Cellular Mechanisms of Drosophila Heart Morphogenesis
Abstract
:1. Introduction
2. Genetics of Drosophila Heart Formation
2.1. Genetic Control of Cardiac Specification
2.2. Genetics of Heart Morphogenesis
2.3. Analyzing the Actomyosin Network during Drosophila Heart Morphogenesis
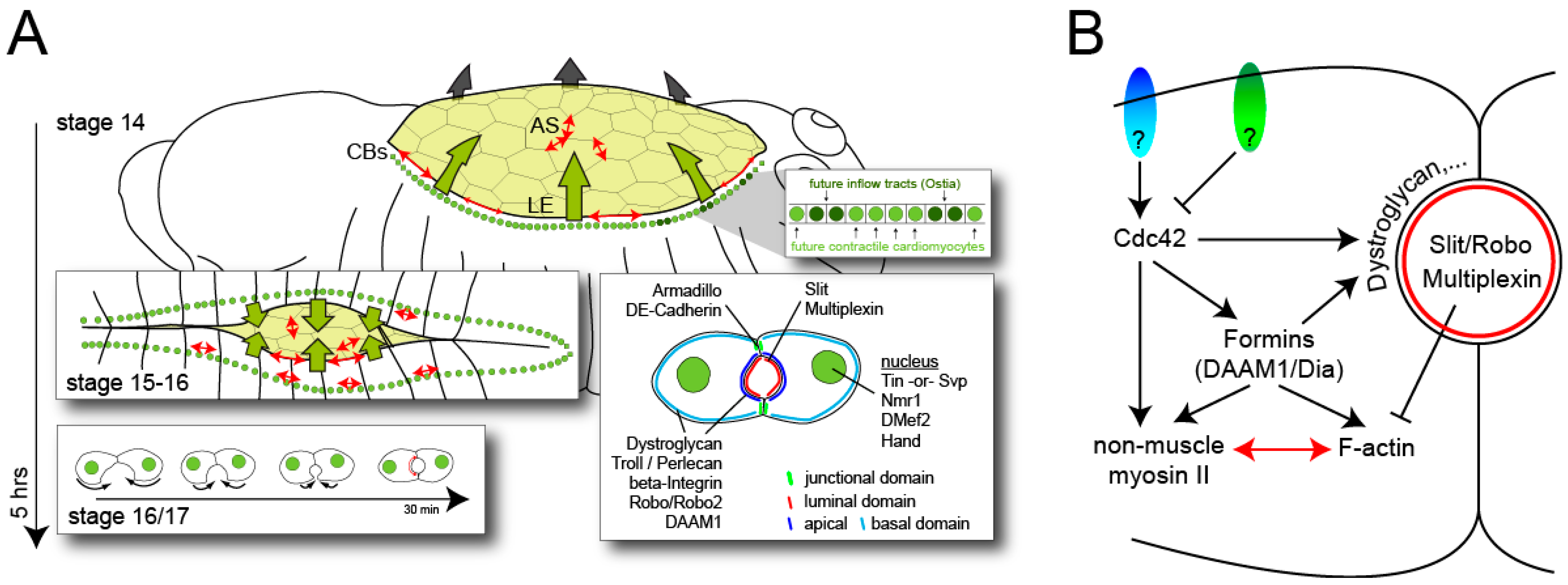
3. Conclusions
| Drosophila Gene | Class | Selected Ref. | Vertebrate Ortholog(s) | Selected Ref. |
|---|---|---|---|---|
| Diaphanous | Formin | [69] | DRF3 | [10] |
| Zipper | Non-muscle myosin | [69] | NMHC-II A/B/C | [43,81] |
| Netrin | Signaling molecule | [82,83] | NET1/3 | [84] |
| Slit | Signaling molecule | [59,60,61,62,69,85] | SLIT1/2/3 | [64,86] |
| Decapentaplegic | Signaling molecule | [87] | BMPs | [88] |
| Wingless | Signaling molecule | [89] | WNTs | [90] |
| Frazzled | Signaling Receptor | [83] | NEO1 | [91] |
| heartless | Signaling Receptor | [92] | FGFR3/4 | [93] |
| Notch | Signaling Receptor | [25] | NOTCH1/2/3 | [94] |
| Robo1/2/3 | Signaling Receptor | [59,61,62,85] | ROBO1/2/3/4 | [64] |
| Unc-5 | Signaling Receptor | [82] | UNC5A/B/C/D | [95] |
| Kuzbanian | ADAM10 metalloprotease | [23] | ADAM10 | [96] |
| Cdc42 | Small Rho GTPase | [69,70] | CDC42 | [68,97] |
| Multiplexin | Collagen type XV/XVIII | [54] | COL15/18 | [98,99] |
| dHand | Transcription factor | [33,100,101] | HAND1/2 | [102,103] |
| DMef2 | Transcription factor | [32,104,105] | MEF2A/C/D | [106,107] |
| Dorsocross1/2/3 | Transcription factor | [29] | TBX2/3/5 | [108,109,110] |
| Ladybird-early/late | Transcription factor | [111,112] | Lbx1/2/3 | [113] |
| Neuromancer1/2 | Transcription factor | [34,35,114] | TBX20 | [115,116] |
| Pannier | Transcription factor | [30,117,118] | GATA4/6 | [119] |
| Seven-up | Transcription factor | [38,45] | COUP-TF II | [120] |
| Tail-up | Transcription factor | [36,37,121] | ISL1 | [122] |
| Tinman | Transcription factor | [27,28] | NKX2.5 | [123] |
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Marelli, A.J.; Ionescu-Ittu, R.; Mackie, A.S.; Guo, L.; Dendukuri, N.; Kaouache, M. Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation 2014. [Google Scholar] [CrossRef]
- Reller, M.D.; Strickland, M.J.; Riehle-Colarusso, T.; Mahle, W.T.; Correa, A. Prevalence of congenital heart defects in metropolitan atlanta, 1998–2005. J. Pediatri. 2008, 153, 807–813. [Google Scholar] [CrossRef]
- Pierpont, M.E.; Basson, C.T.; Benson, D.W.; Gelb, B.D.; Giglia, T.M.; Goldmuntz, E.; McGee, G.; Sable, C.A.; Srivastava, D.; Webb, C.L.; et al. Genetic basis for congenital heart defects: Current knowledge: A scientific statement from the American heart association congenital cardiac defects committee, council on cardiovascular disease in the young: Endorsed by the american academy of pediatrics. Circulation 2007, 115, 3015–3038. [Google Scholar] [CrossRef] [PubMed]
- Fahed, A.C.; Gelb, B.D.; Seidman, J.G.; Seidman, C.E. Genetics of congenital heart disease: The glass half empty. Circ. Res. 2013, 112, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Staudt, D.; Stainier, D. Uncovering the molecular and cellular mechanisms of heart development using the zebrafish. Ann. Rev. Genet. 2012, 46, 397–418. [Google Scholar] [CrossRef] [PubMed]
- Bruneau, B.G. Heart Development; Academic Press: Waltham, MA, USA, 2012; p. 408. [Google Scholar]
- Bruneau, B.G. The developmental genetics of congenital heart disease. Nature 2008, 451, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Kruithof, B.P.T.; Duim, S.N.; Moerkamp, A.T.; Goumans, M.-J. Tgfβ and BMP signaling in cardiac cushion formation: Lessons from mice and chicken. Differentiation 2012, 84, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.M.; Bruneau, B.G. Lessons for cardiac regeneration and repair through development. Trends Mol. Med. 2010, 16, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Christiaen, L.; Davidson, B.; Kawashima, T.; Powell, W.; Nolla, H.; Vranizan, K.; Levine, M. The transcription/migration interface in heart precursors of ciona intestinalis. Science 2008, 320, 1349–1352. [Google Scholar] [CrossRef] [PubMed]
