc. Med. 2009, 19, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Biernacka, A.; Frangogiannis, N.G. Aging and Cardiac Fibrosis. Aging Dis. 2011, 2, 158–173. [Google Scholar] [PubMed]
- Pellman, J.; Zhang, J.; Sheikh, F. Myocyte-fibroblast communication in cardiac fibrosis and arrhythmias: Mechanisms and model systems. J. Mol. Cell. Cardiol. 2016, 94, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Schelbert, E.B.; Diez, J.; Butler, J. Myocardial Interstitial Fibrosis in Heart Failure: Biological and Translational Perspectives. J. Am. Coll. Cardiol. 2018, 71, 1696–1706. [Google Scholar] [CrossRef] [PubMed]
- Segura, A.M.; Frazier, O.H.; Buja, L.M. Fibrosis and heart failure. Heart Fail. Rev. 2014, 19, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Kiani, M.F.; Postlethwaite, A.E.; Weber, K.T. Infarct scar as living tissue. Basic Res. Cardiol. 2002, 97, 343–347. [Google Scholar] [CrossRef]
- Marijianowski, M.M.; Teeling, P.; Mann, J.; Becker, A.E. Dilated cardiomyopathy is associated with an increase in the type I/type III collagen ratio: A quantitative assessment. J. Am. Coll. Cardiol. 1995, 25, 1263–1272. [Google Scholar] [CrossRef]
- Sanderson, J.E.; Lai, K.B.; Shum, I.O.; Wei, S.; Chow, L.T. Transforming growth factor-beta(1) expression in dilated cardiomyopathy. Heart 2001, 86, 701–708. [Google Scholar] [CrossRef]
- Kuhl, U.; Noutsias, M.; Schultheiss, H.P. Immunohistochemistry in dilated cardiomyopathy. Eur. Heart J. 1995, 16 (Suppl. O), 100–106. [Google Scholar] [CrossRef]
- Dixon, I.M.; Hao, J.; Reid, N.L.; Roth, J.C. Effect of chronic AT(1) receptor blockade on cardiac Smad overexpression in hereditary cardiomyopathic hamsters. Cardiovasc. Res. 2000, 46, 286–297. [Google Scholar] [CrossRef]
- Pearlman, E.S.; Weber, K.T.; Janicki, J.S.; Pietra, G.G.; Fishman, A.P. Muscle fiber orientation and connective tissue content in the hypertrophied human heart. Lab. Investig. J. Tech. Methods Pathol. 1982, 46, 158–164. [Google Scholar]
- Brilla, C.G. Aldosterone and myocardial fibrosis in heart failure. Herz 2000, 25, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Bacmeister, L.; Schwarzl, M.; Warnke, S.; Stoffers, B.; Blankenberg, S.; Westermann, D.; Lindner, D. Inflammation and fibrosis in murine models of heart failure. Basic Res. Cardiol. 2019, 114, 19. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, B.; Liu, T.; Chen, X. Reversal of myofibroblast differentiation: A review. Eur. J. Pharmacol. 2014, 734, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Petrov, V.V.; Fagard, R.H.; Lijnen, P.J. Stimulation of Collagen Production by Transforming Growth Factor-β1 During Differentiation of Cardiac Fibroblasts to Myofibroblasts. Hypertension 2002, 39, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Porter, K.E.; Turner, N.A. Cardiac fibroblasts: At the heart of myocardial remodeling. Pharmacol. Ther. 2009, 123, 255–278. [Google Scholar] [CrossRef] [PubMed]
- Long, C.S.; Brown, R.D. The cardiac fibroblast, another therapeutic target for mending the broken heart? J. Mol. Cell. Cardiol. 2002, 34, 1273–1278. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.T.; Brilla, C.G. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin- angiotensin-aldosterone system. Circulation 1991, 83, 1849–1865. [Google Scholar] [CrossRef]
- Brilla, C.G.; Janicki, J.S.; Weber, K.T. Cardioreparative effects of lisinopril in rats with genetic hypertension and left ventricular hypertrophy. Circulation 1991, 83, 1771–1779. [Google Scholar] [CrossRef]
- Brilla, C.G.; Pick, R.; Tan, L.B.; Janicki, J.S.; Weber, K.T. Remodeling of the rat right and left ventricles in experimental hypertension. Circ. Res. 1990, 67, 1355–1364. [Google Scholar] [CrossRef]
- Smits, J.F.; Cleutjens, J.P.; van Krimpen, C.; Schoemaker, R.G.; Daemen, M.J. Cardiac remodeling in hypertension and following myocardial infarction: Effects of arteriolar vasodilators. Basic Res. Cardiol. 1991, 86, 133–139. [Google Scholar] [PubMed]
- Weber, K.T.; Sun, Y.; Tyagi, S.C.; Cleutjens, J.P. Collagen network of the myocardium: Function, structural remodeling and regulatory mechanisms. J. Mol. Cell. Cardiol. 1994, 26, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Swynghedauw, B. Molecular mechanisms of myocardial remodeling. Physiol. Rev. 1999, 79, 215–262. [Google Scholar] [CrossRef] [PubMed]
- Leask, A. Getting to the heart of the matter: New insights into cardiac fibrosis. Circulation Res. 2015, 116, 1269–1276. [Google Scholar] [CrossRef]
- Yue, Z.; Zhang, Y.; Xie, J.; Jiang, J.; Yue, L. Transient receptor potential (TRP) channels and cardiac fibrosis. Curr. Top. Med. Chem. 2013, 13, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Xie, J.; Zhang, Z.; Tsujikawa, H.; Fusco, D.; Silverman, D.; Liang, B.; Yue, L. TRPM7-mediated Ca2+ signals confer fibrogenesis in human atrial fibrillation. Circ. Res. 2010, 106, 992–1003. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.L.; Matera, D.; Doerner, J.F.; Zheng, N.; Del Camino, D.; Mishra, S.; Bian, H.; Zeveleva, S.; Zhen, X.; Blair, N.T.; et al. In vivo selective inhibition of TRPC6 by antagonist BI 749327 ameliorates fibrosis and dysfunction in cardiac and renal disease. Proc. Natl. Acad. Sci. USA 2019, 116, 10156–10161. [Google Scholar] [CrossRef] [PubMed]
- Adapala, R.K.; Thoppil, R.J.; Luther, D.J.; Paruchuri, S.; Meszaros, J.G.; Chilian, W.M.; Thodeti, C.K. TRPV4 channels mediate cardiac fibroblast differentiation by integrating mechanical and soluble signals. J. Mol. Cell. Cardiol. 2013, 54, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Kiseleva, I.S.; Kamkin, A.G.; Kirkheis, R.; Kositskii, G.I. Intercellular electrotonic interactions in the cardiac sinus node in the frog. Doklady Akademii Nauk SSSR 1987, 292, 1502–1505. [Google Scholar] [PubMed]
- Kamkin, A.; Kiseleva, I.; Wagner, K.D.; Pylaev, A.; Leiterer, K.P.; Theres, H.; Scholz, H.; Gunther, J.; Isenberg, G. A possible role for atrial fibroblasts in postinfarction bradycardia. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H842–H849. [Google Scholar] [CrossRef]
- Kamkin, A.; Kiseleva, I.; Wagner, K.D.; Lammerich, A.; Bohm, J.; Persson, P.B.; Gunther, J. Mechanically induced potentials in fibroblasts from human right atrium. Exp. Physiol. 1999, 84, 347–356. [Google Scholar] [CrossRef]
- Kamkin, A.; Kiseleva, I.; Isenberg, G. Activation and inactivation of a non-selective cation conductance by local mechanical deformation of acutely isolated cardiac fibroblasts. Cardiovasc. Res. 2003, 57, 793–803. [Google Scholar] [CrossRef]
- Aguilar, M.; Qi, X.Y.; Huang, H.; Comtois, P.; Nattel, S. Fibroblast electrical remodeling in heart failure and potential effects on atrial fibrillation. Biophys. J. 2014, 107, 2444–2455. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kohl, P.; Noble, D. Mechanosensitive connective tissue: Potential influence on heart rhythm. Cardiovasc. Res. 1996, 32, 62–68. [Google Scholar] [CrossRef]
