Cor Triatriatum Dexter: The Largest Comprehensive Review in the Field on 124 Worldwide Cases (1968–Now)
Abstract
1. Introduction
2. Search Methodology and Data Collection
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nunes, M.A.; Fragata, J.; Lima, M. Echocardiography in cor triatriatum dexter. Rev. Port. Cardiol. 1993, 12, 1043–1048, 1001. [Google Scholar]
- Kilkenny, K.; Frishman, W. Cor Triatriatum: A Review. Cardiol. Rev. 2025, 33, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Yater, W.M. Variations and Anomalies of the Venous Valves of the Right Atrium of the Human Heart. Arch. Pathol. 1929, 7, 418–441. [Google Scholar]
- Rossall, R.; Caldwell, R. Obstruction of Inferior Vena Cava by a Persistent Eustachian Valve in a Young Adult. J. Clin. Pathol. 1957, 10, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Faber, J.W.; Boukens, B.J.; Oostra, R.J.; Moorman, A.F.M.; Christoffels, V.M.; Jensen, B. Sinus Venosus Incorporation: Contentious Issues and Operational Criteria for Developmental and Evolutionary Studies. J. Anat. 2019, 234, 583–591. [Google Scholar] [CrossRef]
- Anderson, R.H. Another Look at Cardiac Embryology. In Progress in Cardiology; Yu, P.N., Goodwin, J.F., Eds.; Lea and Febiger: Philadelphia, PA, USA, 1978; pp. 1–53. [Google Scholar]
- Werner, J.A.; Cheitlin, M.D.; Gross, B.W.; Speck, S.M.; Ivey, T.O. Echocardiographic Appearance of the Chiari Network: Differentiation from Right Heart Pathology. Circulation 1981, 63, 1104–1109. [Google Scholar] [CrossRef]
- Wyss, E.; Ammann, P.; Rickli, H.; Jenni, R. Cor Triatriatum Dexter of an Adult. Z. Kardiol. 1998, 87, 891–893. [Google Scholar] [CrossRef]
- Moreno Granado, F.; Herráiz Sarachaga, I.; Castro Gussoni, C.; Duhagon Cajelli, P. Remnants of the Embryologic Sinus Venosus Valves. “Cor Triatriatum Dexter”. An. Esp. Pediatr. 1976, 9, 407–414. [Google Scholar]
- Battle-Diaz, J.; Stanley, P.; Kratz, C.; Fouron, J.-C.; Guerin, R.; Davignon, A. Echocardiographic Manifestations of Persistence of the Right Sinus Venosus Valve. Am. J. Cardiol. 1979, 43, 850–853. [Google Scholar] [CrossRef]
- Weinberg, P.M.; Peyser, K.; Hackney, J.R. Fetal Hydrops in a Newborn with Hypoplastic Left Heart Syndrome: Tricuspid Valve “Stopper”. J. Am. Coll. Cardiol. 1985, 6, 1365–1369. [Google Scholar] [CrossRef][Green Version]
- Malaki, M.; Willis, A.P.; Jones, R.G. Congenital anomalies of the inferior vena cava. Clin Radiol. 2012, 67, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.N.; Niles, N.R. Spinnaker Formation of Sinus Venosus Valve: Case Report of a Fetal Anomaly in a Ten-Year-Old Boy. Circulation 1965, 38, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Rigatelli, G.; Dell’Avvocata, F.; Giordan, M.; Vassilev, D.; Cardaioli, P. Incomplete Cor Triatriatum Dexter and Its Clinical and Technical Implications in Interatrial Shunt Device-Based Closure: An Intracardiac Echocardiography Study. Congenit. Heart Dis. 2016, 11, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Ullah, W.; Sattar, Y.; Rauf, H.; Roomi, S.; Shah, M.I. A Systematic Review of a Long-Forgotten Cause of Atrial Fibrillation and Stroke: Cor Triatriatum. Cureus 2019, 11, e6371. [Google Scholar] [CrossRef]
- Jha, A.K.; Makhija, N. Cor Triatriatum: A Review. Semin. Cardiothorac. Vasc. Anesth. 2017, 21, 178–185. [Google Scholar] [CrossRef]
- Ather, B.; Meredith, A.; Siddiqui, W.J. Cor Triatriatum. In StatPearls (Internet); StatPearls Publishing: Treasure Island, FL, USA, 2025; Updated 27 July 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK534243/ (accessed on 11 November 2025).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Antes, G.; Atkins, D. Preferred Reporting Items for Systematic Reviews and Meta-analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Runcie, J. A Complicated Case of Cor Triatriatum Dexter. Br. Heart J. 1968, 30, 729–731. [Google Scholar] [CrossRef][Green Version]
- Hansing, C.E.; Young, W.P.; Rowe, G.G. Cor Triatriatum Dexter: Persistent Right Sinus Venosus Valve. Am. J. Cardiol. 1972, 30, 559–564. [Google Scholar] [CrossRef]
- Ott, D.A.; Cooley, D.A.; Angelini, P.; Leachman, R.D. Successful Surgical Correction of Symptomatic Cor Triatriatum Dexter. J. Thorac. Cardiovasc. Surg. 1979, 78, 573–575. [Google Scholar] [CrossRef]
- Mazzucco, A.; Bortolotti, U.; Gallucci, V.; Del Torso, S.; Pellegrino, P. Successful Repair of Symptomatic Cor Triatriatum Dexter in Infancy. J. Thorac. Cardiovasc. Surg. 1983, 85, 140–143. [Google Scholar] [CrossRef]
- Alboliras, E.T.; Edwards, W.D.; Driscoll, D.J.; Seward, J.B. Cor Triatriatum Dexter: Two-Dimensional Echocardiographic Diagnosis. J. Am. Coll. Cardiol. 1987, 9, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Burton, D.A.; Chin, A.; Weinberg, P.M.; Pigott, J.D. Identification of Cor Triatriatum Dexter by Two-Dimensional Echocardiography. Am. J. Cardiol. 1987, 60, 409–410. [Google Scholar] [CrossRef] [PubMed]
- Trakhtenbroit, A.; Majid, P.; Rokey, R. Cor Triatriatum Dexter: Antemortem Diagnosis in an Adult by Cross-Sectional Echocardiography. Br. Heart J. 1990, 63, 314–316. [Google Scholar] [CrossRef] [PubMed]
- Savas, V.; Samyn, J.; Schreiber, T.L.; Hauser, A.; O’Neill, W.W. Cor Triatriatum Dexter: Recognition and Percutaneous Transluminal Correction. Cathet. Cardiovasc. Diagn. 1991, 23, 183–186. [Google Scholar] [CrossRef]
- Adad, S.J.; de Almeida-Ribeiro, R. Persistence of the Right Valve of the Sinus Venosus with Partial Division of the Right Atrium. Rev. Hosp. Clin. Fac. Med. Sao Paulo 1992, 47, 99–102. [Google Scholar]
- Fiorilli, R.; Argento, G.; Tomasco, B.; Serino, W. Cor Triatriatum Dexter Diagnosed by Transesophageal Echocardiography. J. Clin. Ultrasound 1995, 23, 502–504. [Google Scholar] [CrossRef]
- Dobbertin, A.; Warnes, C.A.; Seward, J.B. Cor Triatriatum Dexter in an Adult Diagnosed by Transesophageal Echocardiography: A Case Report. J. Am. Soc. Echocardiogr. 1995, 8, 952–957. [Google Scholar] [CrossRef]