- Vogler, G.; Bodmer, R.; Akasaka, T. A Drosophila model for congenital heart disease. Drug Discov. Today Dis. Models 2009, 6, 47–54. [Google Scholar] [CrossRef]
- Ocorr, K.; Vogler, G.; Bodmer, R. Methods to assess Drosophila heart development, function and aging. Methods 2014, 68, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Reim, I.; Frasch, M. Genetic and genomic dissection of cardiogenesis in the Drosophila model. Pediatr. Cardiol. 2009, 31, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Medioni, C.; Sénatore, S.; Salmand, P.-A.; Lalevée, N.; Perrin, L.; Sémériva, M. The fabulous destiny of the Drosophila heart. Curr. Opin. Genet. Dev. 2009, 19, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Bodmer, R.; Frasch, M. Development and aging of the Drosophila hear. In Heart Development and Regeneration; Harvey, R.P., Rosenthal, N., Eds.; Academic Press: Waltham, MA, USA, 2010; pp. 47–86. [Google Scholar]
- Mohr, S.E.; Hu, Y.; Kim, K.; Housden, B.E.; Perrimon, N. Resources for functional genomics studies in Drosophila melanogaster. Genetics 2014, 197, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Neely, G.G.; Kuba, K.; Cammarato, A.; Isobe, K.; Amann, S.; Zhang, L.; Murata, M.; Elmen, L.; Gupta, V.; Arora, S.; et al. A global in vivo Drosophila RNAi screen identifies Not3 as a conserved regulator of heart function. Cell 2010, 141, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J. An emerging role for hippo-yap signaling in cardiovascular development. J. Biomed. Res. 2014, 28, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Rizki, T.M. The Circulatory System and Associated Cells and Tissues; Ashburner, M., Wright, T.R., Eds.; Academic Press: Waltham, MA, USA, 1978; pp. 397–452. [Google Scholar]
- Zhang, F.; Zhao, Y.; Chao, Y.; Muir, K.; Han, Z. Cubilin and amnionless mediate protein reabsorption in Drosophila nephrocytes. J. Am. Soc. Nephrol. 2013, 24, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.-Y.; Wang, W.; Chen, J.; Ocorr, K.; Bodmer, R. Ros regulate cardiac function via a distinct paracrine mechanism. Cell Rep. 2014, 7, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Bryantsev, A.L.; Cripps, R.M. Cardiac gene regulatory networks in Drosophila. Biochim. Biophys. Acta 2009, 1789, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, S.; Wang, S.; Holz, A.; Bergter, A.; Paululat, A. The adam metalloprotease kuzbanian is crucial for proper heart formation in Drosophila melanogaster. Mech. Dev. 2006, 123, 372–387. [Google Scholar] [CrossRef] [PubMed]
- Ward, E.J.; Skeath, J.B. Characterization of a novel subset of cardiac cells and their progenitors in the Drosophila embryo. Development 2000, 127, 4959–4969. [Google Scholar] [PubMed]
- Han, Z.; Bodmer, R. Myogenic cells fates are antagonized by notch only in asymmetric lineages of the Drosophila heart, with or without cell division. Development 2003, 130, 3039–3051. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Yaich, L.E.; Bodmer, R. Mesodermal cell fate decisions in Drosophila are under the control of the lineage genes numb, Notch, and sanpodo. Mech. Dev. 1998, 75, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Bodmer, R. The gene Tinman is required for specification of the heart and visceral muscles in Drosophila. Development 1993, 118, 719–729. [Google Scholar] [PubMed]
- Azpiazu, N.; Frasch, M. Tinman and bagpipe: Two homeo box genes that determine cell fates in the dorsal mesoderm of Drosophila. Genes Dev. 1993, 7, 1325–1340. [Google Scholar] [CrossRef] [PubMed]
- Reim, I.; Frasch, M. The dorsocross T-box genes are key components of the regulatory network controlling early cardiogenesis in Drosophila. Development 2005, 132, 4911–4925. [Google Scholar] [CrossRef] [PubMed]