- Chen, J.B.; Tao, R.; Sun, H.Y.; Tse, H.F.; Lau, C.P.; Li, G.R. Multiple Ca2+ signaling pathways regulate intracellular Ca2+ activity in human cardiac fibroblasts. J. Cell. Physiol. 2010, 223, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.B.; Zhang, J. Neonatal rat cardiac fibroblasts express three types of voltage-gated K+ channels: Regulation of a transient outward current by protein kinase C. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1010–H1017. [Google Scholar] [CrossRef]
- Shibukawa, Y.; Chilton, E.L.; Maccannell, K.A.; Clark, R.B.; Giles, W.R. K+ currents activated by depolarization in cardiac fibroblasts. Biophys. J. 2005, 88, 3924–3935. [Google Scholar] [CrossRef]
- Dawson, K.; Wu, C.T.; Qi, X.Y.; Nattel, S. Congestive heart failure effects on atrial fibroblast phenotype: Differences between freshly-isolated and cultured cells. PLoS ONE 2012, 7, e52032. [Google Scholar] [CrossRef]
- Wu, C.T.; Qi, X.Y.; Huang, H.; Naud, P.; Dawson, K.; Yeh, Y.H.; Harada, M.; Kuo, C.T.; Nattel, S. Disease and region-related cardiac fibroblast potassium current variations and potential functional significance. Cardiovasc. Res. 2014, 102, 487–496. [Google Scholar] [CrossRef][Green Version]
- Li, G.R.; Sun, H.Y.; Chen, J.B.; Zhou, Y.; Tse, H.F.; Lau, C.P. Characterization of multiple ion channels in cultured human cardiac fibroblasts. PLoS ONE 2009, 4, e7307. [Google Scholar] [CrossRef]
- Chilton, L.; Ohya, S.; Freed, D.; George, E.; Drobic, V.; Shibukawa, Y.; MacCannell, K.A.; Imaizumi, Y.; Clark, R.B.; Dixon, I.M.C.; et al. K+ currents regulate the resting membrane potential, proliferation, and contractile responses in ventricular fibroblasts and myofibroblasts. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H2931–H2939. [Google Scholar] [CrossRef]
- Qi, X.Y.; Huang, H.; Ordog, B.; Luo, X.; Naud, P.; Sun, Y.; Wu, C.T.; Dawson, K.; Tadevosyan, A.; Chen, Y.; et al. Fibroblast inward-rectifier potassium current upregulation in profibrillatory atrial remodeling. Circ. Res. 2015, 116, 836–845. [Google Scholar] [CrossRef]
- Benamer, N.; Moha Ou Maati, H.; Demolombe, S.; Cantereau, A.; Delwail, A.; Bois, P.; Bescond, J.; Faivre, J.F. Molecular and functional characterization of a new potassium conductance in mouse ventricular fibroblasts. J. Mol. Cell. Cardiol. 2009, 46, 508–517. [Google Scholar] [CrossRef]
- Pertiwi, K.R.; Hillman, R.M.; Scott, C.A.; Chilton, E.L. Ischemia Reperfusion Injury Produces, and Ischemic Preconditioning Prevents, Rat Cardiac Fibroblast Differentiation: Role of KATP Channels. J. Cardiovasc. Dev. Dis. 2019, 6. [Google Scholar] [CrossRef]
- Benamer, N.; Vasquez, C.; Mahoney, V.M.; Steinhardt, M.J.; Coetzee, W.A.; Morley, G.E. Fibroblast KATP currents modulate myocyte electrophysiology in infarcted hearts. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1231–H1239. [Google Scholar] [CrossRef]
- Wang, Y.J.; Sung, R.J.; Lin, M.W.; Wu, S.N. Contribution of BK(Ca)-channel activity in human cardiac fibroblasts to electrical coupling of cardiomyocytes-fibroblasts. J. Membr. Biol. 2006, 213, 175–185. [Google Scholar] [CrossRef]
- He, M.L.; Liu, W.J.; Sun, H.Y.; Wu, W.; Liu, J.; Tse, H.F.; Lau, C.P.; Li, G.R. Effects of ion channels on proliferation in cultured human cardiac fibroblasts. J. Mol. Cell. Cardiol. 2011, 51, 198–206. [Google Scholar] [CrossRef]
- Takahashi, K.; Sakamoto, K.; Kimura, J. Hypoxic stress induces transient receptor potential melastatin 2 (TRPM2) channel expression in adult rat cardiac fibroblasts. J. Pharmacol. Sci. 2012, 118, 186–197. [Google Scholar] [CrossRef]
- Stockbridge, L.L.; French, A.S. Stretch-activated cation channels in human fibroblasts. Biophys. J. 1988, 54, 187–190. [Google Scholar] [CrossRef]
- Chatelier, A.; Mercier, A.; Tremblier, B.; Theriault, O.; Moubarak, M.; Benamer, N.; Corbi, P.; Bois, P.; Chahine, M.; Faivre, J.F. A distinct de novo expression of Nav1.5 sodium channels in human atrial fibroblasts differentiated into myofibroblasts. J. Physiol. 2012, 590, 4307–4319. [Google Scholar] [CrossRef]
- Koivumaki, J.T.; Clark, R.B.; Belke, D.; Kondo, C.; Fedak, P.W.; Maleckar, M.M.; Giles, W.R. Na(+) current expression in human atrial myofibroblasts: Identity and functional roles. Front. Physiol. 2014, 5, 275. [Google Scholar] [CrossRef][Green Version]
- Poulet, C.; Kunzel, S.; Buttner, E.; Lindner, D.; Westermann, D.; Ravens, U. Altered physiological functions and ion currents in atrial fibroblasts from patients with chronic atrial fibrillation. Physiol. Rep. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Rook, M.B.; van Ginneken, A.C.; de Jonge, B.; el Aoumari, A.; Gros, D.; Jongsma, H.J. Differences in gap junction channels between cardiac myocytes, fibroblasts, and heterologous pairs. Am. J. Physiol. Cell Physiol. 1992, 263, C959–C977. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Fujiwara, Y.; Fujiwara, R.; Hasegawa, H.; Kibira, S.; Miura, H.; Miura, M. Role of augmented expression of intermediate-conductance Ca2+-activated K+ channels in postischaemic heart. Clin. Exp. Pharmacol. Physiol. 2002, 29, 324–329. [Google Scholar] [CrossRef] [PubMed]
- El Chemaly, A.; Guinamard, R.; Demion, M.; Fares, N.; Jebara, V.; Faivre, J.F.; Bois, P. A voltage-activated proton current in human cardiac fibroblasts. Biochem. Biophys. Res. Commun. 2006, 340, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Mohis, M.; Edwards, S.; Ryan, S.; Rizvi, F.; Tajik, A.J.; Jahangir, A.; Ross, G.R. Aging-related increase in store-operated Ca(2+) influx in human ventricular fibroblasts. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H83–H91. [Google Scholar] [CrossRef] [PubMed]
- Ross, G.R.; Bajwa, T., Jr.; Edwards, S.; Emelyanova, L.; Rizvi, F.; Holmuhamedov, E.L.; Werner, P.; Downey, F.X.; Tajik, A.J.; Jahangir, A. Enhanced store-operated Ca(2+) influx and ORAI1 expression in ventricular fibroblasts from human failing heart. Biol. Open 2017, 6, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Rose, R.A.; Hatano, N.; Ohya, S.; Imaizumi, Y.; Giles, W.R. C-type natriuretic peptide activates a non-selective cation current in acutely isolated rat cardiac fibroblasts via natriuretic peptide C receptor-mediated signalling. J. Physiol. 2007, 580, 255–274. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.; Luo, X.; Qi, X.Y.; Tadevosyan, A.; Maguy, A.; Ordog, B.; Ledoux, J.; Kato, T.; Naud, P.; Voigt, N.; et al. Transient receptor potential canonical-3 channel-dependent fibroblast regulation in atrial fibrillation. Circulation 2012, 126, 2051–2064. [Google Scholar] [CrossRef] [PubMed]