- Ebeid, M.R.; Braden, D.S.; Gaymes, C.H.; Heath, B.; Joransen, J.A. Postsurgical Use of Amplatzer Septal Occluder in Cyanotic Patients with Pulmonary Atresia/Intact Ventricular Septum: Significance of Cor Triatriatum Dexter and Dilated Right Atrium. Catheter. Cardiovasc. Interv. 2000, 51, 186–191. [Google Scholar] [CrossRef]
- Roldán, F.J.; Vargas-Barrón, J.; Espinola-Zavaleta, N.; Romero-Cárdenas, A.; Keirns, C.; Vázquez-Antona, C.; Hernandez, J.P. Cor Triatriatum Dexter: Transesophageal Echocardiographic Diagnosis and Three-Dimensional Reconstruction. J. Am. Soc. Echocardiogr. 2001, 14, 634–636. [Google Scholar] [CrossRef]
- Joe, B.N.; Poustchi-Amin, M.; Woodard, P.K. Case 56: Cor Triatriatum Dexter. Radiology 2003, 226, 701–705. [Google Scholar] [CrossRef]
- Bisinov, E.A.; Dieter, R.S.; Ballantyne, F., III; Wolff, M.R.; Stein, J.H. Echocardiographic Diagnosis and Catheter Treatment of Hypotension Caused by Cor Triatriatum Dexter. J. Am. Soc. Echocardiogr. 2003, 16, 897–898. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, S.T.; Yildirir, A.; Simsek, V.; Bozbas, H.; Bilgi, M.; Ozin, B.; Muderrisoglu, H. Cor Triatriatum Dexter, Atrial Septal Defect, and Ebstein’s Anomaly in an Adult Given a Diagnosis by Transthoracic and Transesophageal Echocardiography: A Case Report. J. Am. Soc. Echocardiogr. 2004, 17, 780–782. [Google Scholar] [CrossRef] [PubMed]
- Kessel-Schaefer, A.; Linka, A.; Pretre, R.; Buser, P. Inferior Sinus Venosus Defect Associated with Incomplete Cor Triatriatum Dexter and Patent Foramen Ovale. Eur. J. Echocardiogr. 2006, 7, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Caliskan, M.; Erdogan, D.; Gullu, H.; Muderrisoglu, H. Cor Triatriatum Dexter in Two Adult Patients. Int. J. Cardiovasc. Imaging 2006, 22, 383–387. [Google Scholar] [CrossRef]
- Steen, H.; Merten, C.; Lehrke, S.; Lossnitzer, D.; Giannitsis, E.; Katus, H.A. Two Rare Cases of Left and Right Atrial Congenital Heart Disease: Cor Triatriatum Dexter and Sinister. Clin. Res. Cardiol. 2007, 96, 122–124. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, K.S.; Lee, J.B.; Kean-Ryu, J.; Choi, J.Y.; Chang, S.G. Cor Triatriatum Dexter Assessed by Three-Dimensional Echocardiography Reconstruction in Two Adult Patients. Echocardiography 2007, 24, 991–994. [Google Scholar] [CrossRef]
- Yarrabolu, T.R.; Simpson, L.; Virani, S.S.; Arora, H.; Navarijo, J.; Stainback, R.F. Cor Triatriatum Dexter. Tex. Heart Inst. J. 2007, 34, 383–385. [Google Scholar]
- Modi, K.; Reddy, P.; Madhusudanannair, V. Diagnosis of a Very Rare Variant of Cor Triatriatum Dexter by Contrast Echocardiography: A Case Report. Echocardiography 2009, 26, 220–223. [Google Scholar] [CrossRef]
- Galli, M.A.; Galletti, L.; Schena, F.; Salvini, L.; Mosca, F.; Danzi, G.B. A Rare Case of Neonatal Cyanosis Due to ‘Cor Triatriatum Dexter’ and a Review of the Literature. J. Cardiovasc. Med. 2009, 10, 535–538. [Google Scholar] [CrossRef]
- Mohan, J.C.; Tomar, D.; Shekhar, C. Congenitally Unguarded Tricuspid Valve Orifice with Multiple Other Defects in a Child with Refractory Heart Failure. Indian Heart J. 2009, 61, 89–92. [Google Scholar]
- Barrea, C.; Rubay, J.; Wagner, K.; Ovaert, C. Images in Cardiovascular Medicine: Cor Triatriatum Dexter Mimicking Ebstein Disease. Circulation 2009, 120, e86–e88. [Google Scholar] [CrossRef] [PubMed]
- Hoye, D.J.; Wilson, E.C.; Fyfe, D.A.; Guzzetta, N.A. Cor Triatriatum Dexter: A Rare Cause of Neonatal Cyanosis. Anesth. Analg. 2010, 110, 716–718. [Google Scholar] [CrossRef] [PubMed]
- Maroules, C.D.; Bhasin, M.; Sposato, J.; Whiting, E.; Mitchell, E. Evaluation of Cor Triatriatum Dexter with Use of 64-Slice Multidetector Computed Tomography. J. Cardiovasc. Comput. Tomogr. 2010, 4, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Brotons, J.A.; López-Pardo, F.J.; Rodríguez-Puras, M.J.; López-Haldón, J.E. Cor Triatriatum Dexter in Adults. Rev. Esp. Cardiol. 2010, 63, 998–999. [Google Scholar] [CrossRef]
- Januszewska, K.; Loeff, M.; Kozlik-Feldmann, R.; Franke, J.; Netz, H.; Malec, E.; Pozza, R.D. Cor Triatriatum Dexter: Rare Case of Neonatal Cyanosis. Clin. Res. Cardiol. 2010, 99, 861–863. [Google Scholar] [CrossRef]
- Salam, S.; Gallacher, D.; Uzun, O. Cor Triatriatum Dexter Masquerading as Ebstein’s Anomaly. Cardiol. Young 2011, 21, 354–356. [Google Scholar] [CrossRef]
- Cartón, A.J.; González Rocafort, Á.; Rubio, D.; García-Guereta, L. Persistent Embryonic Right Venous Valve Giving a Cor Triatriatum Dexter Appearance in a Cyanotic Neonate. J. Thorac. Cardiovasc. Surg. 2011, 142, e147–e148. [Google Scholar] [CrossRef]
- Fesslova, V.; Saracino, A.; Nuri, H.; Pomé, G. Cor Triatriatum Dexter: Unusual Features in Utero and after Birth. Interact. Cardiovasc. Thorac. Surg. 2012, 14, 330–332. [Google Scholar] [CrossRef]
- Martínez-Quintana, E.; Rodríguez-González, F.; Marrero-Santiago, H.; Santana-Montesdeoca, J.; López-Gude, M.J. Cor Triatriatum Dexter versus Prominent Eustachian Valve in an Adult Congenital Heart Disease Patient. Congenit. Heart Dis. 2013, 8, 589–591. [Google Scholar] [CrossRef]
- Zainudin, A.R.; Tiong, K.G.; Mokhtar, S.A. Cor Triatriatum Dexter: A Rare Cause of Childhood Cyanosis. Ann. Pediatr. Cardiol. 2012, 5, 92–94. [Google Scholar] [CrossRef]
- Udovičić, M.; Biočić, S.; Vincelj, J.; Crnogorac, M.; Sakić, I.; Starčević, B. Tetralogy of Fallot with Cor Triatriatum Dexter in an Adult Patient: A Case Report. Congenit. Heart Dis. 2013, 8, E77–E80. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, D.; Sivasankaran, S.; Venkateshwaran, S.; Sasidharan, B. Cor Triatriatum Dexter: A Rare Cause of Isolated Right Atrial Enlargement. Pediatr. Cardiol. 2013, 34, 198–199. [Google Scholar] [CrossRef]
- Aldawoodi, N.N.; Arora, H.; Kumar, P.A. Incidental Discovery of an Unusual Right Atrial Membrane in an Adult Patient. Ann. Card. Anaesth. 2012, 15, 309–311. [Google Scholar] [CrossRef] [PubMed]
- Nageh, M.F.; Watanabe, C.T.; Chou, E.T. Ablation of Isthmus and Non-Isthmus-Dependent Flutters in a Patient with Cor Triatriatum Dexter. Europace 2013, 15, 1573. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yamaguchi, R.; Ota, T.; Tanigawa, T.; Sakai, M.; Norioka, N.; Ishikawa, S.; Kawai, K.; Nishiyama, H.; Tsumori, T.; Kamimori, K.; et al. Cor Triatriatum Dexter with Atrial Septal Defect Evaluated by Real-Time Three-Dimensional Transesophageal Echocardiography. J. Echocardiogr. 2013, 11, 77–79. [Google Scholar] [CrossRef]