- Klinedinst, S.L.; Bodmer, R. GATA factor pannier is required to establish competence for heart progenitor formation. Development 2003, 130, 3027–3038. [Google Scholar] [CrossRef] [PubMed]
- Zaffran, S.; Reim, I.; Qian, L.; Lo, P.C.; Bodmer, R.; Frasch, M. Cardioblast-intrinsic Tinman activity controls proper diversification and differentiation of myocardial cells in Drosophila. Development 2006, 133, 4073–4083. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, K.; Kim, Y.; Lee, Y.M.; Olson, E.N.; Schulz, R.A. D-MEF2 is a target for Tinman activation during Drosophila heart development. EMBO J. 1997, 16, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Yi, P.; Li, X.; Olson, E.N. Hand, an evolutionarily conserved bHLH transcription factor required for Drosophila cardiogenesis and hematopoiesis. Development 2006, 133, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Miskolczi-McCallum, C.M.; Scavetta, R.J.; Svendsen, P.C.; Soanes, K.H.; Brook, W.J. The Drosophila melanogaster T-box genes midline and H15 are conserved regulators of heart development. Dev. Biol. 2005, 278, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Liu, J.; Bodmer, R. Neuromancer TBX20-related genes (H15/midline) promote cell fate specification and morphogenesis of the Drosophila heart. Dev. Biol. 2005, 279, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Wang, J.; Tokusumi, T.; Gajewski, K.; Schulz, R.A. Requirement of the LIM homeodomain transcription factor tailup for normal heart and hematopoietic organ formation in Drosophila melanogaster. Mol. Cell. Biol. 2007, 27, 3962–3969. [Google Scholar] [CrossRef] [PubMed]
- Mann, T.; Bodmer, R.; Pandur, P. The Drosophila homolog of vertebrate Islet1 is a key component in early cardiogenesis. Development 2009, 136, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Lo, P.C.H.; Frasch, M. A role for the coup-tf-related gene seven-up in the diversification of cardioblast identities in the dorsal vessel of Drosophila. Mech. Dev. 2001, 104, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Junion, G.; Spivakov, M.; Girardot, C.; Braun, M.; Gustafson, E.H.; Birney, E.; Furlong, E.E.M. A transcription factor collective defines cardiac cell fate and reflects lineage history. Cell 2012, 148, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Stojnic, R.; Adryan, B.; Ozdemir, A.; Stathopoulos, A.; Frasch, M. Genome-wide screens for in vivo tinman binding sites identify cardiac enhancers with diverse functional architectures. PLOS Genet. 2013, 9, e1003195. [Google Scholar] [CrossRef] [PubMed]
- Rugendorff, A.; Younossi-Hartenstein, A.; Hartenstein, V. Embryonic origin and differentiation of the Drosophila heart. Roux’s Arch. Dev. Biol. 1994, 203, 266–280. [Google Scholar] [CrossRef]
- Holtzman, N.G.; Schoenebeck, J.J.; Tsai, H.J.; Yelon, D. Endocardium is necessary for cardiomyocyte movement during heart tube assembly. Development 2007, 134, 2379–2386. [Google Scholar] [CrossRef] [PubMed]
- Strilić, B.; Kucera, T.; Eglinger, J.; Hughes, M.R.; McNagny, K.M.; Tsukita, S.; Dejana, E.; Ferrara, N.; Lammert, E. The molecular basis of vascular lumen formation in the developing mouse aorta. Dev. Cell 2009, 17, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Zikova, M.; da Ponte, J.-P.; Dastugue, B.; Jagla, K. Patterning of the cardiac outflow region in Drosophila. Proc. Natl. Acad. Sci. USA 2003, 100, 12189–12194. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.R.; Cripps, R.M. Ostia, the inflow tracts of the Drosophila heart, develop from a genetically distinct subset of cardial cells. Mech. Dev. 2001, 109, 51–59. [Google Scholar] [CrossRef] [PubMed]
