Impaired Left Atrial Strain as an Early Marker of Cardiac Involvement in Type 2 Diabetes Mellitus: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Clinical Data Collection
2.4. Statistical Analysis
3. Results
3.1. Baseline Clinical Characteristics
3.2. Echocardiographic Parameters
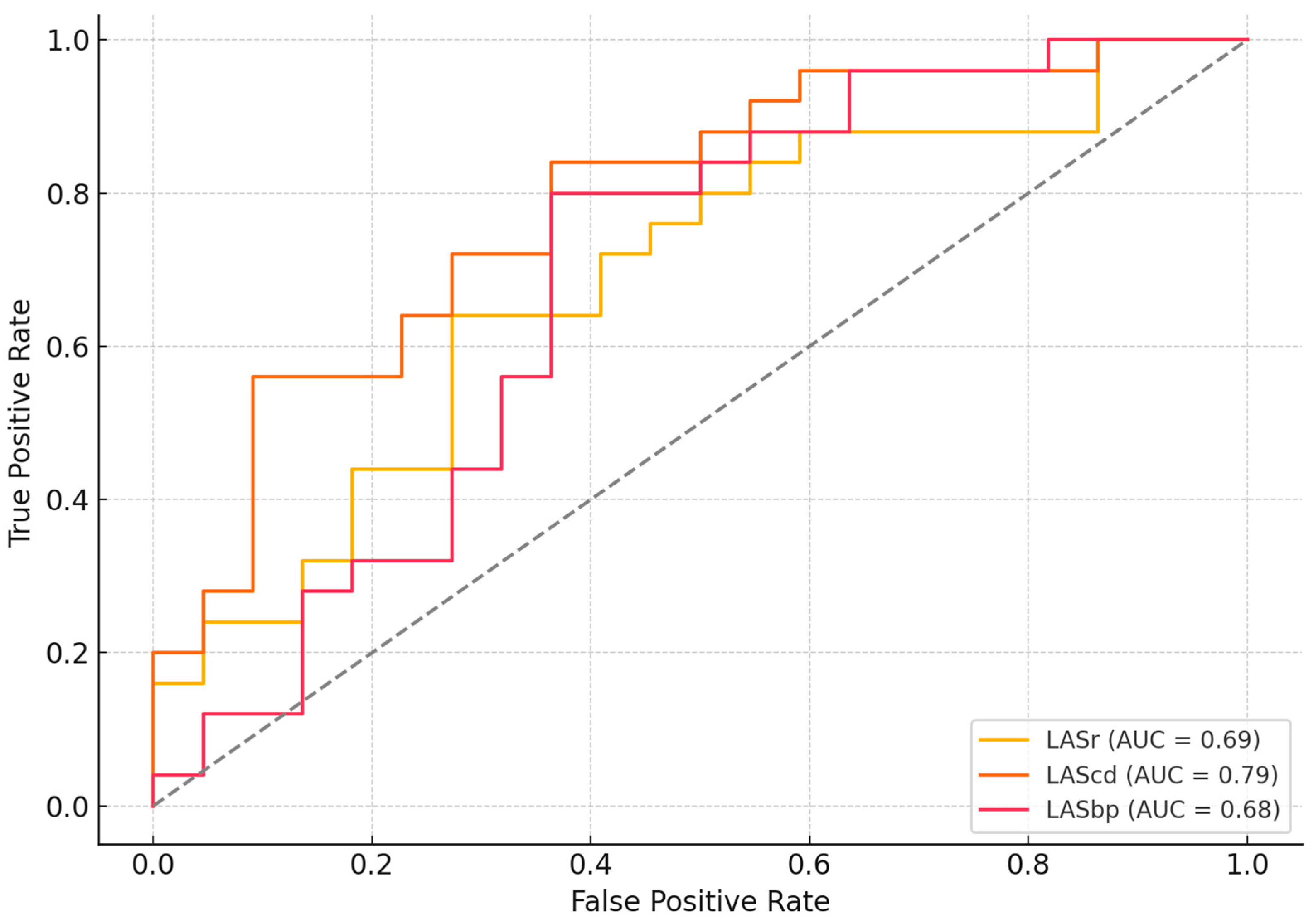
3.3. Importance of LAS Parameters in Predicting Diabetic Cardiomyopathy
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
| LA | left atrium |
| T2DM | type 2 diabetes mellitus |
| LASr | left atrial strain at reservoir |
| LAScd | left atrial strain at conduit |
| LASbp | left atrial strain at booster-pump phase |
| HbA1c | glycosylated hemoglobin |
| DM | diabetes mellitus |
| CVD | cardiovascular disease |
| LV | left ventricular |
| LAVI | left atrial indexed volume |
| LAS | left atrium strain |
| ESC | European Society of Cardiology |
| CCTA | coronary computed tomography angiography |
| BMI | body mass index |
| BSA | body surface area |
| LDL | low-density lipoprotein |
| HDL | high-density lipoprotein |
| NT-proBNP | N-terminal proB-type natriuretic peptide |
| 2D | two-dimensional |
| ASE/EACVI | American Society of Echocardiography/European Association of Cardiovascular Imaging |
| SD | standard deviations |
| ROC | receiver operating characteristic |
| AUC | area under the curve |
| SGLT2 | sodium-glucose cotransporter-2 |
| GLP-1 | glucagon-like peptide-1 |
| DBP | diastolic blood pressure |
| SBP | systolic blood pressure |
| LVEF | left ventricular ejection fraction |
| LVEDV | left ventricular end-diastolic volume |
| LVESV | left ventricular end-systolic volume |
| GLS | global longitudinal strain |
| OR | odds ratio |
| AF | atrial fibrillation |
References
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes prevalence and treatment from 1990 to 2022: A pooled analysis of 1108 population-representative studies with 141 million participants. Lancet 2024, 404, 2077–2093, Erratum in Lancet 2025, 405, 1146. [Google Scholar]