- Tufaro, V.; Slavich, M.; Fisicaro, A.; Ingallina, G.; Margonato, A.; Agricola, E. Right Heart Failure in a Patient with Unexplained Tricuspid Regurgitation and a Rare Congenital Heart Disease. G. Ital. Cardiol. 2013, 14, 555–557. [Google Scholar]
- Low, T.T.; Uy, C.C.; Wong, R.C. Unique Sail-Like Structure of Cor Triatriatum Dexter in Three-Dimensional Echocardiogram. Echocardiography 2014, 31, E212–E214. [Google Scholar] [CrossRef]
- Qureshi, A.U.; Latiff, H.A.; Sivalingam, S. Persistent Valve of Systemic Venous Sinus: A Cause of Neonatal Cyanosis. Cardiol. Young 2014, 24, 756–759. [Google Scholar] [CrossRef]
- Guler, Y.; Akgun, T.; Toprak, C.; Guler, A.; Esen, A.M. Complete A-V Block: Incidental or a Part of Cor Triatriatum Dexter. Perfusion 2014, 29, 238–241. [Google Scholar] [CrossRef]
- Omeje, I.; Christov, G.; Khambadkone, S.; Hsia, T.Y. Cor Triatriatum Dexter and Coarctation of the Aorta—A Rare Association in a 7-Year-Old Child with Type 1 Neurofibromatosis. Cardiol. Young 2015, 25, 308–311. [Google Scholar] [CrossRef]
- Simsek, Z.; Koza, Y.; Tas, H. Cor Triatriatum Dexter, Atrial Septal Defects, and Pulmonary Stenosis—A Rare Association. Echocardiography 2014, 31, E124–E127. [Google Scholar] [CrossRef]
- Yerebakan, C.; Valeske, K.; Esmaeili, A.; Akintuerk, H. Giant Remnant of Fetal Circulation Leading to Cyanosis: Pseudo-Cor Triatriatum Dexter. Interact. Cardiovasc. Thorac. Surg. 2014, 18, 690–692. [Google Scholar] [CrossRef]
- Kilit, C.; Oylumlu, M.; Doğan, A.; Amasyalı, B. Cor Triatriatum Dexter in a Patient with Pectus Excavatum: A Rare Cause of Right Heart Failure. Herz 2015, 40, 725–727. [Google Scholar] [CrossRef]
- Al-Mousily, F.; Baslaim, G.; Kouatli, A.; Al-Ata, J.; Arfi, A.M. Rare Combination of Bilateral Divided Atrial Chambers and Pulmonary Vein Stenosis with Review of the Literature. Cardiol. Young 2015, 25, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Vukovic, P.M.; Kosevic, D.; Milicic, M.; Jovovic, L.; Stojanovic, I.; Micovic, S. Cor Triatriatum Dexter and Atrial Septal Defect in a 43-Year-Old Woman. Tex. Heart Inst. J. 2014, 41, 418–420. [Google Scholar] [CrossRef] [PubMed]
- Mackman, C.A.; Liedel, J.L.; Woods, R.K.; Samyn, M.M. A Case Series of Patients with Cor Triatriatum Dexter: Unique Cause of Neonatal Cyanosis. Pediatr. Cardiol. 2015, 36, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Oh, Y.W. Assessment of Cor Triatriatum Dexter and Giant Eustachian Valve with Cardiac Magnetic Resonance. Circulation 2014, 130, 1727–1729. [Google Scholar] [CrossRef][Green Version]
- Hussain, S.T.; Mawulawde, K.; Stewart, R.D.; Pettersson, G.B. Cor Triatriatum Dexter: A Rare Cause of Myocardial Infarction and Pulmonary Embolism in a Young Adult. J. Thorac. Cardiovasc. Surg. 2015, 149, e48–e50. [Google Scholar] [CrossRef][Green Version]
- Özmen, G.; Demir, M.; Arı, H.; Aktaş, İ. A Rare Association: Concomitant Presence of Mitral Valve Blood Cyst with Atrial Septal Aneurysm and Cor Triatriatum Dexter. Anatol. J. Cardiol. 2015, 15, 502–503. [Google Scholar] [CrossRef]
- Eckersley, L.G.; Clements, B.; Shipton, S. Exercise-Induced Hypoxia Secondary to an Atrial Septal Defect and Cor Triatriatum Dexter. Cardiol. Young 2016, 26, 793–795. [Google Scholar] [CrossRef]
- Alghamdi, M.H. Cor Triatriatum Dexter: A Rare Cause of Cyanosis During Neonatal Period. Ann. Pediatr. Cardiol. 2016, 9, 46–48. [Google Scholar] [CrossRef] [PubMed]
- Elagizi, A.; Marvin, R.; O’Bryan, G.; Vyas, V.; Arcement, L. Three’s a Crowd—An Extremely Rare Case of Cor Triatriatum Dexter. J. La. State Med. Soc. 2017, 169, 50–51. [Google Scholar] [PubMed]
- Anyanwu, L.J.; Mohammad, A.; Muhammad, H.; Aliyu, I.; Abdullahi, L.; Farinyaro, A.; Iya, A. Schinzel-Giedion Syndrome: A Case with Sacrococcygeal Teratoma and Cor-Triatriatum Dexter. Pan Afr. Med. J. 2017, 26, 30. [Google Scholar] [CrossRef] [PubMed]
- Sozzi, F.B.; Montanaro, C.; Bacà, L.; Viani, G.M.; Zilocchi, M.; Canetta, C.; Meazza, R.; Pavone, L.; Lombardi, F. Cor Triatriatum Dexter Associated with Atrial Septal Defect: Management in a Complex Clinical Case. Echocardiography 2017, 34, 1725–1729. [Google Scholar] [CrossRef]
- Bennett, J.M.; Nikolla, I.; Hernandez, A. Deployment of a Sapien 3 Transcatheter Valve for Severe Tricuspid Insufficiency in a Patient with Non-Obstructive Cor Triatriatum Dexter. J. Cardiothorac. Vasc. Anesth. 2018, 32, 423–428. [Google Scholar] [CrossRef]
- León, R.L.; Zaban, N.B.; Schamberger, M.S.; Ho, C.Y.; Mietzsch, U. Cyanosis and Stroke Due to Functional Cor Triatriatum Dexter in a Neonate. Neonatology 2018, 113, 231–234. [Google Scholar] [CrossRef]
- Zoltowska, D.; Kalavakunta, J.K. Cor Triatriatum Dexter. Clin. Case Rep. 2018, 6, 1189–1190. [Google Scholar] [CrossRef]
- Aliyu, I.; Ibrahim, Z.F. Cor-Triatriatum Dexter with Associated Cyanosis in a 3-Month-Old Girl. J. Cardiovasc. Echogr. 2018, 28, 143–145. [Google Scholar] [CrossRef]
- Theodoropoulos, K.C.; Papachristidis, A.; Harries, D.; Sado, D.M.; Monaghan, M.J. Agitated Saline Contrast Echocardiography Reveals Cor Triatriatum Dexter. Echocardiography 2018, 35, 1895–1897. [Google Scholar] [CrossRef]
- Rao, S.; Suntharos, P.; Najm, H.; Komarlu, R. Cor Triatriatum Dexter with Right Ventricular Hypoplasia: Role of Multimodality Imaging in Decision Making. Echocardiography 2018, 35, 2113–2116. [Google Scholar] [CrossRef]
- Rozema, T.K.; Arruda, J.; Snyder, C.S. Cor Triatriatum: A Tale of Two Membranes. CASE 2019, 3, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Haboub, M.; Drighil, A. Successful Balloon Valvuloplasty of a Subpulmonic Membrane Associated with Cor Triatriatum Dexter: A Case Report. J. Med. Case Rep. 2019, 13, 291. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Yoshitomi, H.; Ishikura, M.; Endo, A.; Tanabe, K. Cor Triatriatum Dexter Associated with Atrial Septal Defect and Mitral Valve Regurgitation. J. Echocardiogr. 2021, 19, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Bindra, B.S.; Patel, Z.; Patel, N.; Choudhary, K.V.; Patel, V. Cor Triatriatum Dexter as an Incidental Finding: Role of Two-Dimensional Transthoracic Echocardiography. Cureus 2019, 11, e5683. [Google Scholar] [CrossRef]