- Hatano, N.; Itoh, Y.; Muraki, K. Cardiac fibroblasts have functional TRPV4 activated by 4alpha-phorbol 12,13-didecanoate. Life Sci. 2009, 85, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Runnels, L.W.; Yue, L.; Clapham, D.E. The TRPM7 channel is inactivated by PIP(2) hydrolysis. Nat. Cell Biol. 2002, 4, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Oguri, G.; Nakajima, T.; Yamamoto, Y.; Takano, N.; Tanaka, T.; Kikuchi, H.; Morita, T.; Nakamura, F.; Yamasoba, T.; Komuro, I. Effects of methylglyoxal on human cardiac fibroblast: Roles of transient receptor potential ankyrin 1 (TRPA1) channels. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H1339–H1352. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, C.; Benamer, N.; Morley, G.E. The cardiac fibroblast: Functional and electrophysiological considerations in healthy and diseased hearts. J. Cardiovasc. Pharmacol. 2011, 57, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Ongstad, E.; Kohl, P. Fibroblast-myocyte coupling in the heart: Potential relevance for therapeutic interventions. J. Mol. Cell. Cardiol. 2016, 91, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Oh-hora, M.; Rao, A. Calcium signaling in lymphocytes. Curr. Opin. Immunol. 2008, 20, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E. Calcium signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Nowycky, M.C.; Thomas, A.P. Intracellular calcium signaling. J. Cell Sci. 2002, 115, 3715–3716. [Google Scholar] [CrossRef]
- Lee, H.C.; Aarhus, R. A derivative of NADP mobilizes calcium stores insensitive to inositol trisphosphate and cyclic ADP-ribose. J. Biol. Chem. 1995, 270, 2152–2157. [Google Scholar] [CrossRef]
- Hohenegger, M.; Suko, J.; Gscheidlinger, R.; Drobny, H.; Zidar, A. Nicotinic acid-adenine dinucleotide phosphate activates the skeletal muscle ryanodine receptor. Biochem. J. 2002, 367, 423–431. [Google Scholar] [CrossRef]
- Calcraft, P.J.; Ruas, M.; Pan, Z.; Cheng, X.; Arredouani, A.; Hao, X.; Tang, J.; Rietdorf, K.; Teboul, L.; Chuang, K.T.; et al. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature 2009, 459, 596–600. [Google Scholar] [CrossRef]
- Brailoiu, E.; Churamani, D.; Cai, X.; Schrlau, M.G.; Brailoiu, G.C.; Gao, X.; Hooper, R.; Boulware, M.J.; Dun, N.J.; Marchant, J.S.; et al. Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J. Cell Biol. 2009, 186, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.; Chuang, C.C.; Lewis, A.M.; Aley, P.K.; Brailoiu, E.; Dun, N.J.; Churchill, G.C.; Patel, S. Recruitment of NAADP-sensitive acidic Ca2+ stores by glutamate. Biochem. J. 2009, 422, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Ruas, M.; Rietdorf, K.; Arredouani, A.; Davis, L.C.; Lloyd-Evans, E.; Koegel, H.; Funnell, T.M.; Morgan, A.J.; Ward, J.A.; Watanabe, K.; et al. Purified TPC isoforms form NAADP receptors with distinct roles for Ca(2+) signaling and endolysosomal trafficking. Curr. Biol. CB 2010, 20, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Guse, A.H.; Wolf, I.M. Ca(2+) microdomains, NAADP and type 1 ryanodine receptor in cell activation. Biochimica et Biophysica Acta 2016, 1863, 1379–1384. [Google Scholar] [CrossRef] [PubMed]
- Kiseleva, I.; Kamkin, A.; Kohl, P.; Lab, M.J. Calcium and mechanically induced potentials in fibroblasts of rat atrium. Cardiovasc. Res. 1996, 32, 98–111. [Google Scholar] [CrossRef]
- Ding, Z.; Yuan, J.; Liang, Y.; Wu, J.; Gong, H.; Ye, Y.; Jiang, G.; Yin, P.; Li, Y.; Zhang, G.; et al. Ryanodine Receptor Type 2 Plays a Role in the Development of Cardiac Fibrosis under Mechanical Stretch Through TGFbeta-1. Int. Heart J. 2017, 58, 957–961. [Google Scholar] [CrossRef] [PubMed]
- Higashida, H.; Egorova, A.; Higashida, C.; Zhong, Z.G.; Yokoyama, S.; Noda, M.; Zhang, J.S. Sympathetic potentiation of cyclic ADP-ribose formation in rat cardiac myocytes. J. Biol. Chem. 1999, 274, 33348–33354. [Google Scholar] [CrossRef] [PubMed]
- Gul, R.; Park, D.R.; Shawl, A.I.; Im, S.Y.; Nam, T.S.; Lee, S.H.; Ko, J.K.; Jang, K.Y.; Kim, D.; Kim, U.H. Nicotinic Acid Adenine Dinucleotide Phosphate (NAADP) and Cyclic ADP-Ribose (cADPR) Mediate Ca2+ Signaling in Cardiac Hypertrophy Induced by beta-Adrenergic Stimulation. PLoS ONE 2016, 11, e0149125. [Google Scholar] [CrossRef]
- Macgregor, A.; Yamasaki, M.; Rakovic, S.; Sanders, L.; Parkesh, R.; Churchill, G.C.; Galione, A.; Terrar, D.A. NAADP controls cross-talk between distinct Ca2+ stores in the heart. J. Biol. Chem. 2007, 282, 15302–15311. [Google Scholar] [CrossRef]
- Macgregor, A.T.; Rakovic, S.; Galione, A.; Terrar, D.A. Dual effects of cyclic ADP-ribose on sarcoplasmic reticulum Ca2+ release and storage in cardiac myocytes isolated from guinea-pig and rat ventricle. Cell Calcium 2007, 41, 537–546. [Google Scholar] [CrossRef]
- Snead, A.N.; Insel, P.A. Defining the cellular repertoire of GPCRs identifies a profibrotic role for the most highly expressed receptor, protease-activated receptor 1, in cardiac fibroblasts. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2012, 26, 4540–4547. [Google Scholar] [CrossRef] [PubMed]
- Brilla, C.G.; Scheer, C.; Rupp, H. Angiotensin II and intracellular calcium of adult cardiac fibroblasts. J. Mol. Cell. Cardiol. 1998, 30, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Meszaros, J.G.; Gonzalez, A.M.; Endo-Mochizuki, Y.; Villegas, S.; Villarreal, F.; Brunton, L.L. Identification of G protein-coupled signaling pathways in cardiac fibroblasts: Cross talk between G(q) and G(s). Am. J. Physiol. Cell Physiol. 2000, 278, C154–C162. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ramires, F.J.; Zhou, G.; Ganjam, V.K.; Weber, K.T. Fibrous tissue and angiotensin II. J. Mol. Cell. Cardiol. 1997, 29, 2001–2012. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.T.; Sun, Y.; Bhattacharya, S.K.; Ahokas, R.A.; Gerling, I.C. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat. Rev. Cardiol. 2013, 10, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Bomb, R.; Heckle, M.R.; Sun, Y.; Mancarella, S.; Guntaka, R.V.; Gerling, I.C.; Weber, K.T. Myofibroblast secretome and its auto-/paracrine signaling. Expert Rev. Cardiovasc. Ther. 2016, 14, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Rodriguez, C.; Fernandez, S.; Osorio, J.M.; Olivares, F.; Anfossi, R.; Bolivar, S.; Humeres, C.; Boza, P.; Vivar, R.; Pardo-Jimenez, V.; et al. Expression and function of TLR4- induced B1R bradykinin receptor on cardiac fibroblasts. Toxicol. Appl. Pharmacol. 2018, 351, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Catalan, M.; Aranguiz, P.; Boza, P.; Olmedo, I.; Humeres, C.; Vivar, R.; Anfossi, R.; Ayala, P.; Espinoza, C.; Lavandero, S.; et al. TGF-beta1 induced up-regulation of B1 kinin receptor promotes antifibrotic activity in rat cardiac myofibroblasts. Mol. Biol. Rep. 2019. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Insel, P.A. Cellular mechanisms of tissue fibrosis. 6. Purinergic signaling and response in fibroblasts and tissue fibrosis. Am. J. Physiol. Cell Physiol. 2014, 306, C779–C788. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, E.A.; White, P.J.; May, L.T. Targeting Adenosine Receptors for the Treatment of Cardiac Fibrosis. Front. Pharmacol. 2017, 8, 243. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, E.A.; Chuo, C.H.; Baltos, J.A.; Ford, L.; Scammells, P.J.; Wang, B.H.; Christopoulos, A.; White, P.J.; May, L.T. The hybrid molecule, VCP746, is a potent adenosine A2B receptor agonist that stimulates anti-fibrotic signalling. Biochem. Pharmacol. 2016, 117, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.B.; Liu, W.J.; Che, H.; Liu, J.; Sun, H.Y.; Li, G.R. Adenosine-5'-triphosphate up-regulates proliferation of human cardiac fibroblasts. Br. J. Pharmacol. 2012, 166, 1140–1150. [Google Scholar] [CrossRef] [PubMed]