- Benchea, L.C.; Anghel, L.; Zăvoi, A.; Chiuariu, T.; Birgoan, S.G.; Sascău, R.A.; Stătescu, C. Beyond Blood Sugar: How Left Atrium Strain Predicts Cardiac Outcomes in Type 2 Diabetes. Biomedicines 2024, 12, 1690. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.; Popa, S.G.; Mota, E.; Mitrea, A.; Catrinoiu, D.; Cheta, D.M.; Guja, C.; Hancu, N.; Ionescu-Tirgoviste, C.; Lichiardopol, R.; et al. Prevalence of diabetes mellitus and prediabetes in the adult Romanian population: PREDATORR study. J. Diabetes 2016, 8, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Miyoshi, T.; Yoshida, M.; Akagi, S.; Saito, Y.; Ejiri, K.; Matsuo, N.; Ichikawa, K.; Iwasaki, K.; Naito, T.; et al. Pathophysiology and Treatment of Diabetic Cardiomyopathy and Heart Failure in Patients with Diabetes Mellitus. Int. J. Mol. Sci. 2022, 23, 3587. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Norton, V.; Cui, K.; Zhu, B.; Bhattacharjee, S.; Lu, Y.W.; Wang, B.; Shan, D.; Wong, S.; Dong, Y.; et al. Diabetes and Its Cardiovascular Complications: Comprehensive Network and Systematic Analyses. Front. Cardiovasc. Med. 2022, 9, 841928. [Google Scholar] [CrossRef]
- Siam, N.H.; Snigdha, N.N.; Tabasumma, N.; Parvin, I. Diabetes Mellitus and Cardiovascular Disease: Exploring Epidemiology, Pathophysiology, and Treatment Strategies. Rev. Cardiovasc. Med. 2024, 25, 436. [Google Scholar] [CrossRef]
- Marx, N.; Federici, M.; Schütt, K.; Müller-Wieland, D.; Ajjan, R.A.; Antunes, M.J.; Christodorescu, R.M.; Crawford, C.; Di Angelantonio, E.; Eliasson, B.; et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur. Heart J. 2023, 44, 4043–4140. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 10. Cardiovascular Disease and Risk Management: Standards of Care in Diabetes-2024. Diabetes Care 2024, 47 (Suppl. 1), S179–S218. [Google Scholar] [CrossRef]
- Huo, J.L.; Feng, Q.; Pan, S.; Fu, W.J.; Liu, Z.; Liu, Z. Diabetic cardiomyopathy: Early diagnostic biomarkers, pathogenetic mechanisms, and therapeutic interventions. Cell Death Discov. 2023, 9, 256. [Google Scholar] [CrossRef]
- Gulsin, G.S.; Athithan, L.; McCann, G.P. Diabetic cardiomyopathy: Prevalence, determinants and potential treatments. Ther. Adv. Endocrinol. Metab. 2019, 10, 2042018819834869. [Google Scholar] [CrossRef]
- Kadappu, K.K.; Boyd, A.; Eshoo, S.; Haluska, B.; Yeo, A.E.; Marwick, T.H.; Thomas, L. Changes in left atrial volume in diabetes mellitus: More than diastolic dysfunction? Eur. Heart J. Cardiovasc. Imaging 2012, 13, 1016–1023. [Google Scholar] [CrossRef]