- Tzeis, S.; Asvestas, D.; Sakadakis, E.; Trika, C.; Vardas, P. Atrial Fibrillation Cryoablation in Cor Triatriatum Dexter. Europace 2020, 22, 1. [Google Scholar] [CrossRef]
- Lugtu, I.C.; Hu, Y.F.; Lin, Y.J.; Walia, R.; Liu, C.M.; Chang, S.L.; Chung, F.P.; Liao, J.N.; Lo, L.W.; Chen, S.A. Catheter Ablation of Complex Atrial Tachyarrhythmias in Adult Patients with Cor Triatriatum. J. Interv. Card. Electrophysiol. 2021, 62, 277–283. [Google Scholar] [CrossRef]
- Hurtado-Sierra, D.; Fernández-Gómez, O.; Manrique-Rincón, F.; Buendía-Hernández, A.; Vázquez-Antona, C.A. Cor Triatriatum Dexter: An Unusual Cause of Neonatal Cyanosis. Arch. Cardiol. Mex. 2020, 91, 361–363. [Google Scholar] [CrossRef]
- Alvarez-Santana, R.; Garcia-Diaz, J.A.; Escudero-Salamanca, M.; Cano-Zarate, R.; Espinola-Zavaleta, N. Cor Triatriatum Dexter Associated to Ebstein Anomaly with Tricuspid Double Lesion and Atrial Septal Defect. Arch. Cardiol. Mex. 2021, 91, 508–509. [Google Scholar] [CrossRef]
- Goel, A.; Viswamitra, S.; Reddy, B.N.; Gaduputi, J. Computed Tomography Features of Cor Triatriatum: An Institutional Review. Br. J. Radiol. 2021, 94, 20201252. [Google Scholar] [CrossRef]
- Minciunescu, A.; Ekanem, E.; Nitta, B.; Castro, F.; Dhanani, H. Intraparenchymal Brain Abscess in an Adult Male with Underlying Ebstein Anomaly and Cor Triatriatum Dexter. JACC Case Rep. 2021, 3, 194–197. [Google Scholar] [CrossRef]
- Kalangos, A.; Shatelen, N.; Demyanchuk, V.; Ruban, N.; Sfyridis, P.; Todurov, B. Cor Triatriatum Dexter in Children: Literature Review and Case Report. JTCVS Tech. 2020, 4, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, M.; Pradhan, A.; Vishwakarma, P.; Sethi, R. Cor Triatriatum Dexter with Noncompaction of Right Ventricle and Complete Heart Block: An Unholy Trinity. Int. J. Angiol. 2021, 33, 202–204. [Google Scholar] [CrossRef] [PubMed]
- Derimay, F.; Gamondes, D.; Rioufol, G. First Case of Complete Percutaneous Correction of Isolated Divided Atrium (or Cor Triatriatum) Dexter. Can. J. Cardiol. 2021, 37, 1867–1869. [Google Scholar] [CrossRef] [PubMed]
- Persia-Paulino, Y.R.; Bouzas-Zubeldia, B.; Martinez-Bendayan, I.; Rueda-Nuñez, F. A Red Flag Before Patent Foramen Ovale Closure: Recognizing the Incomplete Cor Triatriatum Dexter. Int. J. Cardiovasc. Imaging 2022, 38, 113–115. [Google Scholar] [CrossRef]
- Hanna, G.; Savoj, J.; Iftikhar, S.; Hu, P. Cor Triatriatum Dexter: A Case Report in a 70-Year-Old Male. J. Med. Cases 2020, 11, 234–238. [Google Scholar] [CrossRef]
- Barbieri, F.; Schröder, M.; Beyhoff, N.; Landmesser, U.; Reinthaler, M.; Kasner, M. Percutaneous Edge-to-Edge Tricuspid Valve Repair in a Patient with Cor Triatriatum Dexter: A Case Report. J. Cardiovasc. Dev. Dis. 2021, 8, 111. [Google Scholar] [CrossRef]
- Poretti, G.; Hoxha, S.; Segreto, A.; Sandrini, C.; Murari, A.; Prioli, M.A.; Faggian, G.; Luciani, G.B. Cor Triatriatum and Intracardiac Anomalous Pulmonary Venous Return: An Inborn Atrial Flow Inversion. Ann. Thorac. Surg. 2022, 113, e453–e455. [Google Scholar] [CrossRef]
- Patel, M.; Vaidhya, N.; Patel, K.; Sheth, M.; Mishra, A. Cor Triatriatum Dexter: A Rare Cause of Aneurysmal Right Atrium. World J. Pediatr. Congenit. Heart Surg. 2022, 13, 253–256. [Google Scholar] [CrossRef]
- Sunthankar, S.; Do, N.L.; Parra, D.; Vera, K.; Soslow, J.H. Mysterious Infantile Cyanosis: An Imaging Case Series. CASE 2021, 5, 267–272. [Google Scholar] [CrossRef]
- Hasnie, U.A.; Prejean, S.P.; Ahmed, A.N.; Ahmed, M.I.; Law, M.A. Transcatheter Balloon Dilatation of Cor Triatriatum Dexter with Percutaneous Atrial Septal Defect Closure. J. Cardiol. Cases 2021, 25, 68–71. [Google Scholar] [CrossRef]
- Chen, P.H.; Liu, Y.C.; Dai, Z.K.; Chen, I.C.; Lo, S.H.; Wu, J.R.; Wu, Y.H.; Hsu, J.H. A Rare Complication During Transcatheter Closure of Double Atrial Septal Defects with Incomplete Cor Triatriatum Dexter: A Case Report. Front. Cardiovasc. Med. 2022, 8, 815312. [Google Scholar] [CrossRef] [PubMed]
- De Michele, F.; Paparella, M.T.; Forte, V.; Chieppa, D.R.R.; Nemore, F.; Guglielmi, G. Cor Triatriatum Dexter: A Rare Incidentaloma. Acta Biomed. 2022, 93, e2022093. [Google Scholar] [PubMed]
- Fuentes Rojas, S.C.; Lawrie, G.; Faza, N.N. Cor Triatriatum Dexter: An Innocent Bystander. Methodist. Debakey Cardiovasc. J. 2022, 18, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Picciolli, I.; Francescato, G.; Colli, A.M.; Cappelleri, A.; Mayer, A.; Raschetti, R.; Di Cosola, R.; Pisaniello, M.; Annoni, G.A.; Papa, M.; et al. Cor Triatriatum Dexter: Contrast Echocardiography Is Key to the Diagnosis of a Rare but Treatable Cause of Neonatal Persistent Cyanosis. Children 2022, 9, 676. [Google Scholar] [CrossRef]
- Liang, L.; Lu, M.J. Multimodality Imaging of Cor Triatriatum Dexter Complicated with Hypertrophic Cardiomyopathy of Restrictive Phenotype. Radiol. Case Rep. 2022, 17, 2598–2602. [Google Scholar] [CrossRef]
- Caputo, A.; Giordano, M.; Iacono, C.; Oppido, G.; Russo, M.G. A Rare Cause of Cyanosis in Neonatal Age: Cor Triatriatum Dexter. Ann. Pediatr. Cardiol. 2022, 15, 429–430. [Google Scholar] [CrossRef]
- He, L.; Du, Y.J.; Wang, X.Y.; Wang, H.Y.; Zhang, Y.S. Percutaneous Closure of a Cor Triatriatum Dexter with an Inferior Sinus Venosus and Secundum Atrial Septal Defect without Distance to Coronary Sinus Ostium and a Patent Foramen Ovale. Eur. Heart J. Case Rep. 2023, 7, ytad127. [Google Scholar] [CrossRef]
- Anastasakis, E.; Grosomianidis, V.; Tossios, P.; Charaf, A.; Sarsam, M.A.I.; Karapanagiotidis, G.T. Cor Triatriatum Dexter as an Incidental Finding Due to Symptomatic Bicuspid Aortic Valve Stenosis. Perfusion 2024, 39, 1274–1276. [Google Scholar] [CrossRef]
- Binder, M.S.; Binder, I.E.; Habib, A.S.; Herold, S.E.; Gay, W.M.; Lystash, J.C. Cor Triatriatum Dexter Associated with a Dysplastic Tricuspid Valve. CASE 2023, 7, 360–364. [Google Scholar] [CrossRef]