- Antonioli, L.; Blandizzi, C.; Pacher, P.; Hasko, G. The Purinergic System as a Pharmacological Target for the Treatment of Immune-Mediated Inflammatory Diseases. Pharmacol. Rev. 2019, 71, 345–382. [Google Scholar] [CrossRef] [PubMed]
- Talasila, A.; Germack, R.; Dickenson, J.M. Characterization of P2Y receptor subtypes functionally expressed on neonatal rat cardiac myofibroblasts. Br. J. Pharmacol. 2009, 158, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, T.; Wu, J.; Yu, X.; Zheng, D.; Yang, F.; Li, T.; Wang, L.; Zhao, Y.; Dong, S.; et al. Calcium sensing receptor promotes cardiac fibroblast proliferation and extracellular matrix secretion. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2014, 33, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Plummer, N.W.; George, M.D.; Abramowitz, J.; Zhu, M.X.; Birnbaumer, L. A role for Orai in TRPC-mediated Ca2+ entry suggests that a TRPC:Orai complex may mediate store and receptor operated Ca2+ entry. Proc. Natl. Acad. Sci. USA 2009, 106, 3202–3206. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Ayaub, E.A.; Murphy, J.; Lu, C.; Kolb, M.; Ask, K.; Janssen, L.J. Disruption of Calcium Signaling in Fibroblasts and Attenuation of Bleomycin-Induced Fibrosis by Nifedipine. Am. J. Respir. Cell Mol. Biol. 2015, 53, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Surprenant, A.; North, R.A. Signaling at purinergic P2X receptors. Annu. Rev. Physiol. 2009, 71, 333–359. [Google Scholar] [CrossRef] [PubMed]
- Solini, A.; Chiozzi, P.; Morelli, A.; Fellin, R.; Di Virgilio, F. Human primary fibroblasts in vitro express a purinergic P2X7 receptor coupled to ion fluxes, microvesicle formation and IL-6 release. J. Cell Sci. 1999, 112 Pt 3, 297–305. [Google Scholar]
- Gentile, D.; Natale, M.; Lazzerini, P.E.; Capecchi, P.L.; Laghi-Pasini, F. The role of P2X7 receptors in tissue fibrosis: A brief review. Purinergic Signal. 2015, 11, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Therkildsen, J.R.; Christensen, M.G.; Tingskov, S.J.; Wehmoller, J.; Norregaard, R.; Praetorius, H.A. Lack of P2X7 Receptors Protects against Renal Fibrosis after Pyelonephritis with alpha-Hemolysin-Producing Escherichia coli. Am. J. Pathol. 2019, 189, 1201–1211. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Liang, B.T. Cardiac P2X(4) receptors: Targets in ischemia and heart failure? Circ. Res. 2012, 111, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Shen, J.B.; Yang, R.; Redden, J.; Dodge-Kafka, K.; Grady, J.; Jacobson, K.A.; Liang, B.T. Novel protective role of endogenous cardiac myocyte P2X4 receptors in heart failure. Circ. Heart Fail. 2014, 7, 510–518. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Novitskaya, T.; Chepurko, E.; Covarrubias, R.; Novitskiy, S.; Ryzhov, S.V.; Feoktistov, I.; Gumina, R.J. Extracellular nucleotide regulation and signaling in cardiac fibrosis. J. Mol. Cell. Cardiol. 2016, 93, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.S. The molecular choreography of a store-operated calcium channel. Nature 2007, 446, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Prakriya, M.; Lewis, R.S. Store-operated calcium channels. Physiol. Rev. 2015, 95, 1383–1436. [Google Scholar] [CrossRef]
- Feske, S. CRAC channels and disease—From human CRAC channelopathies and animal models to novel drugs. Cell Calcium 2019, 80, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, P.; Katz, D.; Bryson, V. SOCE and STIM1 signaling in the heart: Timing and location matter. Cell Calcium 2019, 77, 20–28. [Google Scholar] [CrossRef]
- Voelkers, M.; Salz, M.; Herzog, N.; Frank, D.; Dolatabadi, N.; Frey, N.; Gude, N.; Friedrich, O.; Koch, W.J.; Katus, H.A.; et al. Orai1 and Stim1 regulate normal and hypertrophic growth in cardiomyocytes. J. Mol. Cell. Cardiol. 2010, 48, 1329–1334. [Google Scholar] [CrossRef]
- Correll, R.N.; Goonasekera, S.A.; van Berlo, J.H.; Burr, A.R.; Accornero, F.; Zhang, H.; Makarewich, C.A.; York, A.J.; Sargent, M.A.; Chen, X.; et al. STIM1 elevation in the heart results in aberrant Ca(2)(+) handling and cardiomyopathy. J. Mol. Cell. Cardiol. 2015, 87, 38–47. [Google Scholar] [CrossRef]
- Horton, J.S.; Buckley, C.L.; Alvarez, E.M.; Schorlemmer, A.; Stokes, A.J. The calcium release-activated calcium channel Orai1 represents a crucial component in hypertrophic compensation and the development of dilated cardiomyopathy. Channels 2014, 8, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, F.; Sabourin, J. Cardiac Remodeling and Disease: Current Understanding of STIM1/Orai1-Mediated Store-Operated Ca(2+) Entry in Cardiac Function and Pathology. Adv. Exp. Med. Biol. 2017, 993, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Jiang, J.; Yue, Z.; Liu, S.; Ma, Y.; Yu, N.; Gao, Y.; Sun, S.; Chen, S.; Liu, P. Store-Operated Ca(2+) Entry (SOCE) contributes to angiotensin II-induced cardiac fibrosis in cardiac fibroblasts. J. Pharmacol. Sci. 2016, 132, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Montell, C.; Birnbaumer, L.; Flockerzi, V. The TRP channels, a remarkably functional family. Cell 2002, 108, 595–598. [Google Scholar] [CrossRef]
- Clapham, D.E. TRP channels as cellular sensors. Nature 2003, 426, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Voets, T.; Peters, J. TRP Channels in Disease. Sci. STKE 2005, 2005, re8. [Google Scholar] [CrossRef] [PubMed]
- Story, G.M.; Peier, A.M.; Reeve, A.J.; Eid, S.R.; Mosbacher, J.; Hricik, T.R.; Earley, T.J.; Hergarden, A.C.; Andersson, D.A.; Hwang, S.W.; et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003, 112, 819–829. [Google Scholar] [CrossRef]
- Jordt, S.E.; Bautista, D.M.; Chuang, H.H.; McKemy, D.D.; Zygmunt, P.M.; Hogestatt, E.D.; Meng, I.D.; Julius, D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 2004, 427, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Caterina, M.J. Thermosensation and pain. J. Neurobiol. 2004, 61, 3–12. [Google Scholar] [CrossRef]
- Chubanov, V.; Mederos, Y.S.M.; Waring, J.; Plank, A.; Gudermann, T. Emerging roles of TRPM6/TRPM7 channel kinase signal transduction complexes. Naunyn-Schmiedeberg's Arch. Pharmacol. 2005, 371, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.P.; Wang, X.; Xu, H. TRP channels of intracellular membranes. J. Neurochem. 2010, 113, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Peng, J.B.; Hediger, M.A.; Clapham, D.E. CaT1 manifests the pore properties of the calcium-release-activated calcium channel. Nature 2001, 410, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Vennekens, R.; Prenen, J.; Hoenderop, J.G.; Droogmans, G.; Bindels, R.J. The single pore residue Asp542 determines Ca2+ permeation and Mg2+ block of the epithelial Ca2+ channel. J. Biol. Chem. 2001, 276, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Runnels, L.W.; Yue, L.; Clapham, D.E. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science 2001, 291, 1043–1047. [Google Scholar] [CrossRef]