- Atas, H.; Kepez, A.; Atas, D.B.; Kanar, B.G.; Dervisova, R.; Kivrak, T.; Tigen, M.K. Effects of diabetes mellitus on left atrial volume and functions in normotensive patients without symptomatic cardiovascular disease. J. Diabetes Its Complicat. 2014, 28, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Cameli, M.; Lisi, M.; Focardi, M.; Reccia, R.; Natali, B.M.; Sparla, S.; Mondillo, S. Left atrial deformation analysis by speckle tracking echocardiography for prediction of cardiovascular outcomes. Am. J. Cardiol. 2012, 15, 264–269. [Google Scholar] [CrossRef]
- Bytyçi, I.; D’Agostino, A.; Bajraktari, G.; Lindqvist, P.; Dini, F.L.; Henein, M.Y. Left atrial stiffness predicts cardiac events in patients with heart failure and reduced ejection fraction: The impact of diabetes. Clin. Physiol. Funct. Imaging 2021, 41, 208–216. [Google Scholar] [CrossRef]
- Zhu, S.; Lin, Y.; Zhang, Y.; Wang, G.; Qian, M.; Gao, L.; Ji, M.; Xie, M.; Li, Y.; Zhang, L. Prognostic relevance of left atrial function and stiffness in heart failure with preserved ejection fraction patients with and without diabetes mellitus. Front. Cardiovasc. Med. 2022, 9, 947639. [Google Scholar] [CrossRef]
- Arnautu, D.A.; Arnautu, S.F.; Tomescu, M.C.; Luca, S.; Luca, C.T. Increased Left Atrial Stiffness is Significantly Associated with Paroxysmal Atrial Fibrillation in Diabetic Patients. Diabetes Metab. Syndr. Obes. 2023, 16, 2077–2087. [Google Scholar] [CrossRef] [PubMed]
- Du Bois, D.; Du Bois, E.F. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 1989, 5, 303–313. [Google Scholar] [PubMed]
- McEvoy, J.W.; McCarthy, C.P.; Bruno, R.M.; Brouwers, S.; Canavan, M.D.; Ceconi, C.; Christodorescu, R.M.; Daskalopoulou, S.S.; Ferro, C.J.; Gerdts, E.; et al. 2024 ESC Guidelines for the management of elevated blood pressure and hypertension. Eur. Heart J. 2024, 45, 3912–4018. [Google Scholar] [CrossRef]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Industry representatives, & Reviewers: This document was reviewed by members of the 2016–2018 EACVI Scientific Documents Committee. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar]