- Kazma, H.; Fakih, M.; Raad, A.; Saleh, A.; Mohammed, M. Cor Triatriatum Dexter with a Sinus Venosus Atrial Septal Defect in a 50-Year-Old Woman: A Case Report. Cureus 2024, 16, e53477. [Google Scholar] [CrossRef]
- Roldan, C.A.; Moazez, C.; Yatskowitz, J.; Maoz-Metzl, D.; Castlemain, B.; Fischer, E. Cor Triatriatum Dexter and Right Atrial Mass Causing Severe Inflow Obstruction. CASE 2024, 8, 286–291. [Google Scholar] [CrossRef]
- Kuźma, J.; Buczyński, M.; Lim, G.Y.; Mądry, W.; Kuśmierczyk, M. Saline-Agitated Echocardiography in Diagnosis of Cor Triatriatum Dexter: Case Series and Literature Review. Kardiol. Pol. 2024, 82, 1146–1148. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Benítez, R.; Reyes-Vázquez, H.L. Cor Triatriatum Dexter: An Uncommon Cause of Neonatal Cyanosis. Bol. Med. Hosp. Infant. Mex. 2024, 81, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Alotay, A.; Dowaikh, A.; Alsahari, A.; Momenah, T. Effective Management of Cor Triatriatum Dexter Using Double Balloon Dilatation in a Paediatric Age Group Case-Report Study. CJC Pediatr. Congenit. Heart Dis. 2024, 3, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Daralammouri, Y.; Azamtta, M.; Mahmoud, Q.J.A. An Uncommon Cardiovascular Abnormality: Case Report of Cor Triatriatum Associated with Persistent Left Superior Vena Cava and Coronary Sinus Dilation. Radiol. Case Rep. 2024, 20, 1236–1242. [Google Scholar] [CrossRef]
- Shah, B.; Uppal, A.; Kunal, S.; Prajapati, S.; Gupta, A. Triatrial Appearance in a Patient with Atrial Septal Defect: A Case Report. Cureus 2025, 17, e84011. [Google Scholar] [CrossRef]
- Killen, A.W.; Herbert, A.; Oketcho, M.; Muhoozi, R.M.; Obongnyinge, B.; Lubega, S.; Aliku, T. Symptomatic 9-Year-Old Girl with Cor Triatriatum Dexter, Atrial Septal Defect, and Severe Pulmonary Valve Stenosis. CASE 2025, 9, 237–241. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Zhong, C. Case of a Middle-Aged Woman with Sick Sinus Syndrome and Cor Triatriatum Dexter. Braz. J. Cardiovasc. Surg. 2025, 40, e20240330. [Google Scholar] [CrossRef]
- Taha, A.K.; Abdelaziz Ismaiel, M.; Amer, R.M.; Hesham Hammad, A.; Naji, H. Incidentally Discovered Adult Congenital Heart Disease in a Patient with Decompensated Heart Failure. Cureus 2025, 17, e89496. [Google Scholar] [CrossRef]
- Franco, E.; Rovera, C.; Moretti, C.; Bassareo, P.P. Arrhythmogenic Right Ventricular Cardiomyopathy and Cor Triatriatum Dexter: An Unreported Association. Clin. Case Rep. 2025, 13, e71159. [Google Scholar] [CrossRef]
- Chaaban, B.; Bitar, H.; Hamade, A.; Semaan, I.; Rachid, A. Delayed Diagnosis of Cor Triatriatum Dexter: A Case Report and Comprehensive Review of Embryology, Imaging, and Management. Cureus 2025, 17, e93381. [Google Scholar] [CrossRef]
- Thomka, I.; Bendig, L.; Szente, A.; Arvay, A. Cor Triatriatum Dextrum Simulating Right Ventricular Myxoma and Pulmonary Stenosis. Thorac. Cardiovasc. Surg. 1983, 31, 114–116. [Google Scholar] [CrossRef] [PubMed]
- Lepage, J.R. Cor Triatriatum Dextrum and Persistent Muscle of Lower, Presenting as Budd-Chiari Syndrome: Embryology of a Very Rare Disorder with Unique Morphologic, Physiologic and Clinical Presentations. Angiology 1983, 34, 491–508. [Google Scholar] [CrossRef]
- Sarikouch, S.; Blanz, U.; Sandica, E.; Beerbaum, P. Adult Congenital Heart Disease: Cor Triatriatum Dextrum. J. Thorac. Cardiovasc. Surg. 2006, 132, 164–165. [Google Scholar] [CrossRef] [PubMed]
- Sahin, T.; Bildirici, U.; Kandemir, C.; Celikyurt, U.; Ural, D.; Komsuoglu, B. Infective Endocarditis in the Setting of Infundibular-Valvular Pulmonary Stenosis with Incomplete Cor Triatriatum Dextrum and Patent Foramen Ovale. Int. J. Cardiol. 2008, 127, e129–e131. [Google Scholar] [CrossRef] [PubMed]
- Benyounes, N.; Devys, J.M.; Cohen, A. Diagnosis of Cor Triatriatum Dextrum Using Transoesophageal Echocardiography with a Bubble Study. Arch. Cardiovasc. Dis. 2014, 107, 272–273. [Google Scholar] [CrossRef][Green Version]
- Aljemmali, S.; Bokowski, J.; Morales, R.; Abdulla, R.I. Chiari Network Associated with Hypoxemia in a Neonate: Case Report and Review of the Literature. Pediatr Cardiol. 2020, 41, 1529–1531. [Google Scholar] [CrossRef]
- Xiang, K.; Moukarbel, G.V.; Grubb, B. Permanent Transvenous Pacemaker Implantation in a Patient with Cor Triatriatum Dextrum. World J. Cardiol. 2015, 7, 43–46. [Google Scholar] [CrossRef]
- Montealegre-Gallegos, M.; Bortman, J.; Chaudhry, D.; Mahmood, F. Adult Congenital Heart Defects: How Many Is Too Many? J. Cardiothorac. Vasc. Anesth. 2016, 30, 848–851. [Google Scholar] [CrossRef]
- Aboukhoudir, F.; Aboukhoudir, I.; Rica, O.; Khennine, B.; Pansieri, M.; Rekik, S. Atypical Form of Cor Triatrium in a 59-Year-Old Man. Ann. Cardiol. Angeiol. 2016, 65, 355–358. [Google Scholar] [CrossRef]
- Doucette, J.; Knoblich, R. Persistent Right Valve of the Sinus Venosus: So-Called Cor Triatriatum Dextrum: Review of the Literature and Report of a Case. Arch. Pathol. 1963, 75, 105–112. [Google Scholar]
- Said, S.M. Commentary: Cor Triatriatum Dexter: A Tale of 2 Horns. JTCVS Tech. 2020, 4, 259–260. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A.; Fahey, T.D. Exercise Physiology: Human Bioenergetics and Applications; John Wiley & Sons: New York, NY, USA, 1984; pp. 295–339. [Google Scholar]
- Behrman, R.E.; Nelson, W.E. (Eds.) Textbook of Pediatrics, 14th ed.; WB Saunders: Philadelphia, PA, USA, 1993; pp. 1144–1471. [Google Scholar]
- Mercuro, G.; Bassareo, P.P.; Mariucci, E.; Deidda, M.; Zedda, A.M.; Bonvicini, M. Sex Differences in Congenital Heart Defects and Genetically Induced Arrhythmias. J. Cardiovasc. Med. 2014, 15, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Gold, B.M.; Parekh, D.R.; Kearney, D.L.; Silva, G.V.; Fish, R.D.; Stainback, R.F. Forme Fruste Cor Triatriatum Dexter by Transesophageal Echocardiography and Its Impact on Percutaneous Heart Procedures: A Case Series. CASE 2019, 3, 189–199. [Google Scholar] [CrossRef] [PubMed]
- National Organization for Rare Disorders (NORD). Cor Triatriatum. Available online: https://rarediseases.org/rare-diseases/cor-triatriatum/ (accessed on 23 November 2025).