- LaBeau, E.M.; Trujillo, D.L.; Cripps, R.M. Bithorax complex genes control alary muscle patterning along the cardiac tube of Drosophila. Mech. Dev. 2009, 126, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Boukhatmi, H.; Schaub, C.; Bataillé, L.; Reim, I.; Frendo, J.-L.; Frasch, M.; Vincent, A. An Org-1-Tup transcriptional cascade reveals different types of alary muscles connecting internal organs in Drosophila. Development 2014, 141, 3761–3771. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Shao, H.; Guo, F.; Trimble, R.; Pearce, E.; Abmayr, S.M. Sns and Kirre, the Drosophila orthologs of Nephrin and Neph1, direct adhesion, fusion and formation of a slit diaphragm-like structure in insect nephrocytes. Development 2009, 136, 2335–2344. [Google Scholar] [CrossRef] [PubMed]
- Weavers, H.; Prieto-Sánchez, S.; Grawe, F.; Garcia-López, A.; Artero, R.; Wilsch-Bräuninger, M.; Ruiz-Gómez, M.; Skaer, H.; Denholm, B. The insect nephrocyte is a podocyte-like cell with a filtration slit diaphragm. Nature 2009, 457, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Buechling, T.; Akasaka, T.; Vogler, G.; Ruiz-Lozano, P.; Ocorr, K.A.; Bodmer, R. Non-autonomous modulation of heart rhythm, contractility and morphology in adult fruit flies. Dev. Biol. 2009, 328, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Hollfelder, D.; Frasch, M.; Reim, I. Distinct functions of the laminin β LN domain and collagen IV during cardiac extracellular matrix formation and stabilization of alary muscle attachments revealed by ems mutagenesis in Drosophila. BMC Dev. Biol. 2014, 14, 26. [Google Scholar] [CrossRef] [PubMed]
- Yarnitzky, T.; Volk, T. Laminin is required for heart, somatic muscles, and gut development in the Drosophila embryo. Dev. Biol. 1995, 169, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Chartier, A.; Zaffran, S.; Astier, M.; Sémériva, M.; Gratecos, D. Pericardin, a Drosophila type IV collagen-like protein is involved in the morphogenesis and maintenance of the heart epithelium during dorsal ectoderm closure. Development 2002, 129, 3241–3253. [Google Scholar] [PubMed]
- Harpaz, N.; Ordan, E.; Ocorr, K.; Bodmer, R.; Volk, T. Multiplexin promotes heart but not aorta morphogenesis by polarized enhancement of Slit/Robo activity at the heart lumen. PLOS Genet. 2013, 9, e1003597. [Google Scholar] [CrossRef] [PubMed]
- Volk, T.; Wang, S.; Rotstein, B.; Paululat, A. Matricellular proteins in development: Perspectives from the Drosophila heart. Matrix Biol. 2014, 37, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Vanderploeg, J.; Vazquez Paz, L.L.; MacMullin, A.; Jacobs, J.R. Integrins are required for cardioblast polarisation in Drosophila. BMC Dev. Biol. 2012, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Yi, P.; Johnson, A.N.; Han, Z.; Wu, J.; Olson, E.N. Heterotrimeric g proteins regulate a noncanonical function of septate junction proteins to maintain cardiac integrity in Drosophila. Dev. Cell 2008, 15, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Rothberg, J.M.; Jacobs, J.R.; Goodman, C.S.; Artavanis-Tsakonas, S. Slit: An extracellular protein necessary for development of midline glia and commissural axon pathways contains both EGF and LRR domains. Genes Dev. 1990, 4, 2169–2187. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Liu, J.; Bodmer, R. Slit and Robo control cardiac cell polarity and morphogenesis. Curr. Biol. 2005, 15, 2271–2278. [Google Scholar] [CrossRef] [PubMed]
- Medioni, C.; Astier, M.; Zmojdzian, M.; Jagla, K.; Semeriva, M. Genetic control of cell morphogenesis during Drosophila melanogaster cardiac tube formation. J. Cell Biol. 2008, 182, 249–261. [Google Scholar] [CrossRef] [PubMed]