- Gan, G.C.H.; Ferkh, A.; Boyd, A.; Thomas, L. Left atrial function: Evaluation by strain analysis. Cardiovasc. Diagn. Ther. 2018, 8, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Ghoreyshi-Hefzabad, S.M.; Jeyaprakash, P.; Vo, H.Q.; Gupta, A.; Ozawa, K.; Pathan, F.; Negishi, K. Subclinical systolic dysfunction detected by 2D speckle tracking echocardiography in adults with diabetes mellitus: Systematic review and meta-analysis of 6668 individuals with diabetes mellitus and 7218 controls. Int. J. Cardiovasc. Imaging 2023, 39, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Silverii, G.A.; Toncelli, L.; Casatori, L.; Bossini, R.; Nannelli, F.; Pala, L.; Mannucci, E. Assessment of left ventricular global longitudinal strain in patients with type 2 diabetes: Relationship with microvascular damage and glycemic control. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Romano, L.R.; Scalzi, G.; Malizia, B.; Aquila, I.; Polimeni, A.; Indolfi, C.; Curcio, A. Impact of Percutaneous Mitral Valve Repair on Left Atrial Strain and Atrial Fibrillation Progression. J. Cardiovasc. Dev. Dis. 2023, 10, 320. [Google Scholar] [CrossRef] [PubMed]
| Variable | Patients with DM (n = 25 Patients) | Patients Without DM (n = 22 Patients) | p-Value |
|---|---|---|---|
| Demographic characteristics and cardiovascular risk factors | |||
| Age, mean ± SD | 60.96 ± 9.80 | 53.77 ± 13.66 | 0.047 |
| Female, n (%) | 10 (40%) | 11 (50%) | 0.506 |
| Smoking, n (%) | 9 (36%) | 1 (4.5%) | 0.001 |
| Alcohol user, n (%) | 7 (28%) | 1 (4.5%) | 0.002 |
| Hypertension, n (%) | 23 (92%) | 14 (63.6%) | 0.022 |
| Dyslipidemia, n (%) | 25 (100%) | 15 (68.2%) | 0.005 |
| Admission hemodynamics | |||
| BSA (m2), mean ± SD | 1.99 ± 0.24 | 1.98 ± 0.19 | 0.875 |
| Heart rate (bpm), mean ± SD | 77.92 ± 12.46 | 73.82 ± 11.85 | 0.253 |
| SBP (mmHg), mean ± SD | 152.80 ± 21.69 | 136.18 ± 12.58 | 0.002 |
| DBP (mmHg), mean ± SD | 86.72 ± 11.78 | 84.18 ± 9.15 | 0.411 |
| Biological profile | |||
| NT-proBNP (pg/L), mean ± SD | 140.75 ± 81.66 | 93.19 ± 50.74 | 0.219 |
| Glycosylated hemoglobin (%), mean ± SD | 6.78 ± 1.10 | 4.97 ± 0.42 | 0.125 |
| Total cholesterol (mg/dL), mean ± SD | 192.16 ± 46.89 | 196.91 ± 44.39 | 0.723 |
| LDL cholesterol (mg/dL), mean ± SD | 111.04 ± 41.21 | 122.36 ± 37.86 | 0.331 |
| HDL cholesterol (mg/dL), mean ± SD | 47.16 ± 26.13 | 52.91 ± 13.66 | 0.342 |
| Triglycerides (mg/dL), mean ± SD | 185.00 ± 108.35 | 111.73 ± 53.99 | 0.235 |