- Ogino, H.; Asaka, T.; Inanami, H.; Suzuki, K.; Shiratori, K.; Yoshida, K.; Koizumi, K.; Okumachi, F.; Yanagihara, K.; Kato, H.; et al. Cor Triatriatum Dexter: A Case Report with Particular Reference to the Echocardiographic Features. J. Cardiogr. 1985, 15, 543–550. [Google Scholar]
- Samal, A.K.; Nanda, N.C.; Thakur, A.C.; Aggarwal, R.; Jamil, F.; Kapur, G.; Aaluri, S.R.; Moursi, M.; McGiffin, D.; Kirklin, J. Three-Dimensional Echocardiographic Reconstruction of Atrial Membranes. Echocardiography 1998, 15, 605–610. [Google Scholar] [CrossRef]
- Lanzarini, L.; Lucca, E.; Fontana, A.; Foresti, S. Cor Triatriatum Dextrum Resulting from the Persistence of Embryonic Remnants of the Right Valve of the Sinus Venosus: Prevalence and Echocardiographic Aspects in a Large Consecutive Non-Selected Patient Population. Ital. Heart J. Suppl. 2001, 2, 1209–1216. [Google Scholar]
- Malik, S.B.; Kwan, D.; Shah, A.B.; Hsu, J.Y. The Right Atrium: Gateway to the Heart—Anatomic and Pathologic Imaging Findings. Radiographics 2015, 35, 14–31. [Google Scholar] [CrossRef]
- McLean, G.; Menahem, S.; Teoh, M. Prenatal Diagnosis of Cor Triatriatum Dexter. Ultrasound Obstet. Gynecol. 2010, 36, 777–778. [Google Scholar] [CrossRef]
- Maroun, L.L.; Graem, N.; Skibsted, L. Fetal Cor Triatriatum Dexter: A Report of Two Cases Associated with Nuchal Edema in the Early Second Trimester. Pediatr. Dev. Pathol. 2008, 11, 59–62. [Google Scholar] [CrossRef]