- MacMullin, A.; Jacobs, J.R. Slit coordinates cardiac morphogenesis in Drosophila. Dev. Biol. 2006, 293, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Martínez, E.; Soplop, N.H.; Patel, R.; Kramer, S.G. Repulsion by Slit and roundabout prevents Shotgun/E-cadherin-mediated cell adhesion during Drosophila heart tube lumen formation. J. Cell Biol. 2008, 182, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Fish, J.E.; Wythe, J.D.; Xiao, T.; Bruneau, B.G.; Stainier, D.Y.R.; Srivastava, D.; Woo, S. A Slit/miR-218/Robo regulatory loop is required during heart tube formation in zebrafish. Development 2011, 138, 1409–1419. [Google Scholar] [CrossRef] [PubMed]
- Mommersteeg, M.T.M.; Andrews, W.D.; Ypsilanti, A.R.; Zelina, P.; Yeh, M.L.; Norden, J.; Kispert, A.; Chédotal, A.; Christoffels, V.M.; Parnavelas, J.G. Slit-Robo signaling regulates the development of the cardiac systemic venous return and pericardium. Circ. Res. 2012, 112, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Bashaw, G.J. Son of sevenless directly links the Robo receptor to Rac activation to control axon repulsion at the midline. Neuron 2006, 52, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Hanna, S.; El-Sibai, M. Signaling networks of Rho GTPases in cell motility. Cell. Signal. 2013, 25, 1955–1961. [Google Scholar] [CrossRef] [PubMed]
- Iden, S.; Collard, J.G. Crosstalk between small GTPases and polarity proteins in cell polarization. Nat. Rev. Mol. Cell Biol. 2008, 9, 846–859. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Wythe, J.D.; Liu, J.; Cartry, J.; Vogler, G.; Mohapatra, B.; Otway, R.T.; Huang, Y.; King, I.N.; Maillet, M.; et al. Tinman/Nkx2–5 acts via miR-1 and upstream of Cdc42 to regulate heart function across species. J. Cell Biol. 2011, 193, 1181–1196. [Google Scholar] [CrossRef] [PubMed]
- Vogler, G.; Liu, J.; Iafe, T.W.; Migh, E.; Mihály, J.; Bodmer, R. Cdc42 and formin activity control non-muscle myosin dynamics during Drosophila heart morphogenesis. J. Cell Biol. 2014, 206, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Swope, D.; Kramer, J.; King, T.R.; Cheng, Y.-S.; Kramer, S.G. Cdc42 is required in a genetically distinct subset of cardiac cells during Drosophila dorsal vessel closure. Dev. Biol. 2014, 392, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Veerkamp, J.; Rudolph, F.; Cseresnyes, Z.; Priller, F.; Otten, C.; Renz, M.; Schaefer, L.; Abdelilah-Seyfried, S. Unilateral dampening of BMP activity by nodal generates cardiac left-right asymmetry. Dev. Cell 2013, 24, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Brandt, R.; Paululat, A. Microcompartments in the Drosophila heart and the mammalian brain: General features and common principles. Biol. Chem. 2013, 394, 217–230. [Google Scholar] [PubMed]
- Haack, T.; Schneider, M.; Schwendele, B.; Renault, A.D. Drosophila heart cell movement to the midline occurs through both cell autonomous migration and dorsal closure. Dev. Biol. 2014, 396, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Reim, I.; Hollfelder, D.; Ismat, A.; Frasch, M. The FGF8-related signals Pyramus and Thisbe promote pathfinding, substrate adhesion, and survival of migrating longitudinal gut muscle founder cells. Dev. Biol. 2012, 368, 28–43. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.M.; Jenkins, B.V.; O’Connor-Giles, K.M.; Wildonger, J. A crispr view of development. Genes Dev. 2014, 28, 1859–1872. [Google Scholar] [CrossRef] [PubMed]
- Mohr, S.E.; Smith, J.A.; Shamu, C.E.; Neumüller, R.A.; Perrimon, N. RNAi screening comes of age: Improved techniques and complementary approaches. Nat. Rev. Mol. Cell Biol. 2014, 15, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Gratz, S.J.; Cummings, A.M.; Nguyen, J.N.; Hamm, D.C.; Donohue, L.K.; Harrison, M.M.; Wildonger, J.; O’Connor-Giles, K.M. Genome engineering of Drosophila with the CRISPR RNA-guided Cas9 nuclease. Genetics 2013, 194, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Long, C.; McAnally, J.R.; Shelton, J.M.; Mireault, A.A.; Bassel-Duby, R.; Olson, E.N. Prevention of muscular dystrophy in mice by CRISPR/Cas9-mediated editing of germline DNA. Science 2014, 345, 1184–1188. [Google Scholar] [CrossRef] [PubMed]