| Creatinine clearance (mL/min/1.73 m2), mean ± SD | 81.00 ± 15.69 | 91.46 ± 18.78 | 0.045 |
| Variable | Patients with DM (n = 25 Patients) | Patients Without DM (n = 22 Patients) | p-Value |
|---|---|---|---|
| Demographic characteristics and cardiovascular risk factors | |||
| LVEDV (mL) | 110.68 ± 36.47 | 107.05 ± 32.20 | 0.718 |
| LVESV (mL) | 51.72 ± 20.25 | 47.95 ± 16.11 | 0.481 |
| LVEF (%) | 54.04 ± 5.79 | 54.86 ± 4.71 | 0.593 |
| E/A | 0.96 ± 0.39 | 1.07 ± 0.37 | 0.304 |
| E/E′ septal | 8.96 ± 2.19 | 8.16 ± 2.97 | 0.306 |
| E/E′ lateral | 7.48 ± 2.79 | 6.96 ± 2.57 | 0.510 |
| GLS (%) | −15.18 ± 3.66 | −16.89 ± 4.34 | 0.156 |
| LAVI (mL) | 60.56 ± 17.59 | 53.45 ± 14.52 | 0.136 |
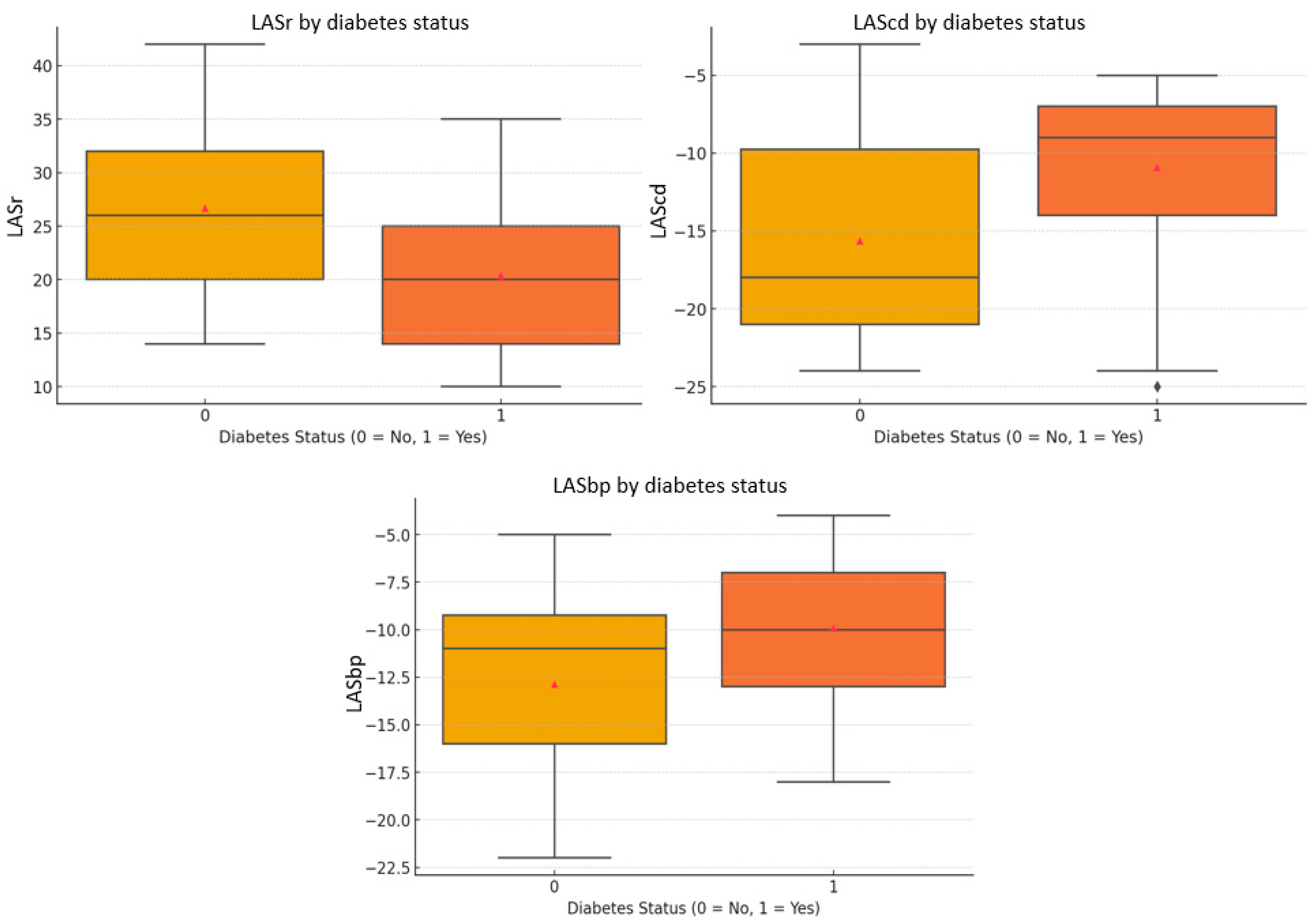
| LASr (%) | 20.40 ± 7.25 | 26.68 ± 8.00 | 0.007 |
| LAScd (%) | −10.92 ± 5.42 | −15.64 ± 6.46 | 0.010 |
| LASbp (5) | −9.92 ± 4.20 | −12.87 ± 4.97 | 0.034 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benchea, L.-C.; Anghel, L.; Dubei, N.; Zanfirescu, R.-L.; Bîrgoan, G.-S.; Sascău, R.A.; Stătescu, C. Impaired Left Atrial Strain as an Early Marker of Cardiac Involvement in Type 2 Diabetes Mellitus: A Cross-Sectional Study. J. Cardiovasc. Dev. Dis. 2025, 12, 369. https://doi.org/10.3390/jcdd12090369
Benchea L-C, Anghel L, Dubei N, Zanfirescu R-L, Bîrgoan G-S, Sascău RA, Stătescu C. Impaired Left Atrial Strain as an Early Marker of Cardiac Involvement in Type 2 Diabetes Mellitus: A Cross-Sectional Study. Journal of Cardiovascular Development and Disease. 2025; 12(9):369. https://doi.org/10.3390/jcdd12090369
Chicago/Turabian StyleBenchea, Laura-Cătălina, Larisa Anghel, Nicoleta Dubei, Răzvan-Liviu Zanfirescu, Gavril-Silviu Bîrgoan, Radu Andy Sascău, and Cristian Stătescu. 2025. "Impaired Left Atrial Strain as an Early Marker of Cardiac Involvement in Type 2 Diabetes Mellitus: A Cross-Sectional Study" Journal of Cardiovascular Development and Disease 12, no. 9: 369. https://doi.org/10.3390/jcdd12090369
APA StyleBenchea, L.-C., Anghel, L., Dubei, N., Zanfirescu, R.-L., Bîrgoan, G.-S., Sascău, R. A., & Stătescu, C. (2025). Impaired Left Atrial Strain as an Early Marker of Cardiac Involvement in Type 2 Diabetes Mellitus: A Cross-Sectional Study. Journal of Cardiovascular Development and Disease, 12(9), 369. https://doi.org/10.3390/jcdd12090369