- Maiques Magraner, E.; Durante-López, A.; Balbacid Domingo, E.; Abelleira Pardeiro, C.; Sánchez-Recalde, Á.; Gutiérrez-Larraya Aguado, F. Incomplete Cor Triatriatum Dexter: An Unsettling Guest in the Percutaneous Closure of Atrial Septal Defects. Rev. Esp. Cardiol. (Engl. Ed.) 2019, 72, 582–583. [Google Scholar] [CrossRef]
- Rigatelli, G.; Zuin, M.; Nghia, N.T. Interatrial Shunts: Technical Approaches to Percutaneous Closure. Expert Rev. Med. Devices 2018, 15, 707–716. [Google Scholar] [CrossRef]
- Sankhyan, L.K.; Anderson, R.H.; Chowdhury, U.K.; George, N.; Pradeep, D.; Vaswani, P.; Pandey, N.N.; Arvind, B. Surgical Management of Divided Atrial Chambers. J. Card. Surg. 2021, 36, 4267–4279. [Google Scholar] [CrossRef]
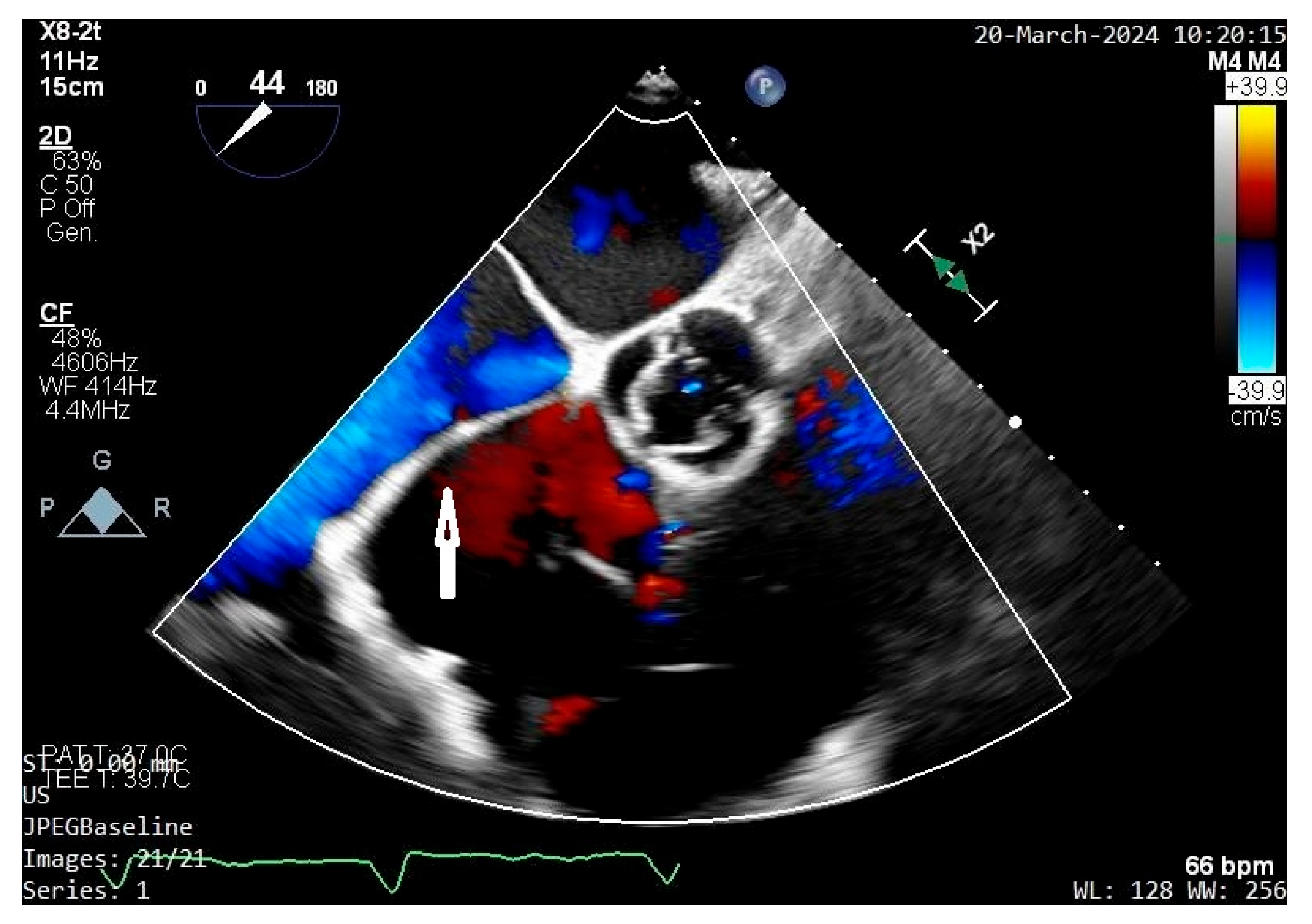
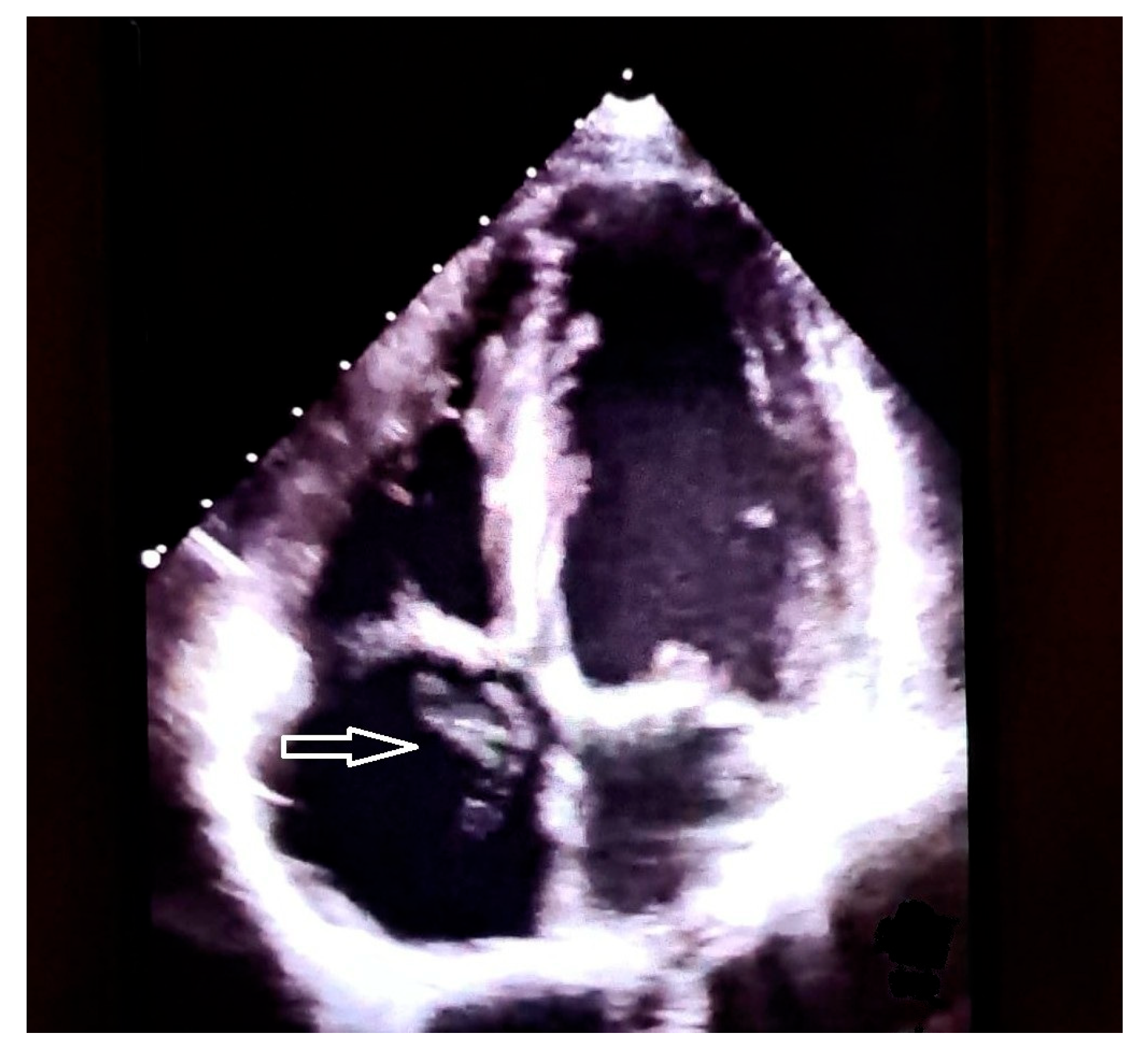
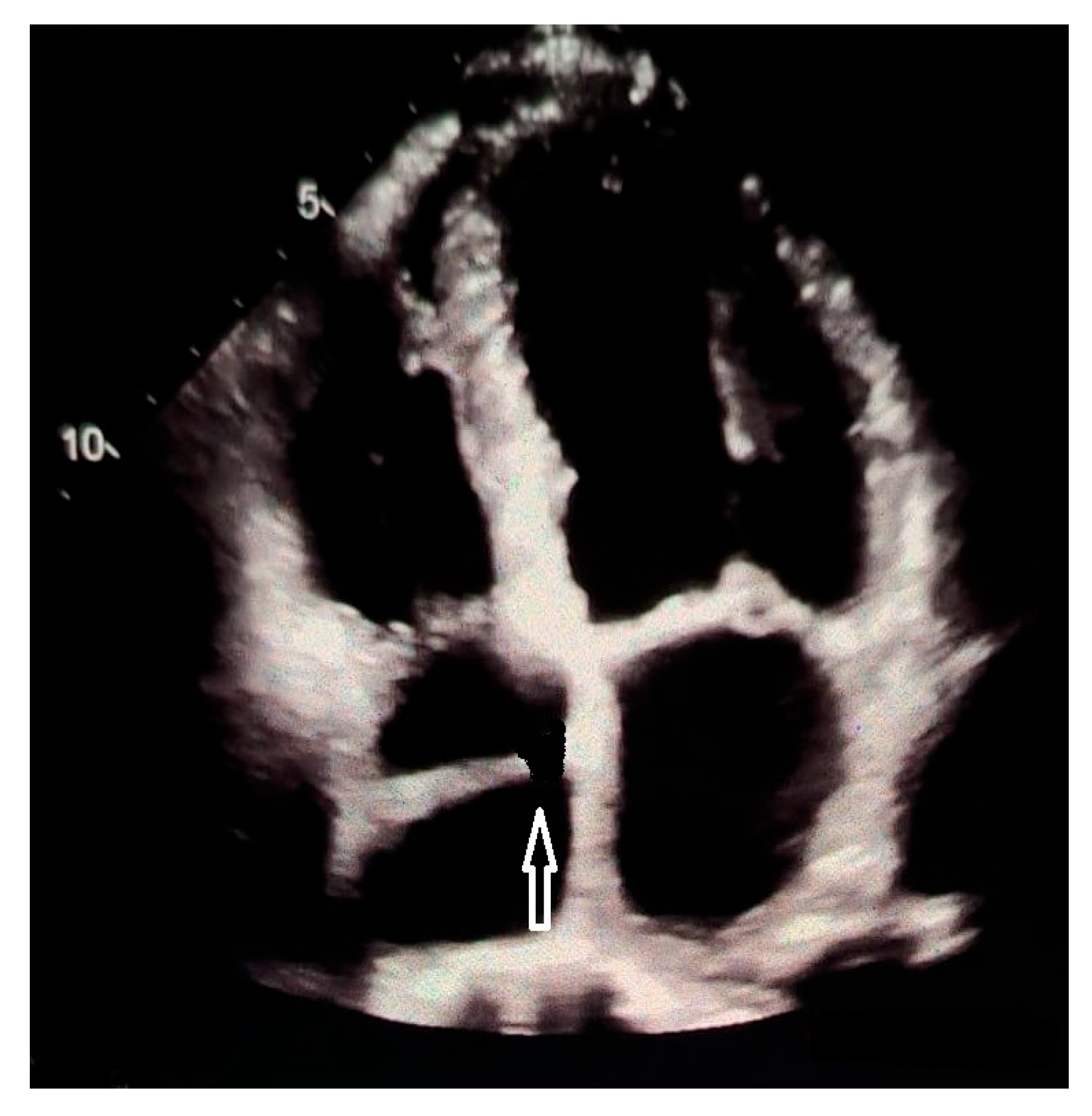
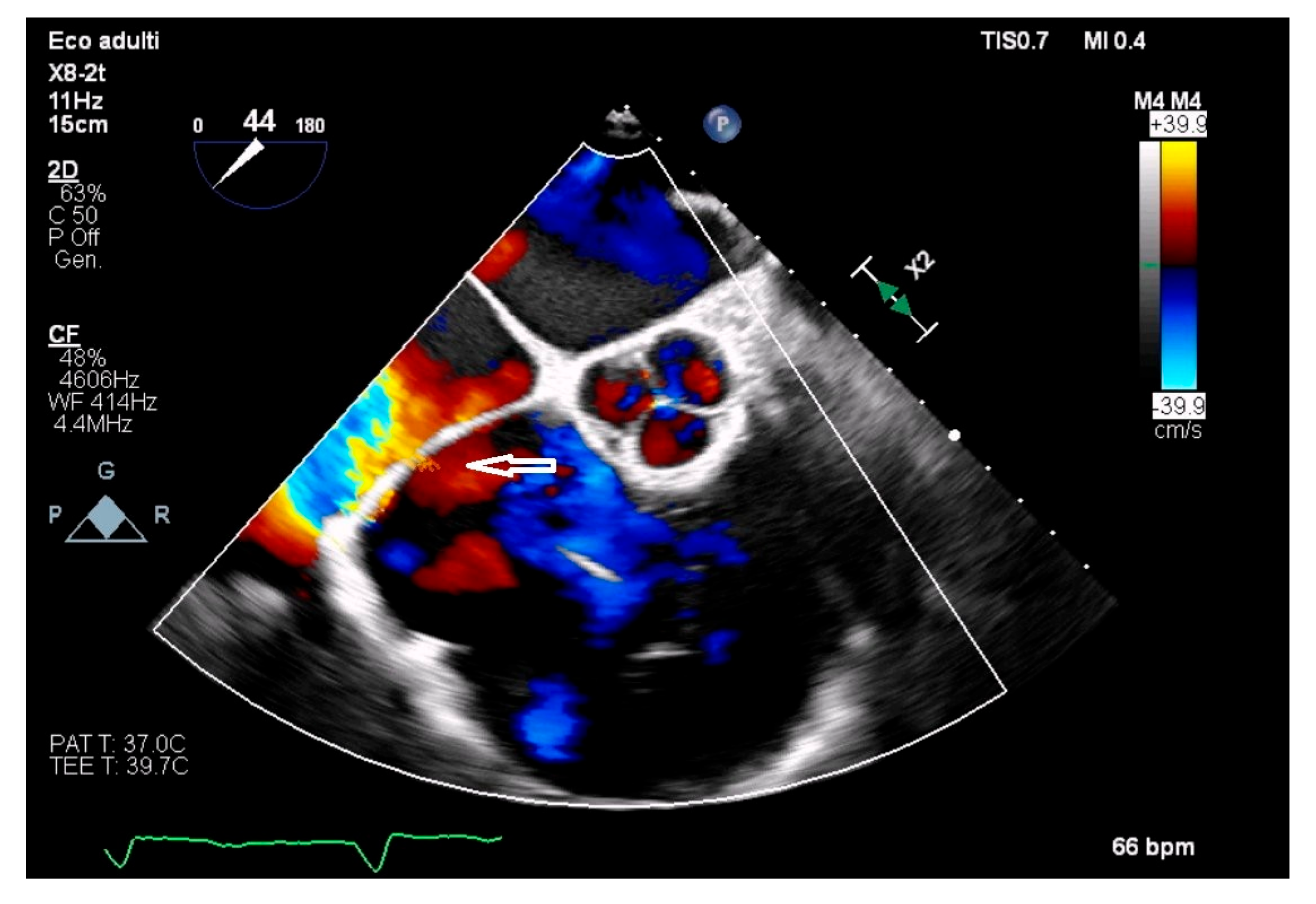
| Features | |
|---|---|
| Age | 33.3 ± 9.4 years |
| Symptoms | dyspnoea 44.3% (54/122) |
| Cyanosis 29.5% (36/122) | |
| TIA/stroke 4.1% (5/122) | |
| ECG | SVT/AF/Afib 33.3% (25/75) |
| RBBB 22.6% (17/75) | |
| RA enlargement 13.3% (10/75) | |
| CHB 35.3% (4/75) | |
| Chest X-ray | Cardiomegaly 46.5% (20/43) |
| Echocardiography | used in 95.2% (118/124) |
| MRI | used in 22.6% (28/124) |
| CT | used in 17.7% (22/124) |
| Concomitant cardiac abnormalities | 67.7% (84/124) |
| Treatment | Surgery 51.6% (50/97) |
| Conservative 43.3% (42/97) | |