- Li, M.; Jiang, J.; Yue, L. Functional Characterization of Homo- and Heteromeric Channel Kinases TRPM6 and TRPM7. J. Gen. Physiol. 2006, 127, 525–537. [Google Scholar] [CrossRef]
- Schlingmann, K.P.; Waldegger, S.; Konrad, M.; Chubanov, V.; Gudermann, T. TRPM6 and TRPM7--Gatekeepers of human magnesium metabolism. Biochimica Et Biophysica Acta 2007, 1772, 813–821. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Montell, C. TRP Channels. Annu. Rev. Biochem. 2007, 76, 387–417. [Google Scholar] [CrossRef]
- Zimmermann, K.; Lennerz, J.K.; Hein, A.; Link, A.S.; Kaczmarek, J.S.; Delling, M.; Uysal, S.; Pfeifer, J.D.; Riccio, A.; Clapham, D.E. Transient receptor potential cation channel, subfamily C, member 5 (TRPC5) is a cold-transducer in the peripheral nervous system. Proc. Natl. Acad. Sci. USA 2011, 108, 18114–18119. [Google Scholar] [CrossRef]
- Nilius, B. TRP channels in disease. Biochimica Et Biophysica Acta 2007, 1772, 805–812. [Google Scholar] [CrossRef]
- Vriens, J.; Owsianik, G.; Hofmann, T.; Philipp, S.E.; Stab, J.; Chen, X.; Benoit, M.; Xue, F.; Janssens, A.; Kerselaers, S.; et al. TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron 2011, 70, 482–494. [Google Scholar] [CrossRef]
- Zufall, F.; Ukhanov, K.; Lucas, P.; Liman, E.R.; Leinders-Zufall, T. Neurobiology of TRPC2: From gene to behavior. Pflug. Arch. Eur. J. Physiol. 2005, 451, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.; Obukhov, A.G.; Schaefer, M.; Harteneck, C.; Gudermann, T.; Schultz, G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 1999, 397, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Beck, B.; Zholos, A.; Sydorenko, V.; Roudbaraki, M.; Lehen’kyi, V.; Bordat, P.; Prevarskaya, N.; Skryma, R. TRPC7 is a receptor-operated DAG-activated channel in human keratinocytes. J. Investig. Dermatol. 2006, 126, 1982–1993. [Google Scholar] [CrossRef] [PubMed]
- Vangeel, L.; Voets, T. Transient Receptor Potential Channels and Calcium Signaling. Cold Spring Harb. Perspect. Biol. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.; Schaefer, M.; Schultz, G.; Gudermann, T. Subunit composition of mammalian transient receptor potential channels in living cells. Proc. Natl. Acad. Sci. USA 2002, 99, 7461–7466. [Google Scholar] [CrossRef] [PubMed]
- Nadler, M.J.; Hermosura, M.C.; Inabe, K.; Perraud, A.L.; Zhu, Q.; Stokes, A.J.; Kurosaki, T.; Kinet, J.P.; Penner, R.; Scharenberg, A.M.; et al. LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature 2001, 411, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Du, J.; Jiang, J.; Ratzan, W.; Su, L.-T.; Runnels, L.W.; Yue, L. Molecular Determinants of Mg2+ and Ca2+ Permeability and pH Sensitivity in TRPM6 and TRPM7. J. Biol. Chem. 2007, 282, 25817–25830. [Google Scholar] [CrossRef]
- Voets, T.; Nilius, B.; Hoefs, S.; van der Kemp, A.W.C.M.; Droogmans, G.; Bindels, R.J.M.; Hoenderop, J.G.J. TRPM6 Forms the Mg2+ Influx Channel Involved in Intestinal and Renal Mg2+ Absorption. J. Biol. Chem. 2004, 279, 19–25. [Google Scholar] [CrossRef]
- Launay, P.; Fleig, A.; Perraud, A.L.; Scharenberg, A.M.; Penner, R.; Kinet, J.P. TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell 2002, 109, 397–407. [Google Scholar] [CrossRef]
- Prawitt, D.; Monteilh-Zoller, M.K.; Brixel, L.; Spangenberg, C.; Zabel, B.; Fleig, A.; Penner, R. TRPM5 is a transient Ca2+-activated cation channel responding to rapid changes in [Ca2+]i. Proc. Natl. Acad. Sci. USA 2003, 100, 15166–15171. [Google Scholar] [CrossRef]
- Liu, D.; Liman, E.R. Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc. Natl. Acad. Sci. USA 2003, 100, 15160–15165. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Xie, J.; Yue, L. Intracellular calcium activates TRPM2 and its alternative spliced isoforms. Proc. Natl. Acad. Sci. USA 2009, 107, 7239–7244. [Google Scholar] [CrossRef] [PubMed]
- Zurborg, S.; Yurgionas, B.; Jira, J.A.; Caspani, O.; Heppenstall, P.A. Direct activation of the ion channel TRPA1 by Ca2+. Nat. Neurosci. 2007, 10, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Wakamori, M.; Ishii, M.; Maeno, E.; Nishida, M.; Yoshida, T.; Yamada, H.; Shimizu, S.; Mori, E.; Kudoh, J.; et al. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol. Cell 2002, 9, 163–173. [Google Scholar] [CrossRef]
- Perraud, A.L.; Fleig, A.; Dunn, C.A.; Bagley, L.A.; Launay, P.; Schmitz, C.; Stokes, A.J.; Zhu, Q.; Bessman, M.J.; Penner, R.; et al. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature 2001, 411, 595–599. [Google Scholar] [CrossRef]
- Sano, Y.; Inamura, K.; Miyake, A.; Mochizuki, S.; Yokoi, H.; Matsushime, H.; Furuichi, K. Immunocyte Ca2+ influx system mediated by LTRPC2. Science 2001, 293, 1327–1330. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.; Xie, J.; Yu, A.S.; Stock, J.; Du, J.; Yue, L. Role of TRP channels in the cardiovascular system. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H157–H182. [Google Scholar] [CrossRef]
- Nishida, M.; Onohara, N.; Sato, Y.; Suda, R.; Ogushi, M.; Tanabe, S.; Inoue, R.; Mori, Y.; Kurose, H. Galpha12/13-mediated up-regulation of TRPC6 negatively regulates endothelin-1-induced cardiac myofibroblast formation and collagen synthesis through nuclear factor of activated T cells activation. J. Biol. Chem. 2007, 282, 23117–23128. [Google Scholar] [CrossRef]
- Xie, J.; Sun, B.; Du, J.; Yang, W.; Chen, H.-C.; Overton, J.D.; Runnels, L.W.; Yue, L. Phosphatidylinositol 4, 5-Bisphosphate (PIP2) Controls Magnesium Gatekeeper TRPM6 Activity. Biophys. J. 2012, 102, 24a. [Google Scholar] [CrossRef]
- Chen, H.C.; Xie, J.; Zhang, Z.; Su, L.T.; Yue, L.; Runnels, L.W. Blockade of TRPM7 channel activity and cell death by inhibitors of 5-lipoxygenase. PLoS ONE 2010, 5, e11161. [Google Scholar] [CrossRef]
- Falcon, D.; Galeano-Otero, I.; Calderon-Sanchez, E.; Del Toro, R.; Martin-Bornez, M.; Rosado, J.A.; Hmadcha, A.; Smani, T. TRP Channels: Current Perspectives in the Adverse Cardiac Remodeling. Front. Physiol. 2019, 10, 159. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.; Burr, A.R.; Davis, G.F.; Birnbaumer, L.; Molkentin, J.D. A TRPC6-dependent pathway for myofibroblast transdifferentiation and wound healing in vivo. Dev. Cell 2012, 23, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Freichel, M.; Berlin, M.; Schurger, A.; Mathar, I.; Bacmeister, L.; Medert, R.; Frede, W.; Marx, A.; Segin, S.; Londono, J.E.C. TRP Channels in the Heart. In Neurobiology of TRP Channels, 2nd ed.; Emir, T.L.R., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 149–185. [Google Scholar]