- Bier, E. Drosophila, the golden bug, emerges as a tool for human genetics. Nat. Rev. Genet. 2005, 6, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Xin, M.; Olson, E.N.; Bassel-Duby, R. Mending broken hearts: Cardiac development as a basis for adult heart regeneration and repair. Nat. Rev. Mol. Cell Biol. 2013, 14, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Tullio, A.N.; Accili, D.; Ferrans, V.J.; Yu, Z.X.; Takeda, K.; Grinberg, A.; Westphal, H.; Preston, Y.A.; Adelstein, R.S. Nonmuscle myosin II-B is required for normal development of the mouse heart. Proc. Natl. Acad. Sci. USA 1997, 94, 12407–12412. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, S.; Altenhein, B.; Paululat, A. The transmembrane receptor uncoordinated5 (UNC5) is essential for heart lumen formation in Drosophila melanogaster. Dev. Biol. 2011, 350, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Macabenta, F.D.; Jensen, A.G.; Cheng, Y.-S.; Kramer, J.J.; Kramer, S.G. Frazzled/DCC facilitates cardiac cell outgrowth and attachment during Drosophila dorsal vessel formation. Dev.Biol. 2013, 380, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Lai Wing Sun, K.; Correia, J.P.; Kennedy, T.E. Netrins: Versatile extracellular cues with diverse functions. Development 2011, 138, 2153–2169. [Google Scholar]
- Santiago-Martínez, E.; Soplop, N.H.; Kramer, S.G. Lateral positioning at the dorsal midline: Slit and roundabout receptors guide Drosophila heart cell migration. Proc. Natl. Acad. Sci. USA 2006, 103, 12441–12446. [Google Scholar] [CrossRef] [PubMed]
- Medioni, C.; Bertrand, N.; Mesbah, K.; Hudry, B.; Dupays, L.; Wolstein, O.; Washkowitz, A.J.; Papaioannou, V.E.; Mohun, T.J.; Harvey, R.P.; et al. Expression of Slit and Robo genes in the developing mouse heart. Dev. Dyn. 2010, 239, 3303–3311. [Google Scholar] [CrossRef] [PubMed]
- Frasch, M. Induction of visceral and cardiac mesoderm by ectodermal Dpp in the early Drosophila embryo. Nature 1995, 374, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.; de Val, S.; Heidt, A.B.; Xu, S.-M.; Bristow, J.; Black, B.L. GATA4 expression in lateral mesoderm is downstream of BMP4 and is activated directly by Forkhead and GATA transcription factors through a distal enhancer element. Development 2005, 132, 3405–3417. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Wu, X.; Golden, K.; Axelrod, J.D.; Bodmer, R. The wingless signaling pathway is directly involved in Drosophila heart development. Dev. Biol. 1996, 177, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Ueno, S.; Weidinger, G.; Osugi, T.; Kohn, A.D.; Golob, J.L.; Pabon, L.; Reinecke, H.; Moon, R.T.; Murry, C.E. Biphasic role for WNT/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc. Natl. Acad. Sci. USA 2007, 104, 9685–9690. [Google Scholar] [CrossRef] [PubMed]
- Wilson, N.H.; Key, B. Neogenin: One receptor, many functions. Int. J. Biochem. Cell Biol. 2007, 39, 874–878. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.; Vogelsang, E.; Leptin, M. FGF signalling and the mechanism of mesoderm spreading in Drosophila embryos. Development 2005, 132, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Marguerie, A.; Bajolle, F.; Zaffran, S.; Brown, N.A.; Dickson, C.; Buckingham, M.E.; Kelly, R.G. Congenital heart defects in Fgfr2-IIIb and Fgf10 mutant mice. Cardiovasc. Res. 2006, 71, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Niessen, K.; Karsan, A. Notch signaling in cardiac development. Circ. Res. 2008, 102, 1169–1181. [Google Scholar] [CrossRef] [PubMed]
- Navankasattusas, S.; Whitehead, K.J.; Suli, A.; Sorensen, L.K.; Lim, A.H.; Zhao, J.; Park, K.W.; Wythe, J.D.; Thomas, K.R.; Chien, C.-B.; et al. The netrin receptor UNC5B promotes angiogenesis in specific vascular beds. Development 2008, 135, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, D.; de Strooper, B.; Serneels, L.; Craessaerts, K.; Herreman, A.; Annaert, W.; Umans, L.; Lubke, T.; Lena Illert, A.; von Figura, K.; et al. The disintegrin/metalloprotease ADAM 10 is essential for notch signalling but not for alpha-secretase activity in fibroblasts. Hum. Mol. Genet. 2002, 11, 2615–2624. [Google Scholar] [CrossRef] [PubMed]