| Device-based 5.1% (5/97) | |
| Death | 8.2% (8/97) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Bassareo, P.P.; Franco, E.; Duignan, S.; Chessa, M.; Cascio, M.; McMahon, C.J.; Walsh, K.P.; Perrone, M.A. Cor Triatriatum Dexter: The Largest Comprehensive Review in the Field on 124 Worldwide Cases (1968–Now). J. Cardiovasc. Dev. Dis. 2026, 13, 76. https://doi.org/10.3390/jcdd13020076
Bassareo PP, Franco E, Duignan S, Chessa M, Cascio M, McMahon CJ, Walsh KP, Perrone MA. Cor Triatriatum Dexter: The Largest Comprehensive Review in the Field on 124 Worldwide Cases (1968–Now). Journal of Cardiovascular Development and Disease. 2026; 13(2):76. https://doi.org/10.3390/jcdd13020076
Chicago/Turabian StyleBassareo, Pier Paolo, Erica Franco, Sophie Duignan, Massimo Chessa, Mariateresa Cascio, Colin Joseph McMahon, Kevin Patrick Walsh, and Marco Alfonso Perrone. 2026. "Cor Triatriatum Dexter: The Largest Comprehensive Review in the Field on 124 Worldwide Cases (1968–Now)" Journal of Cardiovascular Development and Disease 13, no. 2: 76. https://doi.org/10.3390/jcdd13020076
APA StyleBassareo, P. P., Franco, E., Duignan, S., Chessa, M., Cascio, M., McMahon, C. J., Walsh, K. P., & Perrone, M. A. (2026). Cor Triatriatum Dexter: The Largest Comprehensive Review in the Field on 124 Worldwide Cases (1968–Now). Journal of Cardiovascular Development and Disease, 13(2), 76. https://doi.org/10.3390/jcdd13020076