- Kiyonaka, S.; Kato, K.; Nishida, M.; Mio, K.; Numaga, T.; Sawaguchi, Y.; Yoshida, T.; Wakamori, M.; Mori, E.; Numata, T.; et al. Selective and direct inhibition of TRPC3 channels underlies biological activities of a pyrazole compound. Proc. Natl. Acad. Sci. USA 2009, 106, 5400–5405. [Google Scholar] [CrossRef] [PubMed]
- Numaga-Tomita, T.; Oda, S.; Shimauchi, T.; Nishimura, A.; Mangmool, S.; Nishida, M. TRPC3 Channels in Cardiac Fibrosis. Front. Cardiovasc. Med. 2017, 4, 56. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, N.; Numaga-Tomita, T.; Watanabe, M.; Kuroda, T.; Nishimura, A.; Miyano, K.; Yasuda, S.; Kuwahara, K.; Sato, Y.; Ide, T.; et al. TRPC3 positively regulates reactive oxygen species driving maladaptive cardiac remodeling. Sci. Rep. 2016, 6, 37001. [Google Scholar] [CrossRef] [PubMed]
- Numaga-Tomita, T.; Kitajima, N.; Kuroda, T.; Nishimura, A.; Miyano, K.; Yasuda, S.; Kuwahara, K.; Sato, Y.; Ide, T.; Birnbaumer, L.; et al. TRPC3-GEF-H1 axis mediates pressure overload-induced cardiac fibrosis. Sci. Rep. 2016, 6, 39383. [Google Scholar] [CrossRef] [PubMed]
- Saliba, Y.; Karam, R.; Smayra, V.; Aftimos, G.; Abramowitz, J.; Birnbaumer, L.; Fares, N. Evidence of a Role for Fibroblast Transient Receptor Potential Canonical 3 Ca2+ Channel in Renal Fibrosis. J. Am. Soc. Nephrol. 2015, 26, 1855–1876. [Google Scholar] [CrossRef]
- Ikeda, K.; Nakajima, T.; Yamamoto, Y.; Takano, N.; Tanaka, T.; Kikuchi, H.; Oguri, G.; Morita, T.; Nakamura, F.; Komuro, I. Roles of transient receptor potential canonical (TRPC) channels and reverse-mode Na+/Ca2+ exchanger on cell proliferation in human cardiac fibroblasts: Effects of transforming growth factor beta1. Cell Calcium 2013, 54, 213–225. [Google Scholar] [CrossRef]
- Kapur, N.K.; Qiao, X.; Paruchuri, V.; Mackey, E.E.; Daly, G.H.; Ughreja, K.; Morine, K.J.; Levine, J.; Aronovitz, M.J.; Hill, N.S.; et al. Reducing endoglin activity limits calcineurin and TRPC-6 expression and improves survival in a mouse model of right ventricular pressure overload. J. Am. Heart Assoc. 2014, 3. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, S.; Li, D.; Zhang, Y.; Tang, B.; Qiu, C.; Yang, Y.; Yang, D. Dietary capsaicin ameliorates pressure overload-induced cardiac hypertrophy and fibrosis through the transient receptor potential vanilloid type 1. Am. J. Hypertens. 2014, 27, 1521–1529. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Li, D.; Zhang, Y.; Tang, B.; Li, G.; Yang, Y.; Yang, D. Transgenic overexpression of transient receptor potential vanilloid subtype 1 attenuates isoproterenol-induced myocardial fibrosis in mice. Int. J. Mol. Med. 2016, 38, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Sexton, A.; McDonald, M.; Cayla, C.; Thiemermann, C.; Ahluwalia, A. 12-Lipoxygenase-derived eicosanoids protect against myocardial ischemia/reperfusion injury via activation of neuronal TRPV1. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2007, 21, 2695–2703. [Google Scholar] [CrossRef]
- Wang, L.; Wang, D.H. TRPV1 gene knockout impairs postischemic recovery in isolated perfused heart in mice. Circulation 2005, 112, 3617–3623. [Google Scholar] [CrossRef] [PubMed]
- Zhong, B.; Wang, D.H. TRPV1 gene knockout impairs preconditioning protection against myocardial injury in isolated perfused hearts in mice. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H1791–H1798. [Google Scholar] [CrossRef] [PubMed]
- Iwata, Y.; Ohtake, H.; Suzuki, O.; Matsuda, J.; Komamura, K.; Wakabayashi, S. Blockade of sarcolemmal TRPV2 accumulation inhibits progression of dilated cardiomyopathy. Cardiovasc. Res. 2013, 99, 760–768. [Google Scholar] [CrossRef]
- Koch, S.E.; Mann, A.; Jones, S.; Robbins, N.; Alkhattabi, A.; Worley, M.C.; Gao, X.; Lasko-Roiniotis, V.M.; Karani, R.; Fulford, L.; et al. Transient receptor potential vanilloid 2 function regulates cardiac hypertrophy via stretch-induced activation. J. Hypertens. 2017, 35, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.E.; Nieman, M.L.; Robbins, N.; Slone, S.; Worley, M.; Green, L.C.; Chen, Y.; Barlow, A.; Tranter, M.; Wang, H.; et al. Tranilast Blunts the Hypertrophic and Fibrotic Response to Increased Afterload Independent of Cardiomyocyte Transient Receptor Potential Vanilloid 2 Channels. J. Cardiovasc. Pharmacol. 2018, 72, 40–48. [Google Scholar] [CrossRef]
- Entin-Meer, M.; Cohen, L.; Hertzberg-Bigelman, E.; Levy, R.; Ben-Shoshan, J.; Keren, G. TRPV2 knockout mice demonstrate an improved cardiac performance following myocardial infarction due to attenuated activity of peri-infarct macrophages. PLoS ONE 2017, 12, e0177132. [Google Scholar] [CrossRef]
- Zhang, Q.; Qi, H.; Cao, Y.; Shi, P.; Song, C.; Ba, L.; Chen, Y.; Gao, J.; Li, S.; Li, B.; et al. Activation of transient receptor potential vanilloid 3 channel (TRPV3) aggravated pathological cardiac hypertrophy via calcineurin/NFATc3 pathway in rats. J. Cell. Mol. Med. 2018, 22, 6055–6067. [Google Scholar] [CrossRef]
- Liu, Y.; Qi, H.; E, M.; Shi, P.; Zhang, Q.; Li, S.; Wang, Y.; Cao, Y.; Chen, Y.; Ba, L.; et al. Transient receptor potential vanilloid-3 (TRPV3) activation plays a central role in cardiac fibrosis induced by pressure overload in rats via TGF-beta1 pathway. Naunyn-Schmiedeberg's Arch. Pharmacol. 2018, 391, 131–143. [Google Scholar] [CrossRef]
- Thodeti, C.K.; Paruchuri, S.; Meszaros, J.G. A TRP to cardiac fibroblast differentiation. Channels 2013, 7, 211–214. [Google Scholar] [CrossRef]
- Adapala, R.K.; Minasyan, A.; Kanugula, A.K.; Cappelli, H.C.; Paruchuri, S.; Meszaros, G.J.; Thodeti, C.K. Targeting Trpv4 Channels Protects Heart From Pathological Remodeling Following Myocardial Infarction. Circulation 2017, 136 (Suppl. 1), A24061. [Google Scholar]
- Miller, B.A.; Wang, J.; Hirschler-Laszkiewicz, I.; Gao, E.; Song, J.; Zhang, X.Q.; Koch, W.J.; Madesh, M.; Mallilankaraman, K.; Gu, T.; et al. The second member of transient receptor potential-melastatin channel family protects hearts from ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1010–H1022. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, N.E.; Miller, B.A.; Wang, J.; Elrod, J.W.; Rajan, S.; Gao, E.; Song, J.; Zhang, X.Q.; Hirschler-Laszkiewicz, I.; Shanmughapriya, S.; et al. Ca(2)(+) entry via Trpm2 is essential for cardiac myocyte bioenergetics maintenance. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H637–H650. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.T.; Chang, W.L.; Yang, P.C.; Chien, C.L.; Lai, M.S.; Su, M.J.; Wu, M.L. Activation of the transient receptor potential M2 channel and poly(ADP-ribose) polymerase is involved in oxidative stress-induced cardiomyocyte death. Cell Death Differ. 2006, 13, 1815–1826. [Google Scholar] [CrossRef] [PubMed]
- Hiroi, T.; Wajima, T.; Negoro, T.; Ishii, M.; Nakano, Y.; Kiuchi, Y.; Mori, Y.; Shimizu, S. Neutrophil TRPM2 channels are implicated in the exacerbation of myocardial ischaemia/reperfusion injury. Cardiovasc. Res. 2013, 97, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Sah, R.; Mesirca, P.; Mason, X.; Gibson, W.; Bates-Withers, C.; Van den Boogert, M.; Chaudhuri, D.; Pu, W.T.; Mangoni, M.E.; Clapham, D.E. Timing of myocardial trpm7 deletion during cardiogenesis variably disrupts adult ventricular function, conduction, and repolarization. Circulation 2013, 128, 101–114. [Google Scholar] [CrossRef]