- Maillet, M.; Lynch, J.M.; Sanna, B.; York, A.J.; Zheng, Y.; Molkentin, J.D. Cdc42 is an antihypertrophic molecular switch in the mouse heart. J. Clin. Investig. 2009, 119, 3079–3088. [Google Scholar] [CrossRef] [PubMed]
- Rasi, K.; Piuhola, J.; Czabanka, M.; Sormunen, R.; Ilves, M.; Leskinen, H.; Rysä, J.; Kerkelä, R.; Janmey, P.; Heljasvaara, R.; et al. Collagen XV is necessary for modeling of the extracellular matrix and its deficiency predisposes to cardiomyopathy. Circ. Res. 2010, 107, 1241–1252. [Google Scholar] [CrossRef] [PubMed]
- Eklund, L.; Piuhola, J.; Komulainen, J.; Sormunen, R.; Ongvarrasopone, C.; Fássler, R.; Muona, A.; Ilves, M.; Ruskoaho, H.; Takala, T.E.; et al. Lack of type XV collagen causes a skeletal myopathy and cardiovascular defects in mice. Proc. Natl. Acad. Sci. USA 2001, 98, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Lo, P.C.H.; Zaffran, S.; Sénatore, S.; Frasch, M. The Drosophila hand gene is required for remodeling of the developing adult heart and midgut during metamorphosis. Dev. Biol. 2007, 311, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Olson, E.N. Hand is a direct target of tinman and GATA factors during Drosophila cardiogenesis and hematopoiesis. Development 2005, 132, 3525–3536. [Google Scholar] [CrossRef] [PubMed]
- Firulli, A.B.; McFadden, D.G.; Lin, Q.; Srivastava, D.; Olson, E.N. Heart and extra-embryonic mesodermal defects in mouse embryos lacking the bHLH transcription factor hand1. Nat. Genet. 1998, 18, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Lib, L.; Li, Z.; Liu, M.; Yan, J.; Wang, B.; Ma, X. Two novel hand1 mutations in chinese patients with ventricular septal defect. Clin. Chim. Acta 2012, 413, 675–677. [Google Scholar] [CrossRef] [PubMed]
- Cripps, R.M.; Lovato, T.L.; Olson, E.N. Positive autoregulation of the myocyte enhancer factor-2 myogenic control gene during somatic muscle development in Drosophila. Dev. Biol. 2004, 267, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Lilly, B.; Zhao, B.; Ranganayakulu, G.; Paterson, B.M.; Schulz, R.A.; Olson, E.N. Requirement of MADS domain transcription factor D-MEF2 for muscle formation in Drosophila. Science 1995, 267, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Schwarz, J.; Bucana, C.; Olson, E.N. Control of mouse cardiac morphogenesis and myogenesis by transcription factor mef2c. Science 1997, 276, 1404–1407. [Google Scholar] [CrossRef] [PubMed]
- Naya, F.J.; Black, B.L.; Wu, H.; Bassel-Duby, R.; Richardson, J.A.; Hill, J.A.; Olson, E.N. Mitochondrial deficiency and cardiac sudden death in mice lacking the MEF2A transcription factor. Nat. Med. 2002, 8, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Bruneau, B.G.; Nemer, G.; Schmitt, J.P.; Charron, F.; Robitaille, L.; Caron, S.; Conner, D.A.; Gessler, M.; Nemer, M.; Seidman, C.E.; et al. A murine model of holt-oram syndrome defines roles of the T-box transcription factor TBX5 in cardiogenesis and disease. Cell 2001, 106, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Harrelson, Z.; Kelly, R.G.; Goldin, S.N.; Gibson-Brown, J.J.; Bollag, R.J.; Silver, L.M.; Papaioannou, V.E. TBX2 is essential for patterning the atrioventricular canal and for morphogenesis of the outflow tract during heart development. Development 2004, 131, 5041–5052. [Google Scholar] [CrossRef] [PubMed]