- Sah, R.; Mesirca, P.; Van den Boogert, M.; Rosen, J.; Mably, J.; Mangoni, M.E.; Clapham, D.E. Ion channel-kinase TRPM7 is required for maintaining cardiac automaticity. Proc. Natl. Acad. Sci. USA 2013, 110, E3037–E3046. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Wang, T.; Lian, G.; Xu, C.; Wang, H.; Xie, L. TRPM7 regulates angiotensin II-induced sinoatrial node fibrosis in sick sinus syndrome rats by mediating Smad signaling. Heart Vessel. 2018, 33, 1094–1105. [Google Scholar] [CrossRef]
- Zhou, Y.; Yi, X.; Wang, T.; Li, M. Effects of angiotensin II on transient receptor potential melastatin 7 channel function in cardiac fibroblasts. Exp. Ther. Med. 2015, 9, 2008–2012. [Google Scholar] [CrossRef]
- Li, S.; Li, M.; Yi, X.; Guo, F.; Zhou, Y.; Chen, S.; Wu, X. TRPM7 channels mediate the functional changes in cardiac fibroblasts induced by angiotensin II. Int. J. Mol. Med. 2017, 39, 1291–1298. [Google Scholar] [CrossRef]
- Guo, J.L.; Yu, Y.; Jia, Y.Y.; Ma, Y.Z.; Zhang, B.Y.; Liu, P.Q.; Chen, S.R.; Jiang, J.M. Transient receptor potential melastatin 7 (TRPM7) contributes to H2O2-induced cardiac fibrosis via mediating Ca(2+) influx and extracellular signal-regulated kinase 1/2 (ERK1/2) activation in cardiac fibroblasts. J. Pharmacol. Sci. 2014, 125, 184–192. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, S.; Xiao, C.; Jia, Y.; Guo, J.; Jiang, J.; Liu, P. TRPM7 is involved in angiotensin II induced cardiac fibrosis development by mediating calcium and magnesium influx. Cell Calcium 2014, 55, 252–260. [Google Scholar] [CrossRef]
- Andrei, S.R.; Ghosh, M.; Sinharoy, P.; Dey, S.; Bratz, I.N.; Damron, D.S. TRPA1 ion channel stimulation enhances cardiomyocyte contractile function via a CaMKII-dependent pathway. Channels 2017, 11, 587–603. [Google Scholar] [CrossRef]
- Andrei, S.R.; Sinharoy, P.; Bratz, I.N.; Damron, D.S. TRPA1 is functionally co-expressed with TRPV1 in cardiac muscle: Co-localization at z-discs, costameres and intercalated discs. Channels 2016, 10, 395–409. [Google Scholar] [CrossRef]
- Li, S.; Sun, X.; Wu, H.; Yu, P.; Wang, X.; Jiang, Z.; Gao, E.; Chen, J.; Li, D.; Qiu, C.; et al. TRPA1 Promotes Cardiac Myofibroblast Transdifferentiation after Myocardial Infarction Injury via the Calcineurin-NFAT-DYRK1A Signaling Pathway. Oxidative Med. Cell. Longev. 2019, 2019, 6408352. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Y.; Wang, M.; Ye, J.; Liu, J.; Jiang, H.; Ye, D.; Wan, J. TRPA1 inhibition ameliorates pressure overload-induced cardiac hypertrophy and fibrosis in mice. EBioMedicine 2018, 36, 54–62. [Google Scholar] [CrossRef]
- Eder, P.; Molkentin, J.D. TRPC channels as effectors of cardiac hypertrophy. Circ. Res. 2011, 108, 265–272. [Google Scholar] [CrossRef]
- Camacho Londono, J.E.; Tian, Q.; Hammer, K.; Schroder, L.; Camacho Londono, J.; Reil, J.C.; He, T.; Oberhofer, M.; Mannebach, S.; Mathar, I.; et al. A background Ca2+ entry pathway mediated by TRPC1/TRPC4 is critical for development of pathological cardiac remodelling. Eur. Heart J. 2015, 36, 2257–2266. [Google Scholar] [CrossRef]
- Wu, X.; Eder, P.; Chang, B.; Molkentin, J.D. TRPC channels are necessary mediators of pathologic cardiac hypertrophy. Proc. Natl. Acad. Sci. USA 2010, 107, 7000–7005. [Google Scholar] [CrossRef]
- Seo, K.; Rainer, P.P.; Shalkey Hahn, V.; Lee, D.I.; Jo, S.H.; Andersen, A.; Liu, T.; Xu, X.; Willette, R.N.; Lepore, J.J.; et al. Combined TRPC3 and TRPC6 blockade by selective small-molecule or genetic deletion inhibits pathological cardiac hypertrophy. Proc. Natl. Acad. Sci. USA 2014, 111, 1551–1556. [Google Scholar] [CrossRef]
- Certal, M.; Vinhas, A.; Barros-Barbosa, A.; Ferreirinha, F.; Costa, M.A.; Correia-de-Sa, P. ADP-Induced Ca(2+) Signaling and Proliferation of Rat Ventricular Myofibroblasts Depend on Phospholipase C-Linked TRP Channels Activation Within Lipid Rafts. J. Cell. Physiol. 2017, 232, 1511–1526. [Google Scholar] [CrossRef]
- Alfulaij, N.; Meiners, F.; Michalek, J.; Small-Howard, A.L.; Turner, H.C.; Stokes, A.J. Cannabinoids, the Heart of the Matter. J. Am. Heart Assoc. 2018, 7. [Google Scholar] [CrossRef]
- Huang, W.; Rubinstein, J.; Prieto, A.R.; Wang, D.H. Enhanced postmyocardial infarction fibrosis via stimulation of the transforming growth factor-beta-Smad2 signaling pathway: Role of transient receptor potential vanilloid type 1 channels. J. Hypertens. 2010, 28, 367–376. [Google Scholar] [CrossRef]
- Horton, J.S.; Buckley, C.L.; Stokes, A.J. Successful TRPV1 antagonist treatment for cardiac hypertrophy and heart failure in mice. Channels 2013, 7, 17–22. [Google Scholar] [CrossRef]
- Buckley, C.L.; Stokes, A.J. Mice lacking functional TRPV1 are protected from pressure overload cardiac hypertrophy. Channels 2011, 5, 367–374. [Google Scholar] [CrossRef][Green Version]
- Jones, S.; Mann, A.; Worley, M.C.; Fulford, L.; Hall, D.; Karani, R.; Jiang, M.; Robbins, N.; Rubinstein, J.; Koch, S.E. The role of transient receptor potential vanilloid 2 channel in cardiac aging. Aging Clin. Exp. Res. 2017, 29, 863–873. [Google Scholar] [CrossRef]
- Ishii, T.; Uchida, K.; Hata, S.; Hatta, M.; Kita, T.; Miyake, Y.; Okamura, K.; Tamaoki, S.; Ishikawa, H.; Yamazaki, J. TRPV2 channel inhibitors attenuate fibroblast differentiation and contraction mediated by keratinocyte-derived TGF-beta1 in an in vitro wound healing model of rats. J. Dermatol. Sci. 2018, 90, 332–342. [Google Scholar] [CrossRef]
- Rahaman, S.O.; Grove, L.M.; Paruchuri, S.; Southern, B.D.; Abraham, S.; Niese, K.A.; Scheraga, R.G.; Ghosh, S.; Thodeti, C.K.; Zhang, D.X.; et al. TRPV4 mediates myofibroblast differentiation and pulmonary fibrosis in mice. J. Clin. Investig. 2014, 124, 5225–5238. [Google Scholar] [CrossRef]
- Kruse, M.; Schulze-Bahr, E.; Corfield, V.; Beckmann, A.; Stallmeyer, B.; Kurtbay, G.; Ohmert, I.; Brink, P.; Pongs, O. Impaired endocytosis of the ion channel TRPM4 is associated with human progressive familial heart block type I. J. Clin. Investig. 2009, 119, 2737–2744. [Google Scholar] [CrossRef]
- Liu, H.; Chatel, S.; Simard, C.; Syam, N.; Salle, L.; Probst, V.; Morel, J.; Millat, G.; Lopez, M.; Abriel, H.; et al. Molecular genetics and functional anomalies in a series of 248 Brugada cases with 11 mutations in the TRPM4 channel. PLoS ONE 2013, 8, e54131. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; El Zein, L.; Kruse, M.; Guinamard, R.; Beckmann, A.; Bozio, A.; Kurtbay, G.; Megarbane, A.; Ohmert, I.; Blaysat, G.; et al. Gain-of-function mutations in TRPM4 cause autosomal dominant isolated cardiac conduction disease. Circ. Cardiovasc. Genet. 2010, 3, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Stallmeyer, B.; Zumhagen, S.; Denjoy, I.; Duthoit, G.; Hebert, J.L.; Ferrer, X.; Maugenre, S.; Schmitz, W.; Kirchhefer, U.; Schulze-Bahr, E.; et al. Mutational spectrum in the Ca(2+)--activated cation channel gene TRPM4 in patients with cardiac conductance disturbances. Hum. Mutat. 2012, 33, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Desai, B.N.; Navarro, B.; Donovan, A.; Andrews, N.C.; Clapham, D.E. Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science 2008, 322, 756–760. [Google Scholar] [CrossRef]
- Jin, J.; Wu, L.J.; Jun, J.; Cheng, X.; Xu, H.; Andrews, N.C.; Clapham, D.E. The channel kinase, TRPM7, is required for early embryonic development. Proc. Natl. Acad. Sci. USA 2012, 109, E225–E233. [Google Scholar] [CrossRef] [PubMed]
- Rios, F.J.; Zou, Z.G.; Harvey, A.P.; Harvey, K.Y.; Nosalski, R.; Anyfanti, P.; Camargo, L.L.; Lacchini, S.; Ryazanov, A.G.; Ryazanova, L.; et al. Chanzyme TRPM7 protects against cardiovascular inflammation and fibrosis. Cardiovasc. Res. 2019. [Google Scholar] [CrossRef]
- Ryazanova, L.V.; Rondon, L.J.; Zierler, S.; Hu, Z.; Galli, J.; Yamaguchi, T.P.; Mazur, A.; Fleig, A.; Ryazanov, A.G. TRPM7 is essential for Mg(2+) homeostasis in mammals. Nat. Commun. 2010, 1, 109. [Google Scholar] [CrossRef]
- Du, J.; Silverman, D.; Liang, B.; Yue, L. Ca2+-permeable TRP channels in human cardiac fibroblasts. Circulation 2007, 116 (Suppl. 16), 188. [Google Scholar]
- Qin, X.; Yue, Z.; Sun, B.; Yang, W.; Xie, J.; Ni, E.; Feng, Y.; Mahmood, R.; Zhang, Y.; Yue, L. Sphingosine and FTY720 are potent inhibitors of the transient receptor potential melastatin 7 (TRPM7) channels. Br. J. Pharmacol. 2013, 168, 1294–1312. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Ma, N.; Su, F.; Liu, H.; Mei, J. Increased TRPM6 expression in atrial fibrillation patients contribute to atrial fibrosis. Exp. Mol. Pathol. 2015, 98, 486–490. [Google Scholar] [CrossRef]
- Jiang, J.; Li, M.; Yue, L. Potentiation of TRPM7 inward currents by protons. J. Gen. Physiol. 2005, 126, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Bers, D.M. Calcium fluxes involved in control of cardiac myocyte contraction. Circ. Res. 2000, 87, 275–281. [Google Scholar] [CrossRef]
- Stafford, N.; Wilson, C.; Oceandy, D.; Neyses, L.; Cartwright, E.J. The Plasma Membrane Calcium ATPases and Their Role as Major New Players in Human Disease. Physiol. Rev. 2017, 97, 1089–1125. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, T.M.A.; Abou-Leisa, R.; Stafford, N.; Maqsood, A.; Zi, M.; Prehar, S.; Baudoin-Stanley, F.; Wang, X.; Neyses, L.; Cartwright, E.J.; et al. The plasma membrane calcium ATPase 4 signalling in cardiac fibroblasts mediates cardiomyocyte hypertrophy. Nat. Commun. 2016, 7, 11074. [Google Scholar] [CrossRef] [PubMed]
| Types of Ion Channels | Names of the Ion Channels | Cell Types Expressing the Ion Channels |
|---|---|---|
| Voltage-gated Na+ currents | INa.TTX, INa.TTXR, [51]; INa.TTXR [61] NaV1.2, NaV1.9 [62] | Human cardiac fibroblasts [51,63] |
| Voltage-gated K+ channels | Transient outward K+ current, Ito [51] [47,64] | Human cardiac fibroblasts [51], neonatal rat cardiac fibroblasts [47,64] |
| Delayed rectifier K+ currents: IK [48,52], IKf and IKS [47], IKDR [51] | Neonatal [47,64] and adult rat cardiac fibroblasts [48,52]; human cardiac fibroblasts [51] | |
| Inward-rectifier K+ channels | Inward-rectifying K+ currents: Kir [51,52], IK1 [44,53] | Human cardiac fibroblasts [51], adult rat cardiac fibroblasts [52], dog atrial fibroblasts [44,53] |
| ATP-activated K+ channels | KATP [54,56] | Rat ventricular fibroblasts [54,56] |
| Ca2+-activated K+ channels | Big conductance K+ currents activated by Ca2+: BKCa [51,57] | Human cardiac fibroblasts [51,57] |
| Intermediate conductance K+ currents activated by Ca2+: KCa3.1 [59], IKCa [65] | Rat adult cardiac fibroblasts [59,65] | |
| Cl− channels | Swelling-induced Cl− currents, ICl-swell [51] | Human cardiac fibroblasts [51] |
| Voltage-gated H+ channels | Voltage-gated H+-currents [66] | Human cardiac fibroblasts [66] |
| Store-operated Ca2+ channels (Orai/STIM) | Ca2+ release-activated Ca2+ currents: ICRAC [67,68] | Human ventricular fibroblasts [67,68] |
| TRP channels | TRPC-like currents (TRPC3 or TRPC6) [69] | Adult rat ventricular fibroblasts [69] |
| TRPC3 [70] | Rat atrial fibroblasts [70] | |
| TRPV4 [71] | Rat cardiac fibroblasts [71] | |
| TRPM2 [59] | Rat adult fibroblasts [59] | |
| TRPM7 [37,72] | Mouse, rat, and human cardiac fibroblasts [37,72] | |
| TRPA1 [73] | Human cardiac fibroblasts [73] |
| Channels | Expression in Cardiac Cells | Cellular Function in Fibroblasts | Methods Used for In Vivo Pathological Functions | Potential Roles in Heart Diseases | References |
|---|---|---|---|---|---|
| TRPC3 | Myocytes, fibroblasts | Proliferation, differentiation | Specific blockers | Arrhythmia, hypertrophy, heart failure | [70,166,167,168,169,170] |
| TRPC6 | Myocytes, fibroblasts | Proliferation, differentiation | Specific blockers; gene knockout | Arrhythmia, hypertrophy, heart failure | [38,164,171,172] |
| TRPV1 | Fibroblasts | Specific activators and inhibitors | Hypertrophy, heart failure | [173,174,175,176,177] | |
| TRPV2 | Myocytes, fibroblasts | Differentiation | Inhibitors; gene knockout | Dilated and ischemic cardiomyopathy | [178,179,180,181] |
| TRPV3 | Myocytes, fibroblasts | Proliferation | Activators | Hypertrophy | [182,183] |
| TRPV4 | Myocytes, fibroblasts | Differentiation | Inhibitors; gene knockout | Myocardial infarction | [39,184,185] |
| TRPM2 | Myocytes, fibroblasts | Gene knockout | Ischemic injury | [186,187,188,189] | |
| TRPM7 | Myocytes, fibroblasts | Proliferation, differentiation | shRNA | Cardiac fibrosis | [37,72,190,191,192,193,194,195,196] |
| TRPA1 | Myocytes, fibroblasts | Differentiation | Inhibitors, gene knockout | Hypertrophy, heart failure | [197,198,199,200] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, J.; Armillei, M.K.; Yu, A.S.; Liang, B.T.; Runnels, L.W.; Yue, L. Ca2+ Signaling in Cardiac Fibroblasts and Fibrosis-Associated Heart Diseases. J. Cardiovasc. Dev. Dis. 2019, 6, 34. https://doi.org/10.3390/jcdd6040034
Feng J, Armillei MK, Yu AS, Liang BT, Runnels LW, Yue L. Ca2+ Signaling in Cardiac Fibroblasts and Fibrosis-Associated Heart Diseases. Journal of Cardiovascular Development and Disease. 2019; 6(4):34. https://doi.org/10.3390/jcdd6040034
Chicago/Turabian StyleFeng, Jianlin, Maria K. Armillei, Albert S. Yu, Bruce T. Liang, Loren W. Runnels, and Lixia Yue. 2019. "Ca2+ Signaling in Cardiac Fibroblasts and Fibrosis-Associated Heart Diseases" Journal of Cardiovascular Development and Disease 6, no. 4: 34. https://doi.org/10.3390/jcdd6040034
APA StyleFeng, J., Armillei, M. K., Yu, A. S., Liang, B. T., Runnels, L. W., & Yue, L. (2019). Ca2+ Signaling in Cardiac Fibroblasts and Fibrosis-Associated Heart Diseases. Journal of Cardiovascular Development and Disease, 6(4), 34. https://doi.org/10.3390/jcdd6040034