- Bakker, M.L.; Boukens, B.J.; Mommersteeg, M.T.M.; Brons, J.F.; Wakker, V.; Moorman, A.F.M.; Christoffels, V.M. Transcription factor TBX3 is required for the specification of the atrioventricular conduction system. Circ. Res. 2008, 102, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Junion, G.; Bataillé, L.; Jagla, T.; Da Ponte, J.P.; Tapin, R.; Jagla, K. Genome-wide view of cell fate specification: Ladybird acts at multiple levels during diversification of muscle and heart precursors. Genes Dev. 2007, 21, 3163–3180. [Google Scholar] [CrossRef] [PubMed]
- Jagla, K.; Jagla, T.; Heitzler, P.; Dretzen, G.; Bellard, F.; Bellard, M. Ladybird, a tandem of homeobox genes that maintain late wingless expression in terminal and dorsal epidermis of the Drosophila embryo. Development 1997, 124, 91–100. [Google Scholar] [PubMed]
- Schäfer, K.; Neuhaus, P.; Kruse, J.; Braun, T. The homeobox gene Lbx1 specifies a subpopulation of cardiac neural crest necessary for normal heart development. Circ. Res. 2003, 92, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Reim, I.; Mohler, J.P.; Frasch, M. TBX20-related genes, mid and H15, are required for tinman expression, proper patterning, and normal differentiation of cardioblasts in Drosophila. Mech. Dev. 2005, 122, 1056–1069. [Google Scholar] [CrossRef] [PubMed]
- Kirk, E.P.; Sunde, M.; Costa, M.W.; Rankin, S.A.; Wolstein, O.; Castro, M.L.; Butler, T.L.; Hyun, C.; Guo, G.; Otway, R.; et al. Mutations in cardiac T-box factor gene TBX20 are associated with diverse cardiac pathologies, including defects of septation and valvulogenesis and cardiomyopathy. Am. J. Hum. Genet. 2007, 81, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Hammer, S.; Toenjes, M.; Lange, M.; Fischer, J.J.; Dunkel, I.; Mebus, S.; Grimm, C.H.; Hetzer, R.; Berger, F.; Sperling, S. Characterization of TBX20 in human hearts and its regulation by TFAP2. J. Cell. Biochem. 2008, 104, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, A.D.; Shi, W.; Wilson, B.A.; Skeath, J.B. Pannier and pointedP2 act sequentially to regulate Drosophila heart development. Development 2003, 130, 3015–3026. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, K.; Fossett, N.; Molkentin, J.D.; Schulz, R.A. The zinc finger proteins pannier and GATA4 function as cardiogenic factors in Drosophila. Development 1999, 126, 5679–5688. [Google Scholar] [PubMed]
- Zhou, P.; He, A.; Pu, W.T. Regulation of GATA4 transcriptional activity. In Cardiovascular Development and Disease; Academic Press: Waltham, MA, USA, 2012; Volume 100, pp. 143–169. [Google Scholar]
- Pereira, F.A.; Qiu, Y.; Zhou, G.; Tsai, M.J.; Tsai, S.Y. The orphan nuclear receptor COUP-TFII is required for angiogenesis and heart development. Genes Dev. 1999, 13, 1037–1049. [Google Scholar] [CrossRef] [PubMed]
- Zmojdzian, M.; Jagla, K. Tailup plays multiple roles during cardiac outflow assembly in Drosophila. Cell Tissue Res. 2013, 354, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.-L.; Liang, X.; Shi, Y.; Chu, P.-H.; Pfaff, S.L.; Chen, J.; Evans, S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev. Cell 2003, 5, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Akazawa, H.; Komuro, I. Cardiac transcription factor Csx/Nkx2-5: Its role in cardiac development and diseases. Pharmacol. Ther. 2005, 107, 252–268. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vogler, G.; Bodmer, R. Cellular Mechanisms of Drosophila Heart Morphogenesis. J. Cardiovasc. Dev. Dis. 2015, 2, 2-16. https://doi.org/10.3390/jcdd2010002
Vogler G, Bodmer R. Cellular Mechanisms of Drosophila Heart Morphogenesis. Journal of Cardiovascular Development and Disease. 2015; 2(1):2-16. https://doi.org/10.3390/jcdd2010002
Chicago/Turabian StyleVogler, Georg, and Rolf Bodmer. 2015. "Cellular Mechanisms of Drosophila Heart Morphogenesis" Journal of Cardiovascular Development and Disease 2, no. 1: 2-16. https://doi.org/10.3390/jcdd2010002
APA StyleVogler, G., & Bodmer, R. (2015). Cellular Mechanisms of Drosophila Heart Morphogenesis. Journal of Cardiovascular Development and Disease, 2(1), 2-16. https://doi.org/10.3390/jcdd2010002





